Toxicities have limited the use of allogeneic hematopoietic cell transplantation (HCT) to younger, medically fit patients. In a canine HCT model, a combination of postgrafting mycophenolate mofetil (MMF) and cyclosporine (CSP) allowed stable allogeneic engraftment after minimally toxic conditioning with low-dose (200 cGy) total-body irradiation (TBI). These findings, together with the known antitumor effects of donor leukocyte infusions (DLIs), led to the design of this trial. Forty-five patients (median age 56 years) with hematologic malignancies, HLA-identical sibling donors, and relative contraindications to conventional HCT were treated. Immunosuppression involved TBI of 200 cGy before and CSP/MMF after HCT. DLIs were given after HCT for persistent malignancy, mixed chimerism, or both. Regimen toxicities and myelosuppression were mild, allowing 53% of eligible patients to have entirely outpatient transplantations. Nonfatal graft rejection occurred in 20% of patients. Grades II to III acute graft-versus-host disease (GVHD) occurred in 47% of patients with sustained engraftment. With median follow-up of 417 days, survival was 66.7%, nonrelapse mortality 6.7%, and relapse mortality 26.7%. Fifty-three percent of patients with sustained engraftment were in complete remission, including 8 with molecular remissions. This novel allografting approach, based on the use of postgrafting immunosuppression to control graft rejection and GVHD, has dramatically reduced the acute toxicities of allografting. HCT with the induction of potent graft-versus-tumor effects can be performed in previously ineligible patients, largely in an outpatient setting. Future protocol modifications should reduce rejection and GVHD, thereby facilitating studies of allogeneic immunotherapy for a variety of malignancies.
Introduction
Allogeneic hematopoietic cell transplantation (HCT) is effective treatment for patients with hematologic malignancies. Current strategies rely on maximally tolerated doses of systemic chemoradiation to eradicate cancer, and the allografts serve to rescue patients from treatment-induced marrow aplasia. Associated toxicities have limited treatment to younger, medically fit patients, with therapy administered on specialized hospital wards. Almost no HCTs have been done in patients more than 60 years of age, and relatively few in patients older than 50 years.1 Consequently, median patient ages at HCT for chronic myelocytic leukemia (CML), chronic lymphocytic leukemia (CLL), acute myelocytic leukemia (AML), multiple myeloma (MM), and non-Hodgkin lymphoma (NHL) are approximately 2 decades younger than median ages at diagnoses.1 Thus, current allogeneic HCT benefits only a younger minority of patients with candidate diseases.
At least 2 observations have raised doubts as to whether current concepts of allogeneic HCT are universally valid. First, marrow-based malignancies cannot always be eradicated even by the most intensive pretransplantation therapies.2,3 Second, cures may be attributed to graft-versus-tumor (GVT) reactions mediated by donor T cells,4-10 and donor lymphocyte infusions (DLIs) can induce sustained complete remissions (CRs) of malignancies that relapsed after HCT.11-16 These observations, together with a better understanding of how to influence immune cell functions, suggested a reevaluation of how to perform HCT. Specifically, the emphasis is shifting away from relying on high-dose cytotoxic therapy toward using donors' immune cells for eradicating malignancy. Consequently, some investigators have tested reduced-intensity pretransplantation regimens, although these regimens were still given for tumor cytoreduction and had significant toxicities, including temporary severe myelosuppression.17-20
Here, we present a novel concept of allogeneic HCT that uses the knowledge that both host-versus-graft (HVG) and graft-versus-host (GVH) reactions are mediated by T cells. The concept relies on optimizing pharmacologic postgrafting immunosuppression to control both reactions. In canine HCT studies using major histocompatibility complex-matched littermate donors, the synergistic combination of the antimetabolite mycophenolate mofetil (MMF) and the T-cell–activation blocker cyclosporine (CSP) prevented graft-versus-host disease (GVHD) and reduced HVG reactions, thereby allowing a reduction of the single-dose pretransplantation total-body irradiation (TBI) needed for sustained engraftment from 920 cGy to a nonmyeloablative dose of 200 cGy.21-23 Dogs having transplantation this way experienced minimal toxicities and mild pancytopenia, and moreover became stable mixed hematopoietic chimeras. These observations, together with the known GVT effects of allografts, allowed us to apply a novel strategy of HLA-identical sibling HCT using low-dose (200 cGy) TBI/MMF/CSP. In contrast, conventional allografting regimens typically use TBI doses 6 times higher (at least 1200 cGy) combined with high-dose chemotherapy (eg, cyclophosphamide 120 mg/kg). This report describes initial results in 45 patients with hematologic malignancies who were ineligible for conventional HCT because of age or medical contraindications.
Patients, materials, and methods
Patients and eligibility
Forty-five consecutive patients were treated between December 17, 1997, and May 27, 1999, at the Fred Hutchinson Cancer Research Center (FH), University of Washington Medical Center, and Veterans Affairs Medical Center in Seattle; the University of Leipzig (UL); and Stanford University (SU). Consent was obtained according to each institution's institutional review board guidelines.
Major eligibility requirements were as follows: (1) indolent hematologic malignancy or acute leukemia in CR; (2) HLA-identical sibling donor with serologic match at HLA-A, -B, -C, and molecular matching for HLA-DRB124; (3) age greater than 50 years (greater than 60 years for CML) or relative contraindication to conventional HCT in younger patients; and (4) creatinine clearance greater than 50 mL/min, bilirubin less than 2 times normal, and Karnofsky score more than 50. CLL and lymphoma patients had failed at least frontline therapy. MM patients had stage II or III disease at diagnosis or disease progression and had had previous chemotherapy. Three additional poor-risk patients had chemotherapy-resistant acute leukemia (UL607, UL577) and persistent marrow aplasia and aspergillosis after induction chemotherapy for AML (FH14241).25 Table1 shows patient and graft characteristics.
Characteristics of patients and grafts (grouped by rejection status and diagnoses)
| Unique patient no. . | Diagnosis . | Age/sex* . | Previous autograft . | Previous regimens† . | CD34+ cells × 106/kg‡ . | CD3+ cells × 108/kg1-153 . |
|---|---|---|---|---|---|---|
| UL 607 | ALL-REL3 | 60/M | VDAspAra, IdaAra-C, FLAG × 2, FLAG × 2 | 3.9 | 4.5 | |
