Graft-versus-host disease (GVHD) is a major complication of allogeneic bone marrow transplantation. One strategy to treat GVHD is to equip donor T cells with a conditional suicide mechanism that can be triggered when GVHD occurs. The herpes simplex virus thymidine kinase (HSV-tk)/ganciclovir system used clinically has several limitations, including immunogenicity and cell cycle dependence. An alternative switch based on chemically inducible apoptosis was designed and evaluated. A chimeric human protein was expressed comprising an extracellular marker (ΔLNGFR), the Fas intracellular domain, and 2 copies of an FK506-binding protein (FKBP). Primary human T lymphocytes retrovirally transduced with this construct could be purified to homogeneity using immunomagnetic beads. Genetic integrity of the construct was ensured by redesigning repetitive sequences. Transduced T cells behaved indistinguishably from untransduced cells, retaining the ability to mount a specific antiallogeneic immune response. However, they rapidly underwent apoptosis with the addition of subnanomolar concentrations of AP1903, a bivalent “dimerizer” drug that binds FKBP and induces Fas cross-linking. A single 2-hour treatment eliminated approximately 80% of T cells, and multiple exposures induced further apoptosis. T cells were eliminated regardless of their proliferation state, suggesting that the AP1903/Fas system, which contains only human components, is a promising alternative to HSV-tk for treating GVHD.
Introduction
In allogeneic bone marrow transplantation (BMT), the delayed infusion of donor lymphocytes plays a central therapeutic role in the control of disease relapse (graft-versus-leukemia effect [GVL])1 and in the induction of immune reconstitution,2,3 the latter especially in the subset of T-depleted matched transplants and in the context of partially mismatched transplants.4 However, graft-versus-host-disease (GVHD) represents a frequent and often lethal complication of delayed lymphocyte infusions.1 Managing the threat of GVHD while maximizing the beneficial GVL effect would broaden the scope and usefulness of allogeneic BMT procedures.
We and others have previously demonstrated that ex vivo genetic manipulation of donor lymphocytes to insert a conditional, drug-inducible suicide gene provides a means for the specific elimination of donor T cells with the onset of GVHD while maximizing the therapeutic benefit of the GVL effect.2,5,6 Although a number of suicide genes have been proposed,7,8 the herpes simplex virus thymidine kinase (HSV-tk)/ganciclovir (GCV)-based suicide strategy appears to be the most effective and specific and has been widely adopted.9,10 Cells are engineered to express HSV-tk; the addition of ganciclovir leads to cell death through tk-catalyzed metabolism of the drug to a lethal product. In the current clinical trial,6HSV-tk-engineered–donor T cells demonstrated an effective antileukemic effect, and GVHD could be successfully treated through GCV administration.2 11
Despite this demonstration of efficacy, the study revealed limitations of the HSV-tk/GCV approach. First, in 8 of 24 treated patients, a specific cytotoxic CD8-mediated immune response developed against genetically engineered cells that led to the selective elimination of these cells (C.T., unpublished data). Although the cells expressed both HSV-tk and the cell surface marker ΔLNGFR (the extracellular and transmembrane domains of the human low-affinity nerve growth factor receptor), the immune response was directed exclusively against HSV-tk. This suggests that virally derived proteins were recognized by the immune system and eliminated, whereas ectopically expressed human products were not targets of immune recognition. Second, one patient with chronic GVHD exhibited a partial resistance to GCV-mediated elimination of transduced cells. This was attributed to the involvement of slowly proliferating lymphocytes in chronic GVHD and the cell cycle dependence of the HSV-tk–mediated killing. This suggests that the HSV-tk approach may have limited usefulness for the treatment of chronic GVHD.11 Finally, some patients received GCV for concurrent clinical conditions other than GVHD, resulting in the expected, though undesired, clearance of HSV-tk donor lymphocytes.
To circumvent these limitations, we assessed the suitability of a novel suicide switch based on the human Fas receptor to trigger cell death in primary human T lymphocytes. Fas (CD95, APO-1) is a member of the tumor necrosis–nerve growth factor receptor superfamily.12Cross-linking of Fas results in the recruitment of a death-inducing signaling complex, activating a proteolytic cascade of caspases and inducing cell death by apoptosis.12,13 We and others have previously described a system for activating apoptosis at will and demonstrated its function in engineered cell lines.14,15 A chimeric protein containing the membrane-anchored intracellular domain of Fas is fused to the FK506-binding protein, FKBP12. Cross-linking of these proteins by the addition of a bivalent FKBP ligand (a “dimerizer”) triggers the apoptotic death signal. Recently, we refined the system by designing a dimerizer drug, designated AP1903, with specificity for the engineered FKBP over the endogenous protein.16
In this report, we describe the experimental evaluation of the Fas-based suicide switch for the elimination of primary human T lymphocytes for the ultimate purpose of treating GVHD in BMT patients. At the beginning of this work we identified several features that would be required for successful use of the Fas switch—(1) efficient functional expression in primary human T lymphocytes; (2) very high purity of engineered cells to permit the quantitative elimination of cells in vivo; (3) genomic stability of the transgene, for the same reason; (4) rapid, efficient killing of engineered cells with low concentrations of drug; and (5) unaltered immune competence of engineered cells. We describe here the development and characterization of a Fas-based system that meets these criteria. We also use our experimental system to define a drug-dosing regimen that substantially eliminates all cells and, hence, is appropriate for clinical applications. The AP1903/Fas system is, therefore, a promising candidate for clinical use in controlling GVHD and should also be broadly useful for conditional ablation of T-cell subpopulations for research purposes.
Materials and methods
Retroviral plasmid construction
The construct LVVFas was derived from pSRα-LNGFR-2x(F36V-FKBP)-Fas-E14,16,17 by removal of the C-terminal HA tag. LV′VFas was constructed by substitution of the first F36V-FKBP with the “codon-wobbled” F36V′-FKBP (see below). The construct LV′V, which lacks the cytoplasmic domain of Fas, was used as a negative control. The gene cassettes were ligated into the retroviral vector pMX18 that was modified to include a puromycin resistance gene.
F36V′-FKBP construction
The codon-wobbled version of F36V-FKBP, called F36V′-FKBP, was constructed by a polymerase chain reaction (PCR) assembly procedure that contained 3 overlapping oligonucleotides and a 100-fold molar excess of 2 flanking primers (sequences available on request). The expected approximately 335-bp product was gel-purified, cloned, and sequenced to identify a correct clone.
Southern blot analysis
Genomic DNA was isolated from T lymphocytes by standard phenol–chloroform extraction. DNA was digested with XbaI, which cuts between ΔLNGFR and the first F36V-FKBP, and in the 3′ LTR. Blots (Hybond-N nylon membrane; Amersham, Buckinghamshire, United Kingdom) were hybridized with the XbaI–NaeI fragment of LV′VFas encoding for F36V′-F36V-Fas.
Polymerase chain reaction assay of FKBP region in Fas transgenes
Genomic DNA from Fas-engineered cells was prepared by digestion of 0.5 × 106 cells with 50 μg/mL proteinase K. Using a standard PCR reaction, the FKBP region of the Fas suicide cassette was amplified using 2 FKBP flanking primers (D. J. Talbot, unpublished data).
Retroviral transduction
Retroviral plasmids were introduced into amphotropic Phoenix cells (G. P. Nolan, Stanford, CA) by lipofection. Stable transfectants were identified by resistance to 50 μg/mL puromycin. Retroviral supernatants were used to infect human peripheral blood lymphocytes as follows. Peripheral blood was collected from healthy donors, and T lymphocytes were purified by Ficoll-Histopaque (Sigma, St Louis, MO) density centrifugation followed by magnetic selection using anti-CD3 Dynabeads (Dynal, Oslo, Norway). Bead-adherent cells (2-4 × 106/mL) were cultured for 48 hours in RPMI 1640 supplemented with 10% fetal calf serum, 100 ng/mL anti-CD28 (Becton Dickinson, San Jose, CA), and 50 U/mL huIL-2. T lymphocytes (and NIH 3T3 cells) were transduced with retrovirus-containing supernatant either by plating on retronectin-coated wells (BioWhittaker, Walkersville, MD) or by centrifugation (90 minutes, 2000 rpm) in the presence of 4 μg/mL polybrene. Both methods gave similar transduction efficiencies; for T lymphocytes, this ranged from 10% to 50%.
Immunomagnetic selection of Fas-transduced T lymphocytes
Transduced T cells were immunoselected for expression of the cell surface marker ΔLNGFR as previously described.2 6Briefly, cells were incubated with mouse antihuman LNGFR monoclonal antibody 20.4 (Chromaprobe, Mountain View, CA) and selected with goat antimouse IgG-coated magnetic beads (Dynabeads M-450; Dynal). After overnight incubation at 37°C, magnetic beads were separated from the cells by pipetting and were removed magnetically from the culture. T lymphocytes purified in this manner were routinely more than 95% LNGFR-positive after a single round of selection. Cell recovery ranged between 40% and 50%.
Apoptosis–cell death assays
Elimination of T cells was measured by the following assay. Untransduced T cells were loaded with CellTracker Green CMFDA (Molecular Probes, Eugene, OR) as an internal control and were used to spike immunomagnetically sorted LV′VFas-transduced T cells. The cell mixture (2 × 106 cells/mL) was incubated with AP1903 for the indicated time, stained with 7-amino-actinomycin D (7-AAD, 2 μg/mL; Sigma) for 15 minutes on ice, and analyzed on a Becton Dickinson FACSort. The ratio (R) of live-gated (by forward/side scatter and 7-AAD), LV′VFas-transduced cells (nonfluorescent)–untransduced cells (green-fluorescent) was used to calculate specific cell survival using the following formula: %Survival = (R, drug-treated)/(R, untreated) × 100%. For annexin V assays, sorted LV′VFas-transduced T cells (2 × 106 cells/mL) were incubated with 10 nM AP1903. At the indicated time, an aliquot of 2 × 105cells was taken, stained with annexin V–fluorescein isothiocyanate according to the manufacturer's instructions (Clontech, Palo Alto, CA), and analyzed by flow cytometry.
To compare the AP1903/FAS suicide system with the standard ganciclovir–HSV-tk system, human T lymphocytes transduced with the LV′VFas retroviral vector or with the SFCMM-3 retroviral vector11 (carrying the HSV-tk gene) were immunoselected to more than 95% purity. Cells were cultivated for 5 days in the absence and in the presence of 10 nM AP1903 or 50 nM GCV in triplicate. [3H]thymidine (1μ Ci/well; specific activity 87 Ci/mmol; Dupont, Boston, MA) was added 16 hours before harvesting the DNA and counting in a β-scintillation counter (Wallac, Turku, Finland, 1205 β-plate). The effects of the drugs on transduced lymphocytes were expressed as percentage of cell survival, referring to proliferation in the absence of the drug.
Analysis of antiallogeneic response
Antiallogeneic cytotoxic T lymphocytes were induced in vitro in a mixed lymphocyte reaction (MLR) with 2 × 106LV′VFas-transduced donor effector lymphocytes or untransduced cells as control and 1 × 106 irradiated (6 Gy) allogeneic peripheral blood mononuclear cells (PBMC) (fully mismatched). The MLR was performed in Iscoves modified Dulbecco medium supplemented with 10% human serum, glutamine, and antibiotics in the presence of 50 U/mL huIL-2. Equal numbers of lymphocytes were tested in a standard cytotoxicity assay 10 days later, using as target cells51Cr-labeled phytohemagglutinin (PHA)–stimulated PBMCs from the same allogeneic donor and autologous PHA blasts as negative control cells. Natural killer cell–like activity was blocked by cold inhibition with a 30-fold excess of K562 cells over51Cr-labeled specific target cells. A secondary MLR was performed by the addition of irradiated allogeneic PBMC (1 × 106) in the presence or absence of 10 nM AP1903. Ten days later cells were counted, and the cytotoxic activity of equivalent numbers of cells was measured as above.
Results
Development of a conditional Fas suicide switch for use in primary human T cells
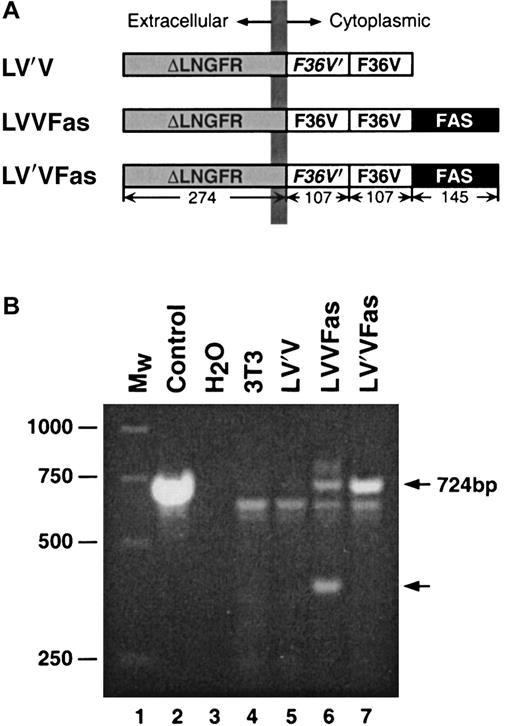
The original format of the inducible Fas suicide system was described by Spencer et al15 and comprised the cytoplasmic domain of the human Fas death receptor fused to 2 copies of human wild-type FKBP12 and an epitope tag. This chimeric protein was fused to an N-terminal myristoylation motif to localize it to the plasma membrane. For this study, we made several modifications to optimize the construct for clinical use in T cells. We replaced the 2 FKBP12 domains with the FKBP point mutant, F36V-FKBP, to allow use of the more potent and specific dimerizer drug, AP1903.16 We removed the C-terminal epitope tag to minimize the potential immunogenicity of the protein. In addition, we replaced the myristoylation motif with the extracellular and transmembrane domains of LNGFR to localize the chimeric protein to the plasma membrane and simultaneously provide a cell surface marker.6 17 The approach physically links the conditional apoptosis cassette with an affinity handle that can be used to purify transduced cells, providing a means to ensure that all resultant cells can be eliminated by adding AP1903. The resultant gene cassette is herein called LVVFas (Figure1A).
Fas constructs and PCR analysis.