| 577 | MF-AML-IF | 62/M | Hu, IFN, IdaTG × 6, IdaAra-C × 2, E Ida (all for MF), HDAC, Mi | 4.8 | 4.3 | |
| FH 14241 | AML-aplasia | 49/M | IdaAra-C × 2 → 10 weeks of aplasia + aspergillosis | 10.7 | 2.7 | |
| 14560 | AML-CR1 | 63/M | IdaAra-C × 2, HDAC | 6.5 | 2.4 | |
| 13461 | AML-CR1 | 60/M | MCE × 2, AraC | 6.1 | 6.7 | |
| UL 646 | AML-CR1 | 36/F | IdaAra-C × 3 | 4.6 | 8.3 | |
| 627 | AML-CR1 | 58/F | IdaAra-C × 3 | 9.1 | 4.2 | |
| 636 | AML-CR1 | 56/F | IdaAra-C × 3 | 8.0 | 4.5 | |
| UL 616 | AML-CR2 | 72/M | TAD, HAM, Mi-FLAG | 3.1 | 2.4 | |
| FH 13361 | AML-CR3 | 66/M | D Ara-C × 3, D Ara-C × 2, IdaAra-C × 2 | 4.0 | 0.7 | |
| SU 1857 | AML-IF | 57/M | CMOPP/CHOP, XRT (for NHL), IdaAra-C for secondary AML | 6.9 | 3.1 | |
| FH 12914 | CLL-REL2 | 53/M | F × 6, F × 8 | 11.3 | 4.9 | |
| SU 1879 | CLL-PR2 | 59/M | FChP × 8, F × 8 | 5.5 | 2.0 | |
| FH 14587 | CLL-REF | 54/M | F × 6, CVP × 2 | 5.2 | 1.9 | |
| 14586 | CLL-REF | 53/M | F × 6 | 9.6 | 3.0 | |
| SU 2028 | CLL-PR2 | 55/M | CHOP × 6, CyE + Rituxin × 4 | 5.1 | 2.6 | |
| FH 14322 | CLL-REF | 46/M | Ch, CVP × 4, F × 4, CED | 10.8 | 3.9 | |
| 14627 | CLL-REF | 54/F | F × 6, CHOP × 4, CVP × 4 | 5.0 | 3.4 | |
| UL 596 | CML-AP | 64/M | Hu/IFN, Hu/TG | 4.7 | 4.3 | |
| UL 605 | CML-CP | 49/F | Hu/IFN, TG | 10.2 | 2.9 | |
| FH 14214 | CML-CP | 70/M | Hu | 3.3 | 1.7 | |
| UL 634 | CML-CP | 60/M | Hu, IdaAra-C, Hu/IFN | 5.9 | 2.5 | |
| FH 14726 | CML-CP | 61/F | Hu, IFN | 6.4 | 4.1 | |
| SU 1955 | HD-CR2 | 61/F | ABVD × 6, CHAD × 3 | 8.6 | 4.6 | |
| FH 13817 | HD-PR | 49/F | XRT, MOPP × 3, ABVD × 6, DHAP × 3, MOPP × 2 | 16.1 | 4.8 | |
| 14226 | HD-REF | 31/M | Yes | ABVD × 6, mantle XRT, MOPP × 3, HDT, Vb, GcCp × 2, Gc | 12.3 | 3.6 |
| 14692 | HD-REF | 39/M | Yes | MOPP/ABVD × 6, ABVD × 5, DHAP × 2, HDT | 4.7 | 2.4 |
| 13527 | MM-PR | 56/M | Yes | MP, VAD × 3, CyTx, HDT | 3.3 | 2.6 |
| 13922 | MM-PR | 58/F | Yes | VAD × 4, CyTx, HDT | 7.2 | 0.8 |
| 11924 | MM-PR | 44/F | Yes | VAD × 4, CyTx, HDT | 17.5 | 5.2 |
| 13541 | MM-REF | 55/F | Yes | VAD × 4, CyTx, HDT | 12.4 | 4.0 |
| 14274 | MM-REF | 63/M | Yes | VAD × 7, CyTx, HDT | 15.5 | 6.0 |
| UL 625 | MM-REF | 59/M | MP, Brendamustine + P | 9.7 | 2.9 | |
| FH 14224 | NHL-REF | 53/F | Ch, FMD × 3, Promace-Cytabom × 4, Rituxin × 4, CE × 2 | 6.0 | 7.2 | |
| 14413 | WM-REF | 50/M | F × 7, Rituxin × 4, Dex × 10 | 10.5 | 2.6 | |
| 14658 | WM-REF | 58/M | 2-CDA × 2, CVP × 2 | 13.2 | 4.9 | |
| UL 644 | AML-CR1 | 52/F | IdaAra-C × 3 | 3.8 | 3.5 | |
| 14284 | CLL-PR | 67/M | Ch, F × 3 | 6.5 | 4.3 | |
| 14091 | CML-AP | 54/M | Hu/INF, 2-CDA | 15.1 | 2.0 | |
| SU 2000 | CML-AP | 40/M | Hu | 7.7 | 4.5 | |
| SU 1948 | CML-CP | 71/M | Hu/INF, Ara-C/Hh, Ara-C | 12.7 | 3.3 | |
| UL 622 | CML-CP | 58/M | Hu | 10.8 | 3.7 | |
| FH 13009 | MDS-RAEB-T | 43/M | None | 4.4 | 2.7 | |
| 13246 | MM-REF | 58/F | VAD × 4 | 9.1 | 1.0 | |
| 13230 | MM-PR | 53/M | VAD × 7 | 10.0 | 3.3 |
| Unique patient no. . | Diagnosis . | Age/sex* . | Previous autograft . | Previous regimens† . | CD34+ cells × 106/kg‡ . | CD3+ cells × 108/kg1-153 . |
|---|---|---|---|---|---|---|
| UL 607 | ALL-REL3 | 60/M | VDAspAra, IdaAra-C, FLAG × 2, FLAG × 2 | 3.9 | 4.5 | |
| 577 | MF-AML-IF | 62/M | Hu, IFN, IdaTG × 6, IdaAra-C × 2, E Ida (all for MF), HDAC, Mi | 4.8 | 4.3 | |
| FH 14241 | AML-aplasia | 49/M | IdaAra-C × 2 → 10 weeks of aplasia + aspergillosis | 10.7 | 2.7 | |
| 14560 | AML-CR1 | 63/M | IdaAra-C × 2, HDAC | 6.5 | 2.4 | |
| 13461 | AML-CR1 | 60/M | MCE × 2, AraC | 6.1 | 6.7 | |
| UL 646 | AML-CR1 | 36/F | IdaAra-C × 3 | 4.6 | 8.3 | |
| 627 | AML-CR1 | 58/F | IdaAra-C × 3 | 9.1 | 4.2 | |
| 636 | AML-CR1 | 56/F | IdaAra-C × 3 | 8.0 | 4.5 | |
| UL 616 | AML-CR2 | 72/M | TAD, HAM, Mi-FLAG | 3.1 | 2.4 | |
| FH 13361 | AML-CR3 | 66/M | D Ara-C × 3, D Ara-C × 2, IdaAra-C × 2 | 4.0 | 0.7 | |
| SU 1857 | AML-IF | 57/M | CMOPP/CHOP, XRT (for NHL), IdaAra-C for secondary AML | 6.9 | 3.1 | |
| FH 12914 | CLL-REL2 | 53/M | F × 6, F × 8 | 11.3 | 4.9 | |
| SU 1879 | CLL-PR2 | 59/M | FChP × 8, F × 8 | 5.5 | 2.0 | |
| FH 14587 | CLL-REF | 54/M | F × 6, CVP × 2 | 5.2 | 1.9 | |
| 14586 | CLL-REF | 53/M | F × 6 | 9.6 | 3.0 | |
| SU 2028 | CLL-PR2 | 55/M | CHOP × 6, CyE + Rituxin × 4 | 5.1 | 2.6 | |
| FH 14322 | CLL-REF | 46/M | Ch, CVP × 4, F × 4, CED | 10.8 | 3.9 | |
| 14627 | CLL-REF | 54/F | F × 6, CHOP × 4, CVP × 4 | 5.0 | 3.4 | |
| UL 596 | CML-AP | 64/M | Hu/IFN, Hu/TG | 4.7 | 4.3 | |
| UL 605 | CML-CP | 49/F | Hu/IFN, TG | 10.2 | 2.9 | |
| FH 14214 | CML-CP | 70/M | Hu | 3.3 | 1.7 | |
| UL 634 | CML-CP | 60/M | Hu, IdaAra-C, Hu/IFN | 5.9 | 2.5 | |
| FH 14726 | CML-CP | 61/F | Hu, IFN | 6.4 | 4.1 | |
| SU 1955 | HD-CR2 | 61/F | ABVD × 6, CHAD × 3 | 8.6 | 4.6 | |
| FH 13817 | HD-PR | 49/F | XRT, MOPP × 3, ABVD × 6, DHAP × 3, MOPP × 2 | 16.1 | 4.8 | |
| 14226 | HD-REF | 31/M | Yes | ABVD × 6, mantle XRT, MOPP × 3, HDT, Vb, GcCp × 2, Gc | 12.3 | 3.6 |
| 14692 | HD-REF | 39/M | Yes | MOPP/ABVD × 6, ABVD × 5, DHAP × 2, HDT | 4.7 | 2.4 |
| 13527 | MM-PR | 56/M | Yes | MP, VAD × 3, CyTx, HDT | 3.3 | 2.6 |
| 13922 | MM-PR | 58/F | Yes | VAD × 4, CyTx, HDT | 7.2 | 0.8 |
| 11924 | MM-PR | 44/F | Yes | VAD × 4, CyTx, HDT | 17.5 | 5.2 |
| 13541 | MM-REF | 55/F | Yes | VAD × 4, CyTx, HDT | 12.4 | 4.0 |
| 14274 | MM-REF | 63/M | Yes | VAD × 7, CyTx, HDT | 15.5 | 6.0 |
| UL 625 | MM-REF | 59/M | MP, Brendamustine + P | 9.7 | 2.9 | |
| FH 14224 | NHL-REF | 53/F | Ch, FMD × 3, Promace-Cytabom × 4, Rituxin × 4, CE × 2 | 6.0 | 7.2 | |
| 14413 | WM-REF | 50/M | F × 7, Rituxin × 4, Dex × 10 | 10.5 | 2.6 | |
| 14658 | WM-REF | 58/M | 2-CDA × 2, CVP × 2 | 13.2 | 4.9 | |
| UL 644 | AML-CR1 | 52/F | IdaAra-C × 3 | 3.8 | 3.5 | |
| 14284 | CLL-PR | 67/M | Ch, F × 3 | 6.5 | 4.3 | |
| 14091 | CML-AP | 54/M | Hu/INF, 2-CDA | 15.1 | 2.0 | |
| SU 2000 | CML-AP | 40/M | Hu | 7.7 | 4.5 | |
| SU 1948 | CML-CP | 71/M | Hu/INF, Ara-C/Hh, Ara-C | 12.7 | 3.3 | |
| UL 622 | CML-CP | 58/M | Hu | 10.8 | 3.7 | |
| FH 13009 | MDS-RAEB-T | 43/M | None | 4.4 | 2.7 | |
| 13246 | MM-REF | 58/F | VAD × 4 | 9.1 | 1.0 | |
| 13230 | MM-PR | 53/M | VAD × 7 | 10.0 | 3.3 |
UL indicates University of Leipzig; ALL, acute lymphocytic leukemia; REL, untreated relapse; MF, myelofibrosis; AML, acute myelogenous leukemia; IF, chemotherapy induction failure; FH, Fred Hutchinson Cancer Research Center; CR, complete remission; SU, Stanford University; CLL, chronic lymphocytic leukemia; PR, partial remission; REF, refractory to conventional chemotherapy; CML, chronic myelogenous leukemia; AP, accelerated phase; CP, chronic phase; HD, Hodgkin disease; MM, multiple myeloma; NHL, non-Hodgkin lymphoma; WM, Waldenström macroglobulinemia; MDS, myelodysplastic syndrome; RAEB-T, refractory anemia with excess blasts in transformation.
Median age = 56 years.
Previous regimens: eg, ×2 = 2 cycles. Abbreviations are expanded in the at the end this article.
Median CD34+ cell dose = 9 × 106/kg.
Median CD3+ cell dose = 3.3 × 108/kg.