(A) Schematic of Fas constructs. The constructs LV′V, LVVFas and LV′VFas contain combinations of the following elements, as indicated: ΔLNGFR, the extracellular and transmembrane domains (residues 1-274) of the human low-affinity nerve growth factor receptor; F36V, Phe36 → Val36 point mutant of human FKBP12;F36V′, codon-wobbled F36V; FAS, cytoplasmic domain (residues 175-319) of human Fas. Sizes (in amino acids) of the individual components are indicated. Expression of the transgenes was driven by the MoMLV LTR using the retroviral vector pMX. (B) PCR analysis of FKBP region in Fas-transduced NIH 3T3 cells. Fas-transduced 3T3 cells were lysed, and the integrity of the FKBP region of the Fas transgene was analyzed by PCR. Lane 1, molecular weight standard; lane 2, control LV′VFas plasmid; lane 3, no DNA control; lane 4, untransduced 3T3 cells; lane 5, LV′V-transduced 3T3 cells; lane 6, LVVFas-transduced 3T3 cells; lane 7, LV′VFas-transduced 3T3 cells. Arrows on the right indicate the sizes of the expected band (724 bp) and a band truncated by approximately 300 bp.
Fas constructs and PCR analysis.
(A) Schematic of Fas constructs. The constructs LV′V, LVVFas and LV′VFas contain combinations of the following elements, as indicated: ΔLNGFR, the extracellular and transmembrane domains (residues 1-274) of the human low-affinity nerve growth factor receptor; F36V, Phe36 → Val36 point mutant of human FKBP12;F36V′, codon-wobbled F36V; FAS, cytoplasmic domain (residues 175-319) of human Fas. Sizes (in amino acids) of the individual components are indicated. Expression of the transgenes was driven by the MoMLV LTR using the retroviral vector pMX. (B) PCR analysis of FKBP region in Fas-transduced NIH 3T3 cells. Fas-transduced 3T3 cells were lysed, and the integrity of the FKBP region of the Fas transgene was analyzed by PCR. Lane 1, molecular weight standard; lane 2, control LV′VFas plasmid; lane 3, no DNA control; lane 4, untransduced 3T3 cells; lane 5, LV′V-transduced 3T3 cells; lane 6, LVVFas-transduced 3T3 cells; lane 7, LV′VFas-transduced 3T3 cells. Arrows on the right indicate the sizes of the expected band (724 bp) and a band truncated by approximately 300 bp.
One of the major safety considerations of using any suicide gene approach for the treatment of GVHD is that all the ex vivo–engineered T lymphocytes to be infused into the patient must express the functional suicide gene. This is of particular concern with the AP1903/Fas system because of the presence of repetitive sequences (2 copies of F36V-FKBP).16 Retrovirally introduced transgenes containing repetitive sequences tend to have a high frequency of rearrangement, which very likely would compromise the function of the chimeric Fas protein. Selection of transduced cells using the LNGFR surface marker does not necessarily prevent exclusion of cells expressing a rearranged transgene because the rearrangement may not affect the LNGFR portion of the protein. Previous studies that used the conditional Fas suicide approach did not report problems with rearrangement, but in these cases nonretroviral approaches or clones of transduced cells were used.14-17 19
To reduce the risk for rearrangement associated with the 2 identical copies of F36V-FKBP, we designed a “codon-wobbled” variant of F36V-FKBP, termed F36V′-FKBP. These 2 constructs encode identical polypeptide chains but share only 62% homology at the nucleotide level by virtue of silent changes of the wobble bases in most codons. The construct LV′VFas incorporates one copy of F36V′-FKBP and one copy of F36V-FKBP and is anticipated to be less recombinogenic than LVVFas (Figure 1A). A control construct, LV′V, lacks the cytoplasmic domain of Fas and thus is incapable of signaling. These transgenes were cloned into the retroviral vector pMX,18 which uses the MoMLV LTR to drive expression and which was found to provide a higher-level expression of the chimeric protein than LXSN-based vectors (data not shown).
F36V′-FKBP prevents rearrangement of the Fas transgene during retroviral transduction
To test the performance and genetic stability of these constructs, they were transiently transfected into amphotropic Phoenix packaging cells, and supernatant collected 48 hours later was used to transduce NIH 3T3 cells. To examine the integrity of the FKBP portion of the Fas transgene, we used a PCR-based assay in which the FKBP portion is amplified from the genomic DNA of transduced cells. Cells transduced with LV′VFas yielded a single PCR product of the expected size, 724 bp (Figure 1B, lane 7). However, cells transduced with LVVFas produced an additional band reduced in size by approximately 300 bp, a size difference consistent with the deletion of one FKBP (Figure 1B, lane 6). This result indicates that there is a significant frequency of rearrangement with the Fas construct containing 2 identical copies of F36V-FKBP but that the use of the modified F36V′-FKBP/ F36V-FKBP combination eliminates this problem.
Engineering of primary human T lymphocytes with Fas suicide genes and immunomagnetic selection of transduced cells
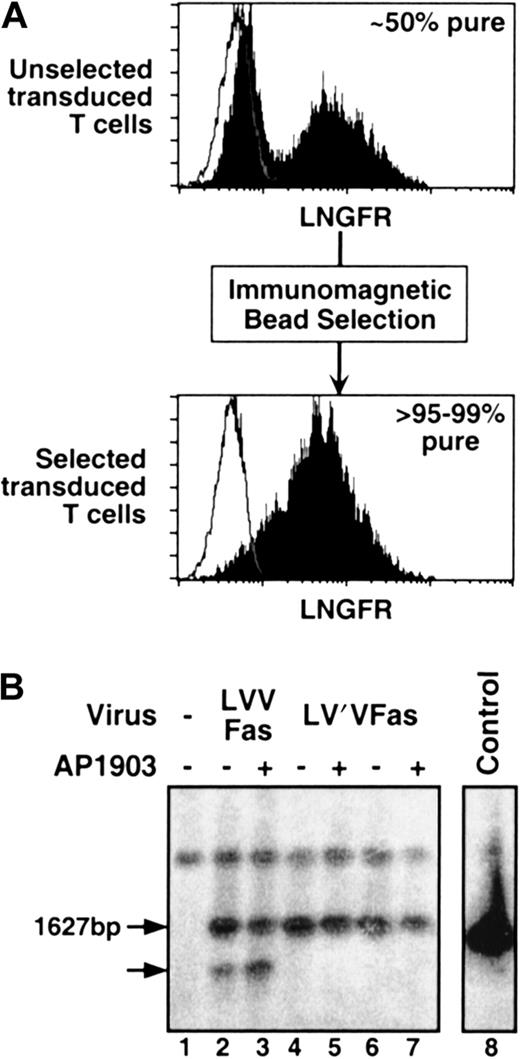
We next evaluated the expression and stability of the conditional Fas constructs in T cells. Primary human T lymphocytes were isolated from peripheral blood obtained from volunteer donors using anti-CD3 magnetic beads. Purified T lymphocytes were stimulated for 48 hours before infection with Fas recombinant retroviruses. We observed high-level expression of the transgene as monitored by flow cytometry to detect LNGFR-positive cells (Figure2A), but there was a complete absence of “toxicity” of the construct (data not shown). To test whether the ΔLNGFR cell surface marker could be used to isolate transduced T cells, LNGFR-positive cells were immunoselected 48 hours after infection with anti-LNGFR magnetic beads as previously described.2 We found that cells sorted in this manner were routinely more than 95% to 99% LNGFR-positive in a single round of purification (Figure 2A). Of note, cell viability was not compromised during the selection process (data not shown). This is an important observation because cross-linking of LNGFR by the antibody-coated beads might be expected to cluster the Fas domains and activate signaling, an outcome that would complicate the use of this procedure to isolate transduced cells. Similarly, ligation of LNGFR on the surface of LV′VFas-transduced T cells with human β nerve growth factor (β-NGF) had no effect on cell viability (data not shown). The fact that these procedures do not trigger apoptosis suggests that the cross-linking of LNGFR brings together the Fas death domains in a structural context that is unproductive for signaling.
Immunomagnetic selection and Southern blot analysis.
(A) Immunomagnetic selection of Fas-engineered T lymphocytes. Fas-transduced primary human T lymphocytes were selected with anti-LNGFR–coated magnetic beads, as described in “Materials and methods.” Before selection, cells were 10% to 50% LNGFR-positive; after selection, they were routinely more than 95% to 99% pure. Histogram plots show the LNGFR staining profile of transduced (filled histograms) and untransduced cells (open histograms). (B) Southern blot analysis of Fas-transduced T lymphocytes. Immunomagnetically sorted, Fas-transduced primary human T lymphocytes were either challenged with 10 nM AP1903 for 10 days (+) or left unchallenged (−). DNA was digested with XbaI, separated by electrophoresis and probed with an FKBP–Fas-specific probe. Lane 1, untransduced control lymphocytes; lanes 2 and 3, LVVFas-transduced lymphocytes; lanes 4 and 5, donor A LV′VFas-transduced lymphocytes; lanes 6 and 7, donor B LV′VFas-transduced lymphocytes; lane 8, control plasmid. Arrows indicate the size of the expected band (1627 bp) and a band truncated by approximately 300 bp.
Immunomagnetic selection and Southern blot analysis.
(A) Immunomagnetic selection of Fas-engineered T lymphocytes. Fas-transduced primary human T lymphocytes were selected with anti-LNGFR–coated magnetic beads, as described in “Materials and methods.” Before selection, cells were 10% to 50% LNGFR-positive; after selection, they were routinely more than 95% to 99% pure. Histogram plots show the LNGFR staining profile of transduced (filled histograms) and untransduced cells (open histograms). (B) Southern blot analysis of Fas-transduced T lymphocytes. Immunomagnetically sorted, Fas-transduced primary human T lymphocytes were either challenged with 10 nM AP1903 for 10 days (+) or left unchallenged (−). DNA was digested with XbaI, separated by electrophoresis and probed with an FKBP–Fas-specific probe. Lane 1, untransduced control lymphocytes; lanes 2 and 3, LVVFas-transduced lymphocytes; lanes 4 and 5, donor A LV′VFas-transduced lymphocytes; lanes 6 and 7, donor B LV′VFas-transduced lymphocytes; lane 8, control plasmid. Arrows indicate the size of the expected band (1627 bp) and a band truncated by approximately 300 bp.
To assess the integrity of the Fas transgene in transduced human T lymphocytes, Southern blot analysis was performed. Similar to our findings in 3T3 cells, primary human T lymphocytes transduced with LVVFas yielded—in addition to a band of the expected size (1627 bp)—a band truncated by approximately 300 bp (Figure 2B, lane 2), probably representing the deletion of one FKBP. Challenge of LVVFas-transduced cells with AP1903 resulted in the enrichment of this fragment (Figure2B, lane 3). This result suggests that approximately 10% to 15% of LVVFas-transduced T lymphocytes harbor a rearranged transgene and that these cells have lost sensitivity to AP1903-induced cell death. In contrast, cells transduced with LV′VFas showed no aberrant-sized bands in the absence or in the presence of AP1903 (Figure 2B, lanes 4-7), demonstrating the lack of rearrangement and confirming the integrity of the LV′VFas transgene. Based on these results, subsequent work was carried out exclusively with the LV′VFas construct.
LV′VFas transgene confers susceptibility to AP1903-induced cell death to primary human T lymphocytes
We next assessed the susceptibility of LV′VFas-transduced T cells to AP1903-induced cell death. The Fas system has previously been tested in vitro in several cell lines, including HT1080, 293, Jurkat, and HeLa cells,14,16,17,19 and in vivo in a transgenic mouse model.15 Interestingly, the killing efficiency varied widely in these diverse cell types, perhaps reflecting an intrinsic, cell type–specific susceptibility to Fas-induced apoptosis. This emphasizes that the performance of the Fas system must be optimized for each target cell type—in the case of this study, primary human T cells.
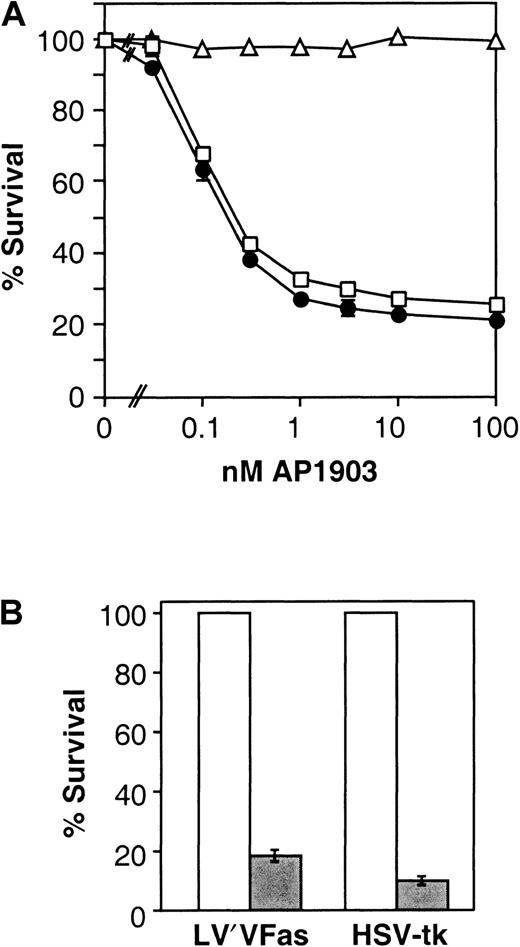
LV′VFas-transduced T lymphocytes were exposed to different concentrations of the dimerizer AP1903, and survival was analyzed 24 and 48 hours later. As shown in Figure3A, the induction of cell death was highly dose dependent. Maximal killing occurred in the presence of 3 to 10 nM AP1903, and the IC50 was approximately 0.2 nM. In a large number of experiments, the maximal killing efficiency was consistently in the range of 60% to 80%, and the IC50 was reproducibly approximately 0.2 nM. We observed no significant donor-to-donor variability of the killing efficiency (data not shown). In addition, when CD4 and CD8 T-cell populations were examined separately, no difference was found in the killing efficiency or in the IC50 value (data not shown). This information is relevant because CD4 and CD8 donor lymphocytes contribute to GVHD, as shown by donor lymphocyte infusion protocols involving the infusion of either CD4-depleted20 or CD8-depleted donor lymphocytes.21 LV′VFas-transduced T lymphocytes demonstrated normal viability in the absence of drug, indicating the absence of autotoxicity—signaling in the absence of added drug (data not shown). AP1903 treatment of LV′V-transduced (Figure 3A) or untransduced control T lymphocytes (data not shown) had no significant effect on their survival, even at the highest concentration of AP1903 tested (1 μM, data not shown). These results demonstrate that the AP1903/Fas suicide system is highly effective and specific to T lymphocytes containing the Fas transgene.
Killing of human T cells.