Treatment and evaluations
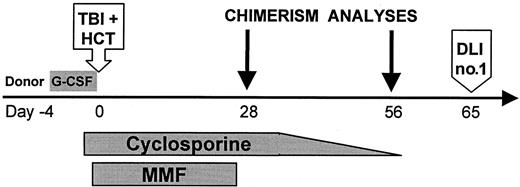
Figure 1 shows the treatment protocol. TBI was given at 7 cGy/min from dual cobalt 60 sources (Fred Hutchinson Cancer Research Center) or linear accelerators (Veterans Affairs Medical Center, University of Leipzig, Stanford University, University of Washington Medical Center). Forty-four patients received 2 Gy TBI alone. One patient (FH14726) with CML received, in addition, fludarabine 30 mg/m2 on days −4, −3, and −2 in an attempt to reduce the risk of graft rejection. CSP levels were targeted to the upper therapeutic range (approximately 500 ng/mL; Abbott TDX, Abbott Park, IL) until day +35. Prophylaxis against Pneumocystis carinii, fungal infections, and cytomegalovirus (CMV) infections was used.26-28 Patients with chronic GVHD requiring systemic immunosuppression received antibiotic prophylaxis againstP carinii and Pneumococcus. For outpatient transplantations, scheduled follow-up included 2 to 3 clinic visits per week for the first month and then once or twice per week or as clinically required thereafter.
Treatment protocol for nonmyeloablative HCT.
Granulocyte colony-stimulating factor (G-CSF)–mobilized peripheral blood stem cells (PBSCs) were infused after TBI on day 0. One patient (FH14726) received fludarabine 30 mg/m2 ×3 intravenously on days −4, −3, and −2 before TBI/MMF/CSP. G-CSF: 16 μg per kg per day on days −4 to 0, aphereses on days −1, 0; TBI: 200 cGy (7 cGy/min) single fraction; HCT: PBSCs infused on day 0; CSP: 1.5 mg/kg intravenously twice daily on days −1 and 0, 6.25 mg/kg orally twice daily on days 1 to +35 (cohort 1), then taper to +56 (cohort 2); MMF: 15 mg/kg orally twice daily on days 0 to +27; DLI: no. 1 equals 107 CD3+ cells/kg, no. 2 equals 3.3 × 107 CD3+ cells/kg.
Treatment protocol for nonmyeloablative HCT.
Granulocyte colony-stimulating factor (G-CSF)–mobilized peripheral blood stem cells (PBSCs) were infused after TBI on day 0. One patient (FH14726) received fludarabine 30 mg/m2 ×3 intravenously on days −4, −3, and −2 before TBI/MMF/CSP. G-CSF: 16 μg per kg per day on days −4 to 0, aphereses on days −1, 0; TBI: 200 cGy (7 cGy/min) single fraction; HCT: PBSCs infused on day 0; CSP: 1.5 mg/kg intravenously twice daily on days −1 and 0, 6.25 mg/kg orally twice daily on days 1 to +35 (cohort 1), then taper to +56 (cohort 2); MMF: 15 mg/kg orally twice daily on days 0 to +27; DLI: no. 1 equals 107 CD3+ cells/kg, no. 2 equals 3.3 × 107 CD3+ cells/kg.
Chimerism among peripheral blood (PB) T cells, granulocytes, and unfractionated marrow was assessed at days 28, 56, 84, 180, and 360 after HCT using fluorescence in situ hybridization to detect X and Y chromosomes for recipients of sex-mismatched transplants and polymerase chain reaction (PCR)–based analysis of polymorphic microsatellite regions for recipients of sex-matched transplants.29
The primary study end point was mixed chimerism at day 28, defined as between 1% and 95% PB donor T cells. Patients with mixed chimerism, no GVHD, and less than 20% increases in donor T cells from day 28 to 56 were eligible for DLI on day +65. In others, monthly chimerism and tumor evaluations were done to assess the need for DLI. DLIs were scheduled to be administered at 2-month intervals starting at 1 × 107 CD3+ cells/kg, with up to 3 subsequent infusions at doses of 3.3 × 107, 1.0 × 108, and 3.3 × 108 CD3+cells/kg to be given based on the presence of stable mixed chimerism and absence of GVHD, or persistent or progressive disease. Patients who had rapidly progressive tumors early after transplantation or graft rejection were considered treatment failures and were eligible for other therapies including DLI off protocol or second transplantations.
Statistical analysis
Survival and progression-free survival were estimated by the Kaplan-Meier method, and mortality from various causes and GVHD were each summarized using cumulative incidence estimates.34The associations of chimerism values at day 28 with acute GVHD and rejection were examined using logistic regression. Associations of various patient characteristics with acute GVHD and rejection were examined using the Wilcoxon rank-sum test for continuous characteristics. The χ2 test was used for categorical characteristics, or Fisher's exact test if assumptions necessary for the χ2 test were not met. Data were analyzed as of April 1, 2000.
Results
Table 2 summarizes the outcomes.
Outcomes after hematopoietic cell transplantation (grouped by rejection status and diagnoses)
| UPN . | Diagnosis . | % donor CD3+ . | Rejection . | DLI . | GVHD . | Survival, days . | Summary of outcome as of 4/1/00 . | ||
|---|---|---|---|---|---|---|---|---|---|
| Day 28 . | Day 56 . | Acute (grade) . | Chronic . | ||||||
| UL 6072-153 | ALL-REL3 | 85 | 100 | Yes* | I | 3562-155 | CR → REL → chemo/DLI → CR → Aspergillus | ||
| 5772-153 | MF-AML-IF | 84 | 85 | III | Lim-S | > 621 | CR | ||
| FH 142412-154 | AML-aplasia | 85 | 95 | 0 | Ext-S, G‡ | > 549 | CR | ||
| 134612-153 | AML-CR1 | 46 | 43 | Yes × 2 | II† | Ext-S | > 759 | Rel → chemo → CR → DLI → REL → chemo | |
| 145602-159 | AML-CR1 | 40 | 99 | Yes | 0 | > 473 | CR | ||
| UL 6462-153 | AML-CR1 | 60 | NE | Yes* | II† | Lim-S | > 341 | Rel → DLI → CR → Rel → chemo/DLI → CR | |
| 6272-159 | AML-CR1 | NE | NE | 0 | > 410 | CR | |||
| 6362-159 | AML-CR1 | 58 | 63 | III | Lim-S | > 383 | CR | ||
| UL 6162-159 | AML-CR2 | 55 | 50 | Yes* | 0 | 1722-155 | Rel → PD | ||
| FH 133612-153 | AML-CR3 | 50 | 90 | Yes* | II | Ext-S, G | 1682-155 | Rel × 2 → DLI → CR mucor infection | |
| SU 18572-153 | AML-IF | 50 | NE | 0 | NE | 382-155 | PD → conventional BMT | ||
| FH 129142-153 | CLL-REL2 | 100 | 100 | II | Ext-S, O‡ | 3612-155 | MCR Pneumococcal sepsis | ||
| SU 18792-153 | CLL-PR2 | 56 | 86 | III | Ext-S, G | > 528 | MCR | ||
| FH 145872-159 | CLL-REF | 10 | 20 | 0 | Ext-S, L, O | > 368 | PR | ||
| 145862-159 | CLL-REF | 90 | 80 | Yes* | II | Ext-S, L | 2442-155 | PD | |
| SU 20282-159 | CLL-PR2 | 30 | 25 | Yes* | 0 | > 375 | PR → PD | ||
| FH 143222-159 | CLL-REF | 65 | 95 | I | Ext-E, O, S | > 319 | MCR | ||
| 146272-159 | CLL-REF | 71 | 96 | III | Ext-L, O, S | > 325 | CR | ||
| UL 5962-153 | CML-AP | 79 | 95 | Yes* | II† | Lim-S | > 530 | MCR | |
| UL 6052-153 | CML-CP | 27 | 32 | 0 | Lim-S | > 488 | MCR | ||
| FH 142142-159 | CML-CP | 45 | 50 | II | Ext-O, S | > 393 | MCR | ||
| UL 6342-159 | CML-CP | 43 | 70 | I | Lim-O | > 390 | MCR | ||
| FH 147262-159 | CML-CP2-160 | 100 | 95 | II | Ext-E, G, L, O, S | > 368 | MCR | ||
| SU 19552-153 | HD-CR2 | 60 | 90 | Yes × 2 | 0 | 1642-155 | PD | ||
| FH 138172-159 | HD-PR | 99 | 99 | II | Ext-E, G, O, S | > 464 | CR | ||
| 142262-159 | HD-REF | 59 | 92 | Yes* | II | Ext-S | > 444 | CR → Rel → XRT/chemo + DLI → PR | |
| 146922-159 | HD-REF | 70 | 90 | Yes* | II† | NE | 1592-155 | PD → Aspergillus infection | |
| 135272-159 | MM-PR | 99 | 97 | II | Ext-E, O, S | > 416 | CR | ||
| 139222-159 | MM-PR | 83 | 94 | Yes | 0 | > 417 | CR2-164 | ||
| 119242-159 | MM-PR | 94 | 97 | 0 | > 473 | CR2-164 | |||
| 135412-159 | MM-REF | 98 | 100 | II | NE | 912-155 | PD | ||
| 142742-159 | MM-REF | 90 | 95 | II | Ext-G, S | > 310 | CR | ||
| UL 6252-159 | MM-REF | 100 | NE | III | NE | 542-155 | PR. Pneumonia, multiorgan failure | ||
| FH 142242-159 | NHL-REF | 88 | 100 | II | NE | 642-155 | CR. Intracranial hemorrhage | ||
| 144132-159 | WM-REF | 58 | 62 | II | Ext-E, O, S | > 380 | SD | ||
| 146582-159 | WM-REF | 85 | 90 | Yes × 2* | 0 | Lim-S, O | > 333 | SD | |
| UL 6442-159 | AML-CR1 | 20 | 5 | Yes | Yes | 0 | 1332-155 | Rel → 2nd NMHCT → engrafted → PD | |
| 142842-159 | CLL-PR | 19 | 24 | Yes | Yes | 0 | > 376 | PD | |
| 140912-153 | CML-AP | 40 | 30 | Yes | Yes | I | 2272-155 | PD → Conventional BMT | |
| SU 20002-159 | CML-AP | 25 | 30 | Yes | Yes | 0 | 4072-155 | PD → 2nd NMHCT → rejected → PD | |
| SU 19482-153 | CML-CP | 60 | 30 | Yes | Yes | 0 | > 501 | PD → 2nd NMHCT → rejected | |
| UL 6222-159 | CML-CP | 10 | 8 | Yes | Yes | 0 | > 341 | PD | |
| FH 130092-153 | MDS-RAEB-T | 40 | 35 | Yes | Yes | 0 | 1832-155 | PD + liver cirrhosis | |
| 132462-153 | MM-REF | 43 | 35 | Yes | Yes | 0 | > 731 | PD psoriasis in CR | |
| 132302-153 | MM-PR | 15 | 5 | Yes | Yes | 0 | > 751 | REL | |
| UPN . | Diagnosis . | % donor CD3+ . | Rejection . | DLI . | GVHD . | Survival, days . | Summary of outcome as of 4/1/00 . | ||
|---|---|---|---|---|---|---|---|---|---|
| Day 28 . | Day 56 . | Acute (grade) . | Chronic . | ||||||
| UL 6072-153 | ALL-REL3 | 85 | 100 | Yes* | I | 3562-155 | CR → REL → chemo/DLI → CR → Aspergillus | ||
| 5772-153 | MF-AML-IF | 84 | 85 | III | Lim-S | > 621 | CR | ||
| FH 142412-154 | AML-aplasia | 85 | 95 | 0 | Ext-S, G‡ | > 549 | CR | ||
| 134612-153 | AML-CR1 | 46 | 43 | Yes × 2 | II† | Ext-S | > 759 | Rel → chemo → CR → DLI → REL → chemo | |
| 145602-159 | AML-CR1 | 40 | 99 | Yes | 0 | > 473 | CR | ||
| UL 6462-153 | AML-CR1 | 60 | NE | Yes* | II† | Lim-S | > 341 | Rel → DLI → CR → Rel → chemo/DLI → CR | |
| 6272-159 | AML-CR1 | NE | NE | 0 | > 410 | CR | |||
| 6362-159 | AML-CR1 | 58 | 63 | III | Lim-S | > 383 | CR | ||
| UL 6162-159 | AML-CR2 | 55 | 50 | Yes* | 0 | 1722-155 | Rel → PD | ||
| FH 133612-153 | AML-CR3 | 50 | 90 | Yes* | II | Ext-S, G | 1682-155 | Rel × 2 → DLI → CR mucor infection | |
| SU 18572-153 | AML-IF | 50 | NE | 0 | NE | 382-155 | PD → conventional BMT | ||
| FH 129142-153 | CLL-REL2 | 100 | 100 | II | Ext-S, O‡ | 3612-155 | MCR Pneumococcal sepsis | ||
| SU 18792-153 | CLL-PR2 | 56 | 86 | III | Ext-S, G | > 528 | MCR | ||
| FH 145872-159 | CLL-REF | 10 | 20 | 0 | Ext-S, L, O | > 368 | PR | ||
| 145862-159 | CLL-REF | 90 | 80 | Yes* | II | Ext-S, L | 2442-155 | PD | |
| SU 20282-159 | CLL-PR2 | 30 | 25 | Yes* | 0 | > 375 | PR → PD | ||
| FH 143222-159 | CLL-REF | 65 | 95 | I | Ext-E, O, S | > 319 | MCR | ||
| 146272-159 | CLL-REF | 71 | 96 | III | Ext-L, O, S | > 325 | CR | ||
| UL 5962-153 | CML-AP | 79 | 95 | Yes* | II† | Lim-S | > 530 | MCR | |
| UL 6052-153 | CML-CP | 27 | 32 | 0 | Lim-S | > 488 | MCR | ||
| FH 142142-159 | CML-CP | 45 | 50 | II | Ext-O, S | > 393 | MCR | ||
| UL 6342-159 | CML-CP | 43 | 70 | I | Lim-O | > 390 | MCR | ||
| FH 147262-159 | CML-CP2-160 | 100 | 95 | II | Ext-E, G, L, O, S | > 368 | MCR | ||
| SU 19552-153 | HD-CR2 | 60 | 90 | Yes × 2 | 0 | 1642-155 | PD | ||
| FH 138172-159 | HD-PR | 99 | 99 | II | Ext-E, G, O, S | > 464 | CR | ||
| 142262-159 | HD-REF | 59 | 92 | Yes* | II | Ext-S | > 444 | CR → Rel → XRT/chemo + DLI → PR | |
| 146922-159 | HD-REF | 70 | 90 | Yes* | II† | NE | 1592-155 | PD → Aspergillus infection | |
| 135272-159 | MM-PR | 99 | 97 | II | Ext-E, O, S | > 416 | CR | ||
| 139222-159 | MM-PR | 83 | 94 | Yes | 0 | > 417 | CR2-164 | ||
| 119242-159 | MM-PR | 94 | 97 | 0 | > 473 | CR2-164 | |||
| 135412-159 | MM-REF | 98 | 100 | II | NE | 912-155 | PD | ||
| 142742-159 | MM-REF | 90 | 95 | II | Ext-G, S | > 310 | CR | ||
| UL 6252-159 | MM-REF | 100 | NE | III | NE | 542-155 | PR. Pneumonia, multiorgan failure | ||
| FH 142242-159 | NHL-REF | 88 | 100 | II | NE | 642-155 | CR. Intracranial hemorrhage | ||
| 144132-159 | WM-REF | 58 | 62 | II | Ext-E, O, S | > 380 | SD | ||
| 146582-159 | WM-REF | 85 | 90 | Yes × 2* | 0 | Lim-S, O | > 333 | SD | |
| UL 6442-159 | AML-CR1 | 20 | 5 | Yes | Yes | 0 | 1332-155 | Rel → 2nd NMHCT → engrafted → PD | |
| 142842-159 | CLL-PR | 19 | 24 | Yes | Yes | 0 | > 376 | PD | |
| 140912-153 | CML-AP | 40 | 30 | Yes | Yes | I | 2272-155 | PD → Conventional BMT | |
| SU 20002-159 | CML-AP | 25 | 30 | Yes | Yes | 0 | 4072-155 | PD → 2nd NMHCT → rejected → PD | |
| SU 19482-153 | CML-CP | 60 | 30 | Yes | Yes | 0 | > 501 | PD → 2nd NMHCT → rejected | |
| UL 6222-159 | CML-CP | 10 | 8 | Yes | Yes | 0 | > 341 | PD | |
| FH 130092-153 | MDS-RAEB-T | 40 | 35 | Yes | Yes | 0 | 1832-155 | PD + liver cirrhosis | |
| 132462-153 | MM-REF | 43 | 35 | Yes | Yes | 0 | > 731 | PD psoriasis in CR | |
| 132302-153 | MM-PR | 15 | 5 | Yes | Yes | 0 | > 751 | REL | |
GVHD indicates graft-versus-host disease; DLI, donor lymphocyte infusion; NE, not evaluable; PD, progressive disease; BMT, bone marrow transplantation; MCR, molecular remission; XRT, radiation therapy; SD, stable disease; NMHCT, nonmyeloablative hematopoietic cell transplantation; TBI, total-body irradiation; MMF, mycophenolate mofetil; and CSP, cyclosporine. For other abbreviations, see Table 1.
Indicates initial DLI given for relapse or progressive disease.
Indicates GVHD first occurred after DLI.
Indicates GVHD resolved; patient off immunosuppressive therapy. GVHD extent: Lim indicates limited GVHD; Ext, extensive GVHD. GVHD organ involvement: E indicates eyes; G, gastrointestinal; L, liver; O, oral; S, skin.
Cohort 1: CSP scheduled day −1 to +35.
Patient died.
CSP taper delayed to day +100 to prevent GVHD because of active fungal infection.
Cohort 2: CSP scheduled day −1 to +56.
Patient 14726 received fludarabine 30 mg/m2 × 3 in addition to TBI/MMF/CSP.
Trace monoclonal protein detected intermittently by immunofixation after CR was achieved.
Regimen-related toxicities and infections
No patient experienced regimen-related painful mucositis, severe nausea and vomiting, pulmonary toxicity, cardiac toxicity, hemorrhagic cystitis, or new-onset alopecia. Mild to moderate nausea and diarrhea due to MMF/CSP were common. Serum creatinine elevations to twice baseline values occurred in 59% of patients because of targeting high serum CSP levels, and resolved in most cases. One MM patient (UL625) with preexisting renal dysfunction (serum creatinine 2.3 mg/dL) required hemodialysis. Reversible hyperbilirubinemia to more than 10 mg/dL occurred in 3 patients because of preexisting liver cirrhosis (FH13009), concurrent amphotericin B treatment (FH14241), and isolated liver GVHD (FH14568). Documented bacterial infections requiring intravenous antibiotics occurred in 10 patients (22%) within 60 days after HCT. CMV reactivations were detected in 6 (24%) of 25 CMV-seropositive patients and were managed successfully with ganciclovir or foscarnet. Thirty-two patients were eligible for outpatient transplantation. Others had transplantation at the University of Leipzig (n = 12) or were already hospitalized (n = 1). Seventeen (53%) of the 32 patients did not require hospitalization within 60 days of HCT. The remainder were hospitalized for 1 to 35 days (median 8 days).