(A) Dose-response curve of AP1903-induced killing of Fas-transduced human T cells. Primary human T lymphocytes were retrovirally infected, and transduced cells were immunomagnetically sorted. Cells were incubated with the indicated concentrations of the synthetic dimerizer AP1903, and specific survival was measured as described in “Materials and methods.” Cells analyzed are control LV′V-transduced cells (▵), LV′VFas-transduced cells analyzed after 24 hours (■), and LV′VFas-transduced cells analyzed after 48 hours (●). Values are the mean ± SD of duplicate points (except for LV′V). The result shown is representative of at least 5 independent experiments. (B) Comparison of killing of human T cells engineered with LV′VFas or HSV-tk. Primary human T lymphocytes were transduced with LV′VFas or SFCMM-3 (HSV-tk), immunomagnetically purified and treated with either 10 nM AP1903 or 50 nM GCV for 5 days. Survival was measured as described in “Materials and methods.” Open bars represent untreated cells; filled bars represent cells treated with drug. Values are the mean ± SD of 3 independent points.
Killing of human T cells.
(A) Dose-response curve of AP1903-induced killing of Fas-transduced human T cells. Primary human T lymphocytes were retrovirally infected, and transduced cells were immunomagnetically sorted. Cells were incubated with the indicated concentrations of the synthetic dimerizer AP1903, and specific survival was measured as described in “Materials and methods.” Cells analyzed are control LV′V-transduced cells (▵), LV′VFas-transduced cells analyzed after 24 hours (■), and LV′VFas-transduced cells analyzed after 48 hours (●). Values are the mean ± SD of duplicate points (except for LV′V). The result shown is representative of at least 5 independent experiments. (B) Comparison of killing of human T cells engineered with LV′VFas or HSV-tk. Primary human T lymphocytes were transduced with LV′VFas or SFCMM-3 (HSV-tk), immunomagnetically purified and treated with either 10 nM AP1903 or 50 nM GCV for 5 days. Survival was measured as described in “Materials and methods.” Open bars represent untreated cells; filled bars represent cells treated with drug. Values are the mean ± SD of 3 independent points.
As shown in Figure 3B, the extent of cell death of drug-treated Fas-engineered T cells is comparable to that of HSV-tk–engineered cells. In this experiment a second-generation vector expressing wild-type HSV-tk(SFCMM-3) was used.11 Because a previous version of this vector (SFCMM-2, which expresses HSV-tk-neo fusion) confers significantly lower sensitivity to GCV11 but was used successfully to ablate T cells and treat GVHD in clinical trials,2 these data suggest that the AP1903-Fas system may also be suitable for clinical use to treat GVHD.
Induction of apoptosis by the AP1903/Fas system occurs within 1 hour of drug addition
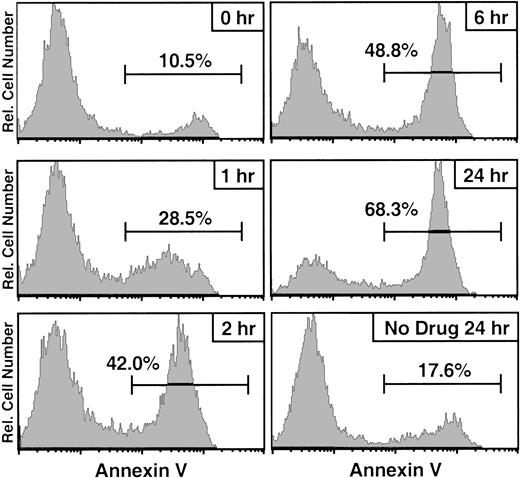
To provide rapid mitigation of GVHD, it is important that the donor T cells be neutralized as quickly as possible. In contrast to HSV-tk–induced cell death, Fas-induced apoptosis is expected to be a rapid event that occurs within hours of the appropriate signal. Some of the earliest detectable events in the apoptotic pathway are the loss of the cytoplasmic polarization of plasma membrane phosphatidylserine and the fragmentation of nuclear DNA.13,22,23 We used the former readout to determine the kinetics of AP1903-induced cell death. LV′VFas-transduced T lymphocytes were treated with 10 nM AP1903 and stained with annexin V24 at different times (Figure4). In as little as 1 hour, apoptotic cells were clearly detectable. The percentage of apoptotic cells continued to increase over time; after 24 hours of drug treatment, 68% of the cells were apoptotic (Figure 4). In contrast, LV′VFas-transduced lymphocytes cultured in the absence of AP1903 showed only a small increase in the proportion of apoptotic cells that could be attributed to spontaneous cell death during culture (Figure 4). Similarly, no significant change in the annexin V staining profile was observed when untransduced T cells were incubated with AP1903 (data not shown). These findings show that AP1903-induced apoptosis is extremely fast. Furthermore, the kinetics observed with AP1903-induced apoptosis are similar to those of primary human lymphocytes (our unpublished observations) or the human Jurkat T-cell line treated with anti-Fas antibodies,24 suggesting that the same signaling cascade is triggered by both agents.
Annexin V kinetics of AP1903/Fas-induced apoptosis.
LV′VFas-transduced primary human T lymphocytes were incubated with 10 nM AP1903, and aliquots of cells were stained with annexin V after 1, 2, 6, and 24 hours. As a control, cells were stained at the beginning of the treatment (0 hour) and after 24 hours in the absence of drug. Cells were analyzed by the use of flow cytometry, and the annexin V staining profile is shown. Percentages of annexin-V–positive (apoptotic) cells are indicated. Untransduced T cells incubated with 10 nM AP1903 showed no significant change in their annexin V staining profile (data not shown).
Annexin V kinetics of AP1903/Fas-induced apoptosis.
LV′VFas-transduced primary human T lymphocytes were incubated with 10 nM AP1903, and aliquots of cells were stained with annexin V after 1, 2, 6, and 24 hours. As a control, cells were stained at the beginning of the treatment (0 hour) and after 24 hours in the absence of drug. Cells were analyzed by the use of flow cytometry, and the annexin V staining profile is shown. Percentages of annexin-V–positive (apoptotic) cells are indicated. Untransduced T cells incubated with 10 nM AP1903 showed no significant change in their annexin V staining profile (data not shown).
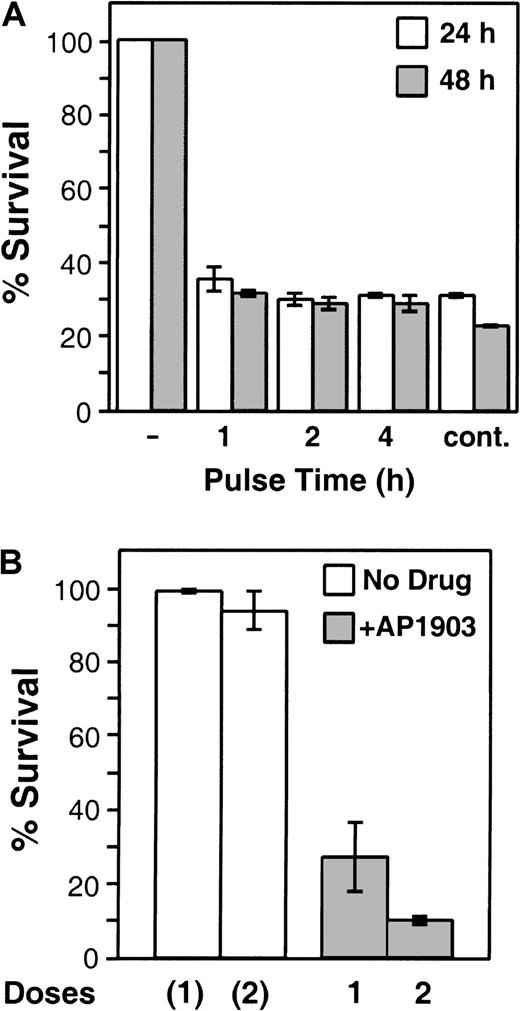
Maximal killing is achieved even by short exposures to AP1903, and additional doses increase the efficiency
To explore the AP1903 dosing regimen and administration schedule likely to be required in a clinical setting, we determined the length of time Fas-engineered T cells must be exposed to the drug to trigger apoptosis. LV′VFas-transduced T lymphocytes were treated with 10 nM AP1903 for 1, 2, or 4 hours, the drug was washed away, and the cells were further incubated until analysis at 24 or 48 hours. Surprisingly, even a 1-hour exposure resulted in near-maximal cell death (Figure5A). Prolonging the duration to 2 or 4 hours did not significantly increase the killing efficiency, and the continuous presence of the drug had only a small additional effect at the 48-hour time point (Figure 5A).