PB cell changes, allogeneic engraftment, and graft rejection
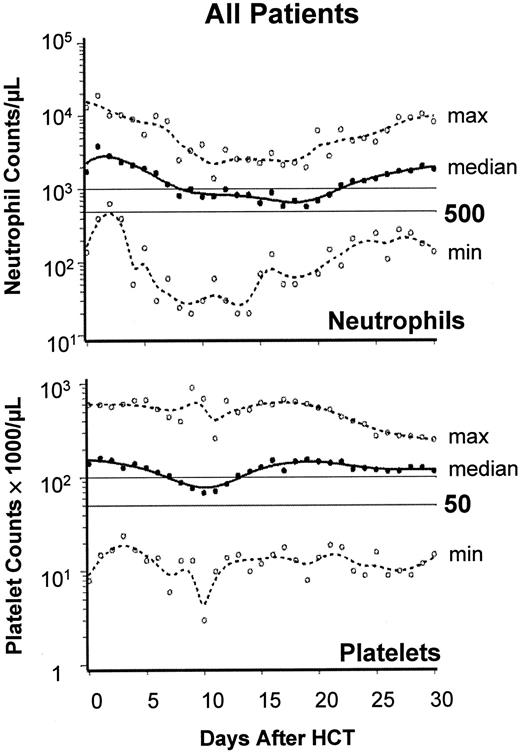
Figure 2 summarizes PB neutrophil and platelet changes to day +30. A median of 0 platelet units (range, 0-38 units) were transfused, and 34 patients (76%) required no transfusions. A median of 2 red blood cell units (range, 0-28 units) were transfused, and 14 patients (31%) required no transfusions. No serious hemolysis occurred in 14 ABO-incompatible transplants. Recipients of 6 major ABO-incompatible transplants (eg, A→O) required 0, 0, 0, 10, 23, and 28 red blood cell units.
Engraftment after nonmyeloablative HCT.
Engraftment profile showing neutrophil and platelet changes after HCT. Graphs show the median (black lines) and range (broken lines) of neutrophil and platelet counts of all 45 patients on days 0 through 30. ○, minimum and maximum values on each day.
Engraftment after nonmyeloablative HCT.
Engraftment profile showing neutrophil and platelet changes after HCT. Graphs show the median (black lines) and range (broken lines) of neutrophil and platelet counts of all 45 patients on days 0 through 30. ○, minimum and maximum values on each day.
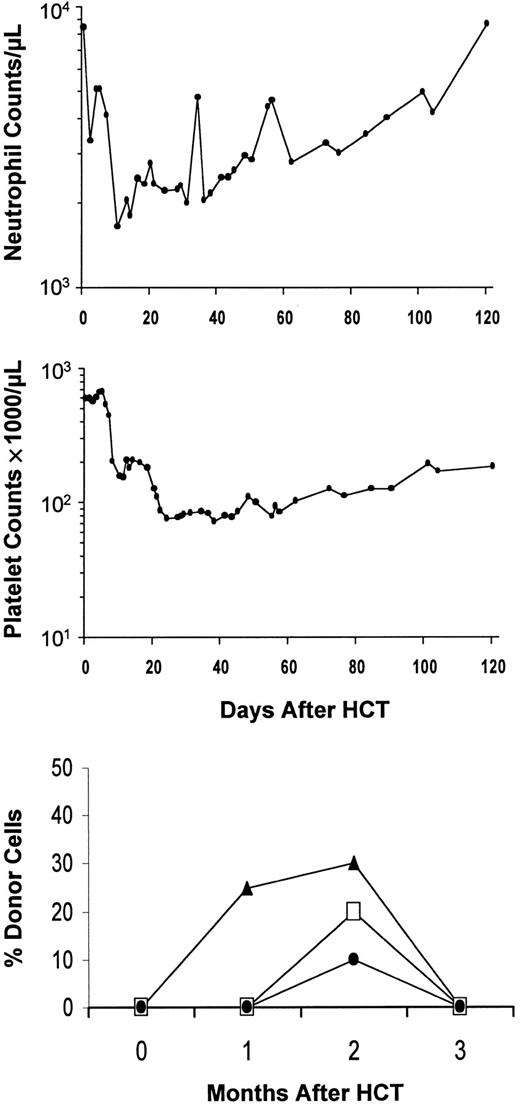
All patients had initial engraftment (Tables 2 and3). Based on T-cell engraftment, 86% were mixed chimeras and 14% were complete chimeras on day 28. This changed to 79% and 21%, respectively, on day 56. Nine (20%) of 44 patients given TBI/CSP/MMF had graft rejection between 2 and 4 months. Four had CML, 2 MM, one AML, one CLL, and one myelodysplasia. Rejections were nonfatal and usually associated with transient minor disturbances of blood counts (Figure 3), except for one patient (FH13246) who had 3 weeks of pancytopenia before recovery. This patient had experienced minimal myelosuppression until the onset of aplasia at 2 months after transplantation. Day-56 chimerism studies showed T cells to be 65% host and granulocytes to be 57% host at a time shortly before rejection when PB counts were not depressed, and these values were increased from 57% (T cells) and 29% (granulocytes) on day 28. Pretransplantation factors predictive of graft rejection were a lack of intensive preceding therapy and a diagnosis of CML (Table 4). Low donor T-cell chimerism at day 28 predicted rejections (Tables5,6). To reduce the risk of graft rejection, fludarabine 30 mg · m−2 · d−1 on days −4, −3, and −2 was added to the regimen. In one patient (FH14726; CML) reported here, T cells at day 28 were 100% donor, as compared with 10% to 79% (median 42%) donor in 8 preceding CML patients.
Median percent and chimerism status
| . | Day 283-150 . | Day 563-151 . |
|---|---|---|
| Median % (range) of donor cells | ||
| T cells | 60 (10-100) | 91 (5-100) |
| PB neutrophils | 91 (0-100) | 99 (4-100) |
| Bone marrow | 87 (0-100) | 95 (2-100) |
| Chimerism | ||
| % Mixed3-152 | 86 | 79 |
| % Full donor3-153 | 14 | 21 |
| . | Day 283-150 . | Day 563-151 . |
|---|---|---|
| Median % (range) of donor cells | ||
| T cells | 60 (10-100) | 91 (5-100) |
| PB neutrophils | 91 (0-100) | 99 (4-100) |
| Bone marrow | 87 (0-100) | 95 (2-100) |
| Chimerism | ||
| % Mixed3-152 | 86 | 79 |
| % Full donor3-153 | 14 | 21 |
There were 44 evaluable patients. One additional patient-donor pair (UL627) had no identifiable DNA polymorphism, and evidence of donor cell engraftment was based on red blood cell polymorphisms.
There were 41 evaluable patients.
At least 1 and no more than 95% T cells of donor origin.
More than 95% T cells of donor origin.
Graft rejection after HCT.
Example of graft rejection after HCT, showing neutrophil and platelet changes in patient SU2000 with CML. Initial engraftment and subsequent rejection between 2 and 3 months were documented by chimerism studies (bottom panel). ▴, CD3+; ■, polymorphonuclear leukocytes (PMNs); ●, bone marrow (BM).
Graft rejection after HCT.
Example of graft rejection after HCT, showing neutrophil and platelet changes in patient SU2000 with CML. Initial engraftment and subsequent rejection between 2 and 3 months were documented by chimerism studies (bottom panel). ▴, CD3+; ■, polymorphonuclear leukocytes (PMNs); ●, bone marrow (BM).
Rejection and graft-versus-host disease: analysis of pretransplantation factors
| Factor . | Rejectors (n = 9) . | Nonrejectors (n = 35) . | P value (rejection) . | Grades II-IV GVHD (n = 17) . | Grades 0-I GVHD (n = 19) . | P value (GVHD) . |
|---|---|---|---|---|---|---|
| Male patient | 7/9 (78%) | 24/35 (67%) | .70 | 11/17 (65%) | 13/19 (68%) | .81 |
| Male donor | 4/9 (44%) | 21/35 (58%) | .47 | 9/17 (53%) | 12/19 (63%) | .54 |
| Intensive prior therapy4-150 | 1/9 (11%) | 31/35 (86%) | < .0001 | 15/17 (88%) | 16/19 (84%) | 1.0 |
| Donor age in years | 48 (40-72) | 57 (33-73) | .80 | 53 (33-73) | 58 (38-65) | .78 |
| Patient age in years | 54 (40-71) | 56 (31-72) | .76 | 56 (31-70) | 57 (36-72) | .87 |
| CD34+ cell dose × 106/kg | 9.1 (3.8-15.1) | 6.9 (3.1-17.5) | .51 | 6.4 (3.3-16.1) | 6.9 (3.1-17.5) | .57 |
| CD3+ cell dose × 108/kg | 3.3 (1.0-4.5) | 3.4 (0.7-8.3) | .58 | 3.6 (0.7-7.2) | 3.1 (0.8-8.3) | .86 |
| Diagnosis of CML | 4/9 (44%) | 4/35 (11%) | .04 | 2/17 (12%) | 3/19 (16%) | 1.0 |
| Factor . | Rejectors (n = 9) . | Nonrejectors (n = 35) . | P value (rejection) . | Grades II-IV GVHD (n = 17) . | Grades 0-I GVHD (n = 19) . | P value (GVHD) . |
|---|---|---|---|---|---|---|
| Male patient | 7/9 (78%) | 24/35 (67%) | .70 | 11/17 (65%) | 13/19 (68%) | .81 |
| Male donor | 4/9 (44%) | 21/35 (58%) | .47 | 9/17 (53%) | 12/19 (63%) | .54 |
| Intensive prior therapy4-150 | 1/9 (11%) | 31/35 (86%) | < .0001 | 15/17 (88%) | 16/19 (84%) | 1.0 |
| Donor age in years | 48 (40-72) | 57 (33-73) | .80 | 53 (33-73) | 58 (38-65) | .78 |
| Patient age in years | 54 (40-71) | 56 (31-72) | .76 | 56 (31-70) | 57 (36-72) | .87 |
| CD34+ cell dose × 106/kg | 9.1 (3.8-15.1) | 6.9 (3.1-17.5) | .51 | 6.4 (3.3-16.1) | 6.9 (3.1-17.5) | .57 |
| CD3+ cell dose × 108/kg | 3.3 (1.0-4.5) | 3.4 (0.7-8.3) | .58 | 3.6 (0.7-7.2) | 3.1 (0.8-8.3) | .86 |
| Diagnosis of CML | 4/9 (44%) | 4/35 (11%) | .04 | 2/17 (12%) | 3/19 (16%) | 1.0 |
Data are presented as n (%) or median (range). Rejection analysis includes 44 patients who received low-dose TBI/CSP/MMF and excludes patient FH14726, who also received fludarabine.
Intensive prior therapy includes prior autograft, prior intensive chemotherapy for acute leukemia, or more than 3 cycles of a fludarabine-containing regimen.
Association of blood and marrow chimerism status at day 28 with subsequent rejection and acute graft-versus-host disease
| Cell fraction . | Percent donor chimerism as a continuous (0-100%) variable . | |||||
|---|---|---|---|---|---|---|
| Rejection . | GVHD . | |||||
| OR5-150 . | 95% CI . | Pvalue5-151 . | OR5-150 . | 95% CI . | Pvalue5-151 . | |
| Marrow | 0.4 | 0.2-0.8 | .009 | 0.7 | 0.3-1.6 | .42 |
| PB neutrophils | 0.4 | 0.2-0.7 | .004 | 1.2 | 0.6-2.5 | .62 |
| PB T cells | 0.1 | 0.04-0.5 | .003 | 2.6 | 1.1-6.4 | .03 |
| Cell fraction . | Percent donor chimerism as a continuous (0-100%) variable . | |||||
|---|---|---|---|---|---|---|
| Rejection . | GVHD . | |||||
| OR5-150 . | 95% CI . | Pvalue5-151 . | OR5-150 . | 95% CI . | Pvalue5-151 . | |
| Marrow | 0.4 | 0.2-0.8 | .009 | 0.7 | 0.3-1.6 | .42 |
| PB neutrophils | 0.4 | 0.2-0.7 | .004 | 1.2 | 0.6-2.5 | .62 |
| PB T cells | 0.1 | 0.04-0.5 | .003 | 2.6 | 1.1-6.4 | .03 |
Analysis of GVHD restricted to 35 patients with sustained engraftment and without GVHD before day 28. Patient UL627 was excluded from analysis because of lack of DNA polymorphism.
GVHD indicates graft-versus-host disease; CI, confidence interval; OR, odds ratio; PB, peripheral blood.
OR presented as an increase of 25% in donor chimerism. For example, the odds of rejection among patients with 50% donor T-cell chimerism are 0.4 times the odds of rejection among patients with 25% donor T-cell chimerism.
P value obtained from logistic regression, Wald test.
Association of donor T-cell chimerism with rejection and acute graft-versus-host disease
| Percent donor PB T-cell chimerism as a categorical variable . | ||
|---|---|---|
| % donor T cells on day 28 . | No. rejection/no. at risk (%) . | No. GVHD/no. at risk (%) . |
| 0-25 | 5/6 (83%) | 0/1 (0%) |
| 26-50 | 3/11 (27%) | 2/8 (25%) |
| 51-75 | 1/11 (9%) | 5/10 (50%) |
| 76-100 | 0/16 (0%) | 9/15 (63%) |
| Pvalue | .00016-150 | .326-150 |
| Percent donor PB T-cell chimerism as a categorical variable . | ||
|---|---|---|
| % donor T cells on day 28 . | No. rejection/no. at risk (%) . | No. GVHD/no. at risk (%) . |
| 0-25 | 5/6 (83%) | 0/1 (0%) |
| 26-50 | 3/11 (27%) | 2/8 (25%) |
| 51-75 | 1/11 (9%) | 5/10 (50%) |
| 76-100 | 0/16 (0%) | 9/15 (63%) |
| Pvalue | .00016-150 | .326-150 |
GVHD indicates graft-versus-host disease; PB, peripheral blood.
Analysis of GVHD restricted to 35 patients with sustained engraftment and without GVHD before day 28. Patient UL627 was excluded from analysis because of lack of DNA polymorphism.
P value obtained from Fisher's exact test.
Patients with sustained allografts
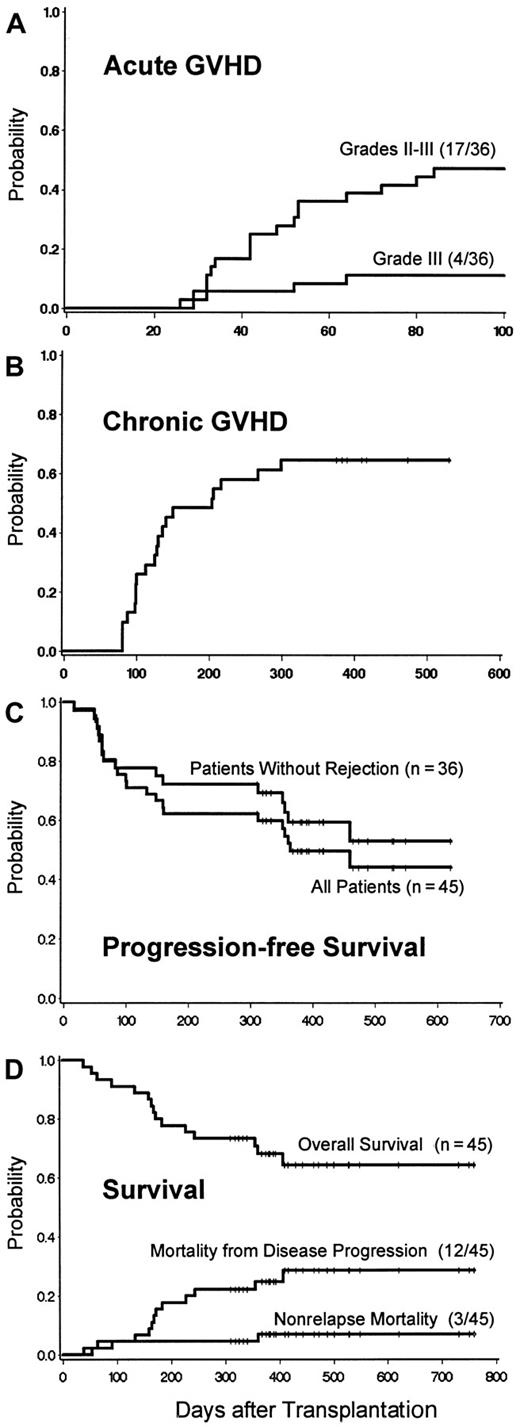
Of 36 patients, 17 (47%) developed acute GVHD, which was grade II in 13 (36%) and grade III in 4 (11%) (Figure4A). The day-28 levels of donor T-cell chimerism predicted subsequent acute GVHD (Tables 5 and 6), which occurred in all patients with full donor chimerism. With one exception, acute GVHD began after discontinuation of MMF. In most cases, it responded to continuation of CSP and a standard course of methylprednisolone.35 Twenty-three (74%) of 31 evaluable patients developed chronic GVHD; 19 (61%) were treated with systemic immunosuppressive therapy, one with topical steroids, and 3 received no specific therapy.31 The extent of chronic GVHD and organ involvement are shown in Table 2. Of the patients treated for chronic GVHD, 9 had excellent responses, 9 had partial responses (PRs), and 2 were unevaluable because of death. Two patients discontinued therapy without a flare of GVHD.
GVHD and survival after HCT.
(A) Incidence of acute GVHD: cumulative incidence curves for patients with sustained engraftment. (B) Chronic GVHD: cumulative incidence curve for patients with sustained engraftment. Curve includes both chronic extensive and limited GVHD. (C) Survival, transplantation-related mortality, and relapse mortality after HCT. Survival estimates were 68.6% (95% CI = 54.9% to 82.2%) at 12 months and 65.0% (95% CI = 50.3% to 79.6%) at 18 months. Transplantation-related mortality (TRM) was regarded as a competing risk for death from relapse, relapse as a competing risk for TRM, and death without GVHD as a competing risk for GVHD. (D) Progression-free survival. Freedom from disease progression is shown among the entire cohort and among patients without graft rejection. Disease progression was defined as relapse from CR or disease progression that required additional chemotherapy and/or DLI after transplantation or > 25% increase in tumor measurements over those present at transplantation baseline.
GVHD and survival after HCT.
(A) Incidence of acute GVHD: cumulative incidence curves for patients with sustained engraftment. (B) Chronic GVHD: cumulative incidence curve for patients with sustained engraftment. Curve includes both chronic extensive and limited GVHD. (C) Survival, transplantation-related mortality, and relapse mortality after HCT. Survival estimates were 68.6% (95% CI = 54.9% to 82.2%) at 12 months and 65.0% (95% CI = 50.3% to 79.6%) at 18 months. Transplantation-related mortality (TRM) was regarded as a competing risk for death from relapse, relapse as a competing risk for TRM, and death without GVHD as a competing risk for GVHD. (D) Progression-free survival. Freedom from disease progression is shown among the entire cohort and among patients without graft rejection. Disease progression was defined as relapse from CR or disease progression that required additional chemotherapy and/or DLI after transplantation or > 25% increase in tumor measurements over those present at transplantation baseline.