Short-term and multidose treatment.
(A) Short-term treatment (“pulsing”) of Fas-transduced T cells with AP1903. LV′VFas-transduced primary human T lymphocytes were incubated with 10 nM AP1903 for 1, 2, or 4 hours and were washed extensively, and survival was assayed after 24 (open bars) or 48 (shaded bars) hours, as described in “Materials and methods.” As a control, cells were incubated in the absence of AP1903 (−) or in the continuous presence of 10 nM AP1903 (cont.). Values are the mean ± SD of duplicate points. (B) Multidose treatment of Fas-transduced T cells. LV′VFas-transduced T lymphocytes were treated once or twice (48 hours apart) without drug (open bars) or with two 2-hour pulses (doses) of 10 nM AP1903 (shaded bars). In parallel, as a control, cells were treated identically without the addition of drug (open bars). Survival was assayed 48 hours after drug treatment. Values are the mean ± SD of 2 independent experiments.
Short-term and multidose treatment.
(A) Short-term treatment (“pulsing”) of Fas-transduced T cells with AP1903. LV′VFas-transduced primary human T lymphocytes were incubated with 10 nM AP1903 for 1, 2, or 4 hours and were washed extensively, and survival was assayed after 24 (open bars) or 48 (shaded bars) hours, as described in “Materials and methods.” As a control, cells were incubated in the absence of AP1903 (−) or in the continuous presence of 10 nM AP1903 (cont.). Values are the mean ± SD of duplicate points. (B) Multidose treatment of Fas-transduced T cells. LV′VFas-transduced T lymphocytes were treated once or twice (48 hours apart) without drug (open bars) or with two 2-hour pulses (doses) of 10 nM AP1903 (shaded bars). In parallel, as a control, cells were treated identically without the addition of drug (open bars). Survival was assayed 48 hours after drug treatment. Values are the mean ± SD of 2 independent experiments.
The fact that a single AP1903 dose eliminates 60% to 80% of engineered cells raises the question of whether the remaining cells are intrinsically resistant to AP1903-induced apoptosis. This is a significant question because clinical use to mitigate GVHD would be optimal if all engineered cells could be eliminated. We investigated whether cells surviving the first drug challenge could be eliminated by a second drug administration. As shown in Figure 5B, a first dose of AP1903 (2-hour pulse with 10 nM) resulted in the elimination of approximately 72% of LV′VFas-transduced T lymphocytes. Forty-eight hours after the first dose, the surviving cells were treated with a second 2-hour pulse of AP1903. This second dose resulted in the killing of more than 60% of the remaining cells. Together, the 2 pulses (doses) resulted in an aggregate killing efficiency of approximately 90% (Figure 5B). In contrast, control LV′VFas-engineered cells subjected to the same procedure but without the addition of AP1903 showed only a small decrease in viability (Figure 5B). These findings indicate that cells not killed by the first AP1903 treatment have not acquired resistance and are only temporarily refractory to AP1903/Fas-induced cell death. This is supported by the absence of rearranged transgene-bearing cells before or after AP1903 treatment (Figure 2B, lanes 4 to 7). Rather, the presence of cells surviving AP1903 treatment is probably attributable to properties intrinsic to T-cell biology25 and is consistent with previous observations that cross-linking of endogenous Fas on the surface of stimulated primary human T lymphocytes26,27 or human Jurkat T cells28,29 kills only 40% to 80% of the cells. Although the phenomenon is not yet fully understood, temporary resistance of T cells to Fas-induced cell death may reflect stochastic variations in intracellular levels of antiapoptotic molecules such as bcl-2 and bcl-xL,30,31 XIAP,32 or c-FLIP,33 leading to transient blockage of the Fas signal.
Interestingly, AP1903 treatment of Fas-engineered lymphocytes caused the rapid down-regulation of the LV′VFas protein in cells that were not killed by the drug (data not shown). This disappearance from the surface is dependent on the Fas signaling domain; the expression level of the LV′V control transgene is not affected by the drug (data not shown). However, the down-regulation of LV′VFas expression is only temporary. Expression levels recover within 24 to 48 hours after withdrawal of the drug (data not shown).
AP1903/Fas-induced cell death of primary human T lymphocytes is not dependent on the proliferation state of the cell
T lymphocytes are a highly heterogeneous population of cells with different antigen specificities, and they exist in various states of activation and differentiation. One of the inherent limitations of the HSV-tk suicide system is that only cells that progress through the S-phase of the cell cycle are killed by GCV administration. In essence, this means that only activated, rapidly proliferating T cells are eliminated efficiently, whereas cells that are in a resting, nonproliferation state are relatively resistant. In the clinical trial involving the infusion of HSV-tk–engineered lymphocytes, this resulted in the partial resistance of chronic GVHD to GCV treatment.2,11 By contrast, Fas-induced apoptosis does not require S-phase entry.27 The AP1903/Fas suicide system is, therefore, expected to function independently of the proliferative state of the cell. In addition, the transgene-derived Fas-fusion protein is expressed constitutively, and there is less than a 2-fold difference in expression levels between activated and resting T cells (data not shown). Therefore, AP1903-induced apoptosis should operate independently of activation-induced Fas-receptor up-regulation,34 and it should be dependent only on the sequential activation of caspases, which are known to be constitutively expressed.35-37
The cell cycle dependence of the AP1903/Fas system was tested by taking advantage of the transient nature of the activation of in vitro–stimulated T lymphocytes. After the initial stimulation, cells proliferated rapidly and remained in a highly activated state for approximately 2 weeks, after which proliferation slowed down and they gradually returned to a less activated (“resting”) state. To measure the proliferation/activation state of the Fas-engineered T cells, we examined 2 parameters: the expression of the IL-2 receptor α chain (CD25) and the incorporation of [3H]thymidine.
As shown in Figure 6A, LV′VFas-transduced T lymphocytes expressing high levels of CD25 (top panel) were eliminated by AP1903 with 66% ± 7.5% (n = 10) efficiency. When cells were examined after CD25 expression returned to basal levels (Figure 6A, bottom panel), 63% ± 4.7% (n = 9) killing was observed after AP1903 treatment. Taken together, these results show that there is no statistically significant difference (P = .17) between the killing efficiencies of highly activated (CD25hi) T cells and cells that have returned to a less activated state (CD25−). We then analyzed cycling and noncycling LV′VFas-transduced T cells based on their incorporation of [3H]thymidine. Again, there was no significant difference between rapidly cycling and noncycling cells with respect to their killing efficiencies (Figure 6B). These results demonstrate that the AP1903/Fas suicide system functions equally well in rapidly cycling, highly activated T cells and in noncycling, resting T cells. This is in good agreement with the finding that stimulated primary human T cells expressing endogenous Fas could be killed with anti-Fas antibodies or FasL, regardless of their proliferation or activation state.27
AP1903-induced killing is independent of the proliferation state of human T lymphocytes.
(A) LV′VFas-transduced primary human T cells were stained for CD25 at different times after the initial stimulation, and the killing efficiency of CD25hi (top panel) and CD25−(bottom panel) cells was measured 24 hours after treatment with 10 nM AP1903. Representative CD25 staining profiles (filled histograms) with the isotype control (open histograms) are shown on the left side. Survival percentages of AP1903-treated cells are shown on the right side. (B) Five, 12, or 21 days after the initial stimulation LV′VFas-transduced primary human T cells were incubated with [3H]thymidine in the absence of AP1903 to assess proliferation activity (open bars) or in the presence of 10 nM AP1903 to assess relative survival (closed bars). Values are the mean ± SD of 3 independent points.
AP1903-induced killing is independent of the proliferation state of human T lymphocytes.
(A) LV′VFas-transduced primary human T cells were stained for CD25 at different times after the initial stimulation, and the killing efficiency of CD25hi (top panel) and CD25−(bottom panel) cells was measured 24 hours after treatment with 10 nM AP1903. Representative CD25 staining profiles (filled histograms) with the isotype control (open histograms) are shown on the left side. Survival percentages of AP1903-treated cells are shown on the right side. (B) Five, 12, or 21 days after the initial stimulation LV′VFas-transduced primary human T cells were incubated with [3H]thymidine in the absence of AP1903 to assess proliferation activity (open bars) or in the presence of 10 nM AP1903 to assess relative survival (closed bars). Values are the mean ± SD of 3 independent points.
Fas-transduced T lymphocytes are able to mount a specific antiallogeneic response
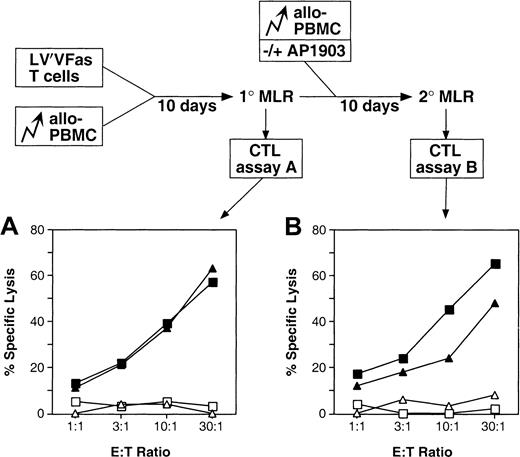
To determine whether Fas-transduced T lymphocytes are immune functional, and in particular whether they retain the ability to generate a specific antiallogeneic response, an MLR was performed. LV′VFas-transduced lymphocytes were stimulated with irradiated, allogeneic PBMCs, and specific cytotoxic activity was measured in a chromium release assay 10 days later. As shown in Figure7A, LV′VFas-transduced T cells exhibited the same specific lytic activity against allogeneic target cells as untransduced T cells, suggesting that transduced cells are immune functional and that activation on encounter with a specific antigen does not trigger spontaneous apoptosis. Because donor T cells play a central therapeutic role during allo-BMT in providing a GVL effect, these properties are important for the eventual use of the AP1903/Fas system to combat GVHD.
Immune potential of Fas-engineered T lymphocytes.
A schematic illustration of the mixed lymphocyte reactions carried out is shown (see also “Materials and methods”). Untransduced or LV′VFas-transduced primary human T cells were incubated with allogeneic PBMC for 10 days (1° MLR). Cultures were divided in half, and a second MLR was performed by another addition of allo-PBMC and in the absence or presence of 10 nM AP1903 (2° MLR). The cytotoxic activity of equivalent numbers of cells from the 1° MLR or the 2° MLR was measured in a standard chromium release assay. (A) Cytotoxic T-lymphocyte (CTL) assay with cells from the 1° MLR: specific antiallo (▴) and antiautologous (▵) response of LV′VFas-engineered cells; and specific antiallo (▪) and antiautologous (■) response of untransduced cells. (B) CTL assay with cells from the 2° MLR: specific antiallo response of LV′VFas-engineered cells cultured in the absence (▪) or the presence (▴) of 10 nM AP1903; and antiautologous control response of LV′VFas-engineered (▵) and untransduced cells (■).
Immune potential of Fas-engineered T lymphocytes.
A schematic illustration of the mixed lymphocyte reactions carried out is shown (see also “Materials and methods”). Untransduced or LV′VFas-transduced primary human T cells were incubated with allogeneic PBMC for 10 days (1° MLR). Cultures were divided in half, and a second MLR was performed by another addition of allo-PBMC and in the absence or presence of 10 nM AP1903 (2° MLR). The cytotoxic activity of equivalent numbers of cells from the 1° MLR or the 2° MLR was measured in a standard chromium release assay. (A) Cytotoxic T-lymphocyte (CTL) assay with cells from the 1° MLR: specific antiallo (▴) and antiautologous (▵) response of LV′VFas-engineered cells; and specific antiallo (▪) and antiautologous (■) response of untransduced cells. (B) CTL assay with cells from the 2° MLR: specific antiallo response of LV′VFas-engineered cells cultured in the absence (▪) or the presence (▴) of 10 nM AP1903; and antiautologous control response of LV′VFas-engineered (▵) and untransduced cells (■).
To test whether the cytotoxic activity of Fas-engineered T cells can be inhibited by AP1903 treatment, cells from the primary MLR were restimulated with allogeneic PBMCs in the absence or presence of 10 nM AP1903. As expected, 60% to 80% of the Fas-engineered cells were killed by the drug in the secondary MLR (data not shown). Interestingly, cells surviving the drug treatment exhibited significant reductions in lytic activity relative to Fas-engineered cells restimulated in the absence of AP1903 (Figure 7B). This result suggests that in addition to the 60% to 80% reduction of cytotoxic activity associated with AP1903-induced cell death, the lytic activity against an allogeneic target cell of Fas-engineered T cells is significantly reduced after exposure to AP1903, an issue particularly relevant in the treatment of GVHD. This result is in good agreement with the previous observation that antiallogeneic lymphocytes challenged with the relevant target cell showed a reduction in proliferation of approximately 35% when they were preincubated with anti-Fas antibody.38
Discussion
In this report, we examined the feasibility of using the AP1903/Fas suicide strategy for the treatment of GVHD after allogeneic BMT by examining its performance in vitro in genetically engineered primary human T lymphocytes. We showed that a modified Fas cassette that incorporates a cell surface marker allows transduced cells to be purified to homogeneity without activating apoptosis. We demonstrated that a “codon-wobbled” version of F36V-FKBP is a crucial element to prevent the rearrangement of the Fas transgene, an important safety consideration in gene therapy applications using retroviral vectors. We showed that primary human T lymphocytes can be engineered to express high levels of the Fas transgene and can then be eliminated by the AP1903/Fas system with high efficiency, potency, and specificity. Importantly, Fas-transduced lymphocytes retain their immune potential, a relevant issue for the GVL effect. The redesigned Fas cassette, therefore, fulfills the criteria we identified at the beginning of this study, required for clinical usefulness for combating GVHD.