Status of underlying diseases: patients with graft rejection
Eight of 9 patients had persistent disease, and one MM patient (FH13230) achieved CR before relapse (Table 2). Patient FH13246 (MM) experienced a sustained CR of long-standing (30 years) psoriatic arthritis.
Status of underlying diseases: patients with sustained engraftment
Of 36 patients, 25 were alive, with 19 (53%) in CR, 2 in PR, and 4 without responses (Table 2). Twenty-nine of 36 patients had measurable disease before transplantation, and 19 (66%) achieved CR sometime after HCT. Progression-free survival for the entire group and survival for patients with sustained engraftment are shown in Figure 4C.
Five of 6 AML patients having transplantation in first CR or during aplasia were in remission. Of 2 AML patients in second or third remission, one died of relapse and one of infection while in remission. Of 3 patients with refractory acute leukemia, 2 died of relapse and one remained in remission at more than 23 months. All 5 CML patients were alive in molecular CR (Figure 5A). Of 7 CLL patients, 3 were in CR including 2 in molecular CR (Figure 5B), one was in PR, and one had progressive disease. One died of infection while in molecular CR and one died of progressive disease. Molecular CRs were first documented 5 to 12 months after transplantation and occurred gradually (Figure 5A,B). These remissions occurred in all 8 patients while they were receiving immunosuppressive therapy for management of GVHD. Of 6 MM patients, 4 were in CR (Figure 5C), one died of progressive disease, and one died of infection. Of 4 Hodgkin disease patients, one was in CR, one was alive in relapse, and 2 died of progressive disease. One NHL patient died of cerebral hemorrhage while in CR at 2 months. Two patients with Waldenström macroglobulinemia were alive with stable disease.
Complete disease responses after HCT.
(A) Example of molecular remission of CML (patient FH14726) induced by HCT without DLI, as documented by failure of reverse transcription–PCR to detect bcr-abl transcripts. The lane described “−ve” is a negative control of normal bone marrow (BM). K562 is a positive control for bcr-abl. (B) Example of molecular remission of CLL induced by HCT (patient FH12914) without DLI, as documented by PCR to detect a tumor-specific immunoglobulin heavy-chain gene rearrangement (arrow). Each posttransplantation sample was amplified in duplicate. The lanes designated 10−1 to 10−5 show a dilution series of the patient's pretransplantation sample (more than 90% tumor cells) into normal bone marrow. (C) Example of complete remission of MM (patient FH13922) after allogeneic HCT. The patient was initially treated with 4 cycles of vincristine, adriamycin, and dexamethasone (VAD). High-dose cytoreduction with melphalan 200 mg/m2 and autologous transplantation were performed 3 months before allogeneic HCT. DLI was given 4 months after HCT because of persistent tumor. After CR was achieved, trace levels of serum monoclonal paraprotein detected by immunofixation were present intermittently in follow-up testing.
Complete disease responses after HCT.
(A) Example of molecular remission of CML (patient FH14726) induced by HCT without DLI, as documented by failure of reverse transcription–PCR to detect bcr-abl transcripts. The lane described “−ve” is a negative control of normal bone marrow (BM). K562 is a positive control for bcr-abl. (B) Example of molecular remission of CLL induced by HCT (patient FH12914) without DLI, as documented by PCR to detect a tumor-specific immunoglobulin heavy-chain gene rearrangement (arrow). Each posttransplantation sample was amplified in duplicate. The lanes designated 10−1 to 10−5 show a dilution series of the patient's pretransplantation sample (more than 90% tumor cells) into normal bone marrow. (C) Example of complete remission of MM (patient FH13922) after allogeneic HCT. The patient was initially treated with 4 cycles of vincristine, adriamycin, and dexamethasone (VAD). High-dose cytoreduction with melphalan 200 mg/m2 and autologous transplantation were performed 3 months before allogeneic HCT. DLI was given 4 months after HCT because of persistent tumor. After CR was achieved, trace levels of serum monoclonal paraprotein detected by immunofixation were present intermittently in follow-up testing.
Donor lymphocyte infusions
Twenty-three patients (51%) underwent the first DLI on days 56 to 326, including all 9 patients who subsequently rejected their grafts. Thus, DLIs given at 1 × 107 CD3+ cells/kg were unable to prevent progression to complete graft rejection. Twelve of 14 remaining patients had measurable disease. Four achieved CR, 5 had no response, and 3 were unevaluable because of concurrent other therapy (n = 2) or early death due to infection (FH14692). Three patients developed their first GVHD after DLI.
Survival and causes of death
With median follow-up at 417 days (range, 310-769 days), 30 of 45 patients (66.7%) were alive (Figure 4D). Twelve patients (26.7%) died of progressive disease, including 4 from DLI-induced GVHD and infections and 2 after conventional second HCT. Three (6.7%) died of transplantation complications without disease progression, including pneumonia at 2 months (UL625), central nervous system hemorrhage at 2 months (FH14224), and streptococcal sepsis after sinusitis at 1 year while off all systemic immunosuppression (FH12914).
Discussion
This clinical protocol was a direct translation of preclinical canine HCT studies.22 The new concept for HCT, which relies on GVT effects rather than extreme chemoradiation to eradicate malignancies, has several advantages. First, serious organ damage characteristic of conventional transplantation is avoided. Second, a lack of profound pancytopenia reduces infection risks. These 2 features allow application of HCT to otherwise ineligible patients. Third, there may be an immunologic advantage that is the key to the success of this approach. Specifically, minimally cytotoxic pretransplantation conditioning allows persistence of host antigen-presenting cells, thus enhancing presentation of host minor antigens to donor T cells and initiation of GVT (and GVH) effects.
By exploiting postgrafting immunosuppression to control graft rejection, most of the pretransplantation TBI required for engraftment was eliminated in canine studies.22 Encouraged by the effectiveness and safety of these transplantations together with the therapeutic potential of donor lymphocytes,11-16 we initiated this study. Several conclusions are possible.
First, the regimen was safe and minimally toxic. Alopecia, severe mucositis, severe protracted pancytopenias, and fatal regimen-related toxicities typical of conventional HCT were not encountered. This allowed 53% of eligible patients to be treated entirely as outpatients, with others having relatively short hospitalizations (median 8 days), as compared with medians of more than 30 days with conventional HCT.36,37 Most patients required neither antibiotic treatment for infections nor platelet transfusions. Given the modest declines in blood counts, it was difficult to estimate the speed of engraftment. However, in one patient who was aplastic at transplantation (FH14241), neutrophils rose to more than 500/μL by day 8 and platelets to more than 20 000/μL by day 13.25Thus, the degree and exact tempo of myelosuppression induced by 200 cGy TBI in humans not given stem cell support cannot be estimated from this study. However, in dogs given this TBI dose without stem cell transplantation, the granulocyte nadir was 750/μL on day 20 and platelet nadirs were at 16 to 24 days after transplantation. Marrow recovery occurred in 17 of 18 dogs and all 17 survived beyond 30 days, with recovery complete by day 40 for granulocytes and by day 50 for platelets.22 CMV reactivations occurred in only 24% of CMV-seropositive patients, as compared with 79% after conventional transplantation.38 Three patients (6.7%) died of transplantation complications without disease progression. Overall, this toxicity profile was impressive considering the ages, preceding therapies, and preexisting organ dysfunction or infections among patients who were not conventional allografting candidates. These observations support the concept that the use of potent postgrafting immunosuppression permits one to reduce the intensity of pretransplantation immunosuppressive and myelosuppressive therapy, thereby reducing toxicities usually associated with allografting.
As with any radiation, there is a risk of late malignancies even from the low dose rate of 200 cGy TBI used here. Nevertheless, evidence suggests that this risk might be small. Among 95 673 nuclear workers exposed to low-level radiation, the excess relative risk of cancer was estimated to be only −0.02 (95% confidence interval [CI] −0.34 to 0.35) per sievert (approximately 1 Gy γ-radiation).39 In a large retrospective study, TBI-associated cancer risks after conventional HCT were dose related, but were not increased with TBI-based regimens using less than 10 Gy as compared with chemotherapy-only regimens.40 Long-term follow-up of individuals exposed to ionizing radiation after atomic-bomb explosions also supports the concept that the risks from low-dose TBI are relatively small.41 Others have reported a significant incidence of late leukemia or cancer after low-dose TBI when patients received fractionated high-dose-rate (150 cGy/min) TBI and, in most cases, additional high-dose local radiation and/or systemic alkylating agents.42 Based on those observations, it is difficult to estimate the late cancer risks from single-fraction low-dose-rate TBI as used here. The low dose rate used here (7 cGy/min), together with allografting, which largely eliminates host hematopoiesis through GVH reactions, should provide protection against secondary AML. Nonetheless, concerns about long-term safety have provided impetus to further reduce reliance on TBI as immunosuppression, particularly when treating nonmalignant disorders in younger patients. Additional canine studies have provided evidence that alternative, less toxic immunosuppression may eventually allow reduction or elimination of TBI from the current protocols.43 The risks of late malignancies from alternative reduced-intensity allografting regimens that use fludarabine with intermediate-dose or high-dose alkylating agents17-19 44 also remain uncertain and must be determined by longer follow-up.