Spencer et al15,19 have found that expression of Fas in transiently transfected cells induces apoptosis in the absence of drug addition. Most likely this basal toxicity (“autotoxicity”) is caused by the self-association of the death domain.39Consistent with these findings, we observed high autotoxicity with the LV′VFas construct in transiently transfected cell lines. However, when the LV′VFas construct was introduced in cells by retroviral infection, there was a complete lack of autotoxicity, both in cell lines and in primary human T lymphocytes. These findings suggest that autotoxicity is only observed when Fas is expressed at extremely high levels, as is the case in transiently transfected cells. This is in good agreement with the finding that Fas toxicity is significantly decreased when the amount of DNA transfected is reduced.19
Although this study was undertaken to investigate potential clinical use of the Fas T-cell suicide system, the conditional elimination of T is also useful as a research tool. Conditional ablation of subpopulations of T cells, for example in transgenic mouse models,15 40 provides a means to probe their importance in specific aspects of the immune response. Our data suggest that the modified Fas construct with an integral cell surface marker could also be useful in these research applications.
Recent clinical experience with the HSV-tk suicide system has identified several significant limitations to its use in treating GVHD associated with allo-BMT.11,41 Immune responses against infused HSV-tk–engineered T lymphocytes developed in several patients, resulting in the elimination of the cells and precluding the possibility of future infusions. In addition, chronic GVHD could only be partially controlled by GCV administration, a finding that was attributed to the cell cycle dependence of HSV-tk killing.11 Finally, GCV administration for concurrent clinical conditions other than GVHD resulted in the undesired clearance of HSV-tk donor lymphocytes and, hence, in the loss of the GVL effect.
The AP1903/Fas system offers several potential advantages over the HSV-tk/GCV strategy. Unlike HSV-tk, all the functional protein components of AP1903/Fas are of human origin and are less likely to elicit immune responses. The only potential for immunogenicity in the construct are the point mutation in F36V-FKBP and 3 junction peptides. We demonstrate that with a single treatment of AP1903, 60% to 80% of Fas-engineered cells are eliminated. This is in comparison with 30% to 50% in vitro killing of HSV-tk–neo-engineered T cells11 using the same construct (SFCMM-2) that was used successfully in clinical trials.2 The most recent retroviral vectors carrying the wild-type HSV-tk suicide gene are more efficient in killing highly proliferating cells—a single administration of the prodrug results in more than 90% T cell deaths in vitro.11Although a single exposure of LV′VFas-transduced lymphocytes to AP1903 is not as efficient as GCV in killing transduced lymphocytes, LV′VFas-engineered T cells can be eliminated nearly completely by repeated exposure to the drug. Furthermore, killing of T cells expressing the Fas suicide gene is more rapid than that of cells expressing HSV-tk in vitro. Fas-engineered cells show signs of apoptosis within 1 hour of drug addition and killing is nearly complete after 24 hours, whereas cell death induced by HSV-tk occurs over a time course of several days. We provide evidence that AP1903/Fas-induced cell death, unlike HSV-tk,11 occurs independently of the proliferation state of the cell. Thus, engineered T cells should be eliminated with high efficiency even in patients with chronic GVHD, in which GVHD effector cells likely are low-proliferating cells.11 The drug used to trigger suicide in the Fas system is AP1903, a synthetic, small-molecule compound that was specifically designed to interact with the engineered F36V-FKBP but not with endogenous FKBP12.16 In contrast, the HSV-tk system requires GCV, an antiviral drug with a low therapeutic index because of myelotoxicity42 and moreover a drug broadly used for treatment of cytomegalovirus infection.43
Our experiments provide information that will be useful in defining the dosing regimen for use in GVHD applications. Specifically, delivery of short (1- to 2-hour) pulses of drug that are repeated at approximately 24- to 48-hour intervals should mimic the conditions found to induce efficient elimination of engineered T cells in vitro. The pharmacologic properties of AP1903 in healthy human volunteers were recently determined in a phase 1 trial that demonstrated the safety of the drug and established a direct relation between the plasma concentrations and the dose administered (J. D. Iuliucci, unpublished data). No clinically meaningful adverse effects were observed at plasma AP1903 concentrations up to 100-fold above the maximally effective in vitro concentration.
Based on our experiments showing safety, specificity, killing efficiency, and immune competence of LV′VFas-engineered lymphocytes and on the results of the phase 1 clinical trial of AP1903, we propose the AP1903/Fas system as a novel, safe, and efficacious suicide strategy to induce a controlled GVL effect in allogeneic marrow transplantation. Our data indicate that Fas-engineered donor lymphocytes should be as efficacious as HSV-tk–engineered donor lymphocyte infusions in controlling disease relapse but able to bypass the intrinsic limitations of the HSV-tk/GCV suicide system.
We thank Zulma Magnani, Franklin Cerasoli Jr, and Patrizia Rovere for helpful discussion; Carl Rollins, Daniela Maggioni, and Scott Wardwell for technical assistance; and Dale Talbot for developing the FKBP PCR assay. In addition, we thank the various blood donors for enabling us to conduct these experiments.
Supported in part by a grant from the Italian Association for Cancer Research, Milano, Italy.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
References
Author notes
Daniel C. Thomis or Tim Clackson, ARIAD Gene Therapeutics, 26 Landsdowne St, Cambridge, MA 02139; e-mail:clackson@ariad.com.

![Fig. 6. AP1903-induced killing is independent of the proliferation state of human T lymphocytes. / (A) LV′VFas-transduced primary human T cells were stained for CD25 at different times after the initial stimulation, and the killing efficiency of CD25hi (top panel) and CD25−(bottom panel) cells was measured 24 hours after treatment with 10 nM AP1903. Representative CD25 staining profiles (filled histograms) with the isotype control (open histograms) are shown on the left side. Survival percentages of AP1903-treated cells are shown on the right side. (B) Five, 12, or 21 days after the initial stimulation LV′VFas-transduced primary human T cells were incubated with [3H]thymidine in the absence of AP1903 to assess proliferation activity (open bars) or in the presence of 10 nM AP1903 to assess relative survival (closed bars). Values are the mean ± SD of 3 independent points.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/97/5/10.1182_blood.v97.5.1249.h8001249_1249_1257/6/m_h80510740006.jpeg?Expires=1769227675&Signature=TPZ-PjgtHrRCvxmuNay0GeoKybH1xZcu~qgOILv41lzYbHt~tH1-FQWtMZjjUBTv41xsoJyA3UFHjQGr5xWUw0T0kpcL2tnDEGgGdCVcFuLthVq8VxZBppmzbiEdatbK7GbY4vtytpYXqYd~6QOuT6WZCMGpzRo7Oz9BRs45C-PfMeJvPtlgzQhahL3KvGqTkCuPn0nlBhi~oZcUA07euvaGXCu48n0bFAmQjjYFopJIjwLaiWbPx659Tvx8LAAxVkKN3wvJ3pH4WGN7JlBX4rC2UIPKwjdWdkQfnVciHWpNnCMEfI4yIS77AgEW~P5H9d-M5u6R2XTAVeEhj8ZoNQ__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)
This feature is available to Subscribers Only
Sign In or Create an Account Close Modal