Second, stable allogeneic engraftment was achieved in most patients despite using only 200 cGy TBI before transplantation. It is known from canine studies that 200 cGy is by itself not sufficiently immunosuppressive to permit stable allogeneic engraftment. However, when 200 cGy TBI before HCT was combined with postgrafting MMF/CSP, stable engraftment was seen in 11 of 12 dogs, whereas no long-term engraftment was seen with CSP alone. To achieve the same rate of engraftment without postgrafting immunosuppression, 920 cGy TBI was required. Put another way, MMF/CSP after grafting provided a degree of host immunosuppression that was otherwise achieved with more than 700 cGy TBI before transplantation. These considerations suggest that postgrafting CSP/MMF had an important role in facilitating engraftment in this clinical study. Nonfatal graft rejection occurred in 20% of the 44 patients who received TBI/MMF/CSP, and autologous hematopoietic recovery occurred in each case, as in the canine studies,22 providing evidence that host hematopoiesis was not ablated by the conditioning therapy. Rejection episodes occurred at 2 to 4 months, and one patient had 3 weeks of severe pancytopenia before recovery. The relatively large and increasing admixture of host cells, together with relatively normal PB counts shortly before rejection, suggests that TBI-induced suppression was not responsible for pancytopenia in this patient and implicates another mechanism, possibly similar to that observed with DLI-induced aplasia. The factor most closely associated with rejection was the absence of intensive previous chemotherapy. The current approach was most effective for heavily pretreated patients, with 30 of 31 (97%) having sustained engraftment. To reduce the rejection risk, we added the potent immunosuppressive purine analogue fludarabine to the pretransplantation conditioning for one patient reported here, and this approach is being evaluated in further studies. The high-level day-28 T-cell engraftment in CML patient FH14726 together with uniformly stable engraftment in 43 subsequent HLA-identical siblings (unpublished observations) and 11 unrelated donor HCT recipients45 suggests that fludarabine/TBI more effectively prevents rejection than TBI alone. Fludarabine synergizes with radiation to kill tumor cells,46 and this may also be true for its effect on T lymphocytes. It has been a component of most other reported reduced-intensity allografting conditioning regimens.17-19 44
Third, 47% of patients with sustained engraftment developed acute grades II to III GVHD, with no grade IV disease. By comparison, rates of acute GVHD after conventional allografting using standard methotrexate/CSP prophylaxis47 were 35% and 44% among 44648 and 16449 younger patients, respectively, and 60% among 57 older patients (50-60 years) (R. Storb, unpublished data, Fred Hutchinson Cancer Research Center). The lack of stable mixed chimerism in most patients together with the development of GVHD in some patients after discontinuation of CSP on day 35 led us to extend CSP administration to day +56 for better GVHD protection. GVHD was effectively managed in most current patients. Whereas the median onset of acute GVHD was day 16 after conventional transplantation,48 the current median was 40 days. The almost complete absence of GVHD during days of combined MMF/CSP prophylaxis suggests that this is a potent immunosuppressive combination, consistent with canine GVHD studies.50 The contributions of low-intensity pretransplantation conditioning and initial mixed chimerism to the relatively benign courses of GVHD in these older patients remain unclear. Given the importance of inducing GVT effects for successful nonmyeloablative transplantation and the appreciable incidence of both acute and chronic GVHD encountered, GVHD control remains a critical research objective. Because MMF/CSP was given for relatively short periods after transplantation in this study, GVHD control might be improved simply by extending the duration of postgrafting immunosuppression.
Fourth, an important initial goal was establishing mixed chimerism, anticipating that it might protect from GVHD and it would provide an immunologic platform for subsequent DLI.51 Although approximately 80% of patients had mixed chimerism early after transplantation, it was usually unstable, progressing to either full donor chimerism or graft rejection and restricting DLI to 22 patients, including 8 who rejected their grafts. The differences between dogs and human patients with respect to establishing stable mixed chimerism may relate to differences in age and thymic function, previous chemotherapy and transfusions in patients, and minor interspecies variations in responses to the immunosuppressive agents.
Finally, persisting antitumor responses occurred exclusively in patients with sustained engraftment, indicating the importance of donor T cells for inducing and maintaining these responses. Nineteen of 29 patients (66%) with measurable disease before transplantation achieved CRs, and 3 others achieved PRs. Among these, 8 molecular remissions in CML (n = 5) and CLL (n = 3) patients were documented 5 to 12 months after HCT, and 7 of these occurred without the need for DLI. The molecular remissions occurred while patients received immunosuppression for treatment of GVHD, indicating that powerful GVT responses were generated and continued even with control of other GVHD manifestations. Although low-dose TBI given with or without alkylating agents may induce remissions in some patients with untreated CLL and NHL,52,53 it has not been reported that low-dose TBI can induce CRs and molecular remissions in patients with chemotherapy-resistant CLL, and in particular after failure of previous fludarabine-containing regimens. Similarly, although low-dose TBI may have transient suppressive effects on CML, even high-dose myeloablative regimens (at least 1200 cGy TBI plus intensive chemotherapy) are by themselves often incapable of providing sustained disease control, as indicated by the frequent relapses observed after using T-cell–depleted allografts.54 Thus, it is very unlikely that the low-dose and low-dose-rate TBI was responsible for the major disease responses observed here. Further, the molecular remissions occurred gradually, with clearing of tumor over many months and long after conditioning therapies were given. These observations also suggest that for some indolent malignancies, cytoreductive therapy given before transplantation may not be necessary and that DLI in this setting may be used sparingly and even avoided if disease is stable or responding. Factors that may influence the extent and tempo of disease responses include degrees of minor non-HLA disparities between donors and recipients, effects of immunosuppressive therapy given for GVHD, and expression of target antigens by tumor cells. For aggressive malignancies, it may be necessary to control tumor growth before GVT effects occur. Whereas others have used systemic chemotherapy, we are exploring whether this can be achieved by adding tumor-directed monoclonal antibody conjugates.55 56
The induction of molecular remissions suggests that this treatment approach has curative potential for CML and CLL and may be a future alternative for younger patients who wish to avoid complications from high-dose regimens. Observations of durable molecular remissions of CML after DLI was given for relapse after conventional HCT support this notion.57 58 The current study included a heterogeneous group of patients, which allowed us to demonstrate antitumor effects in several different diseases and to identify patients at increased risk of graft rejection. Disease-specific phase 2 and 3 studies with prolonged follow-up will be required to fully evaluate the benefits and limitations of this approach regarding its curative potential, consequences of GVHD, late side effects, and comparisons with conventional allografting.
In smaller series reported recently, reduced-intensity regimens were used for allografting.17-20 Conceptually, achieving engraftment relied on immunosuppressive (and myelosuppressive) chemotherapy of varying intensities given before transplantation to suppress tumors and host immunity. Sustained engraftment was usually achieved, and impressive GVT activity was observed in some patients. Investigators from Houston used conventional chemotherapy regimens incorporating the purine analogues fludarabine or 2-chloro-deoxyadenosine in patients with advanced malignancies who were ineligible for conventional allografts.17,19 Postgrafting GVHD prophylaxis was omitted in their initial patients but added later to control GVHD. Fludarabine, 30 mg/m2 ×3, and cyclophosphamide, 300 mg/m2 ×3, their least intensive regimen, resulted in failed donor engraftment in 3 of 5 heavily pretreated CLL patients,19 necessitating dose escalation. These results suggest that this regimen was less immunosuppressive than TBI/MMF/CSP reported here. Others have used modifications of fludarabine/cyclophosphamide.44,59 Slavin et al18 reported using an attenuated conventional transplantation regimen of busulfan, fludarabine, and antithymocyte globulin with CSP alone as GVHD prophylaxis. Among 26 patients, veno-occlusive disease occurred in 50% and fatal acute GVHD occurred in 15%. Although the 14-month survival of 77.5% was impressive, the median patient age was 32 years (range, 1-56 years), and all appeared to be candidates for conventional allografting. Using high-dose cyclophosphamide and antithymocyte globulin with or without thymic irradiation, Sykes et al20 have established grafts from HLA-matched and HLA-mismatched donors in patients with advanced lymphoma.
By focusing on postgrafting immunosuppression to control rejection and GVHD, we have further reduced the intensity and toxicity of conditioning therapy for recipients of HLA-identical grafts. This allografting approach appears broadly applicable and suitable for studies of adoptive immunotherapy in hematologic and solid tumors, given that the transplantations can be performed effectively in an outpatient setting with minimal toxicity and limited supportive-care requirements. The development of effective targeting strategies to focus T-cell reactions against tumor antigens or minor antigens with hematopoietic restriction60,61 might enhance future outcomes through more effective tumor killing and by reducing the risks of GVHD. As reviewed elsewhere,51 this new allografting concept also has potentially important applications in the treatment of some nonmalignant diseases.
We thank the medical, nursing, data processing, and clinical staffs at the listed institutions for their important contributions to this study through their careful and dedicated care of the patients. We thank Bonnie Larson for her assistance in preparation of this manuscript.
Abbreviations for therapies listed in Table 1: ABVD indicates adriamycin, bleomycin, vinblastine, dacarbazine; Ara-C, cytosine arabinoside; Asp, asparaginase; CE ± D, high-dose cyclophosphamide, etoposide ± decadron; Ch, chlorambucil; CHAD, cisplatin, cytosine arabinoside, dexamethasone; CHOP, vincristine, adriamycin, cyclophosphamide, prednisone; CMOPP, cyclophosphamide, vincristine, procarbazine, prednisone; Cp, cisplatin; CVP, cyclophosphamide, vincristine, prednisone; Cy, cyclophosphamide; CyTx, high-dose cyclophosphamide, Taxol; D, daunorubicin; Dex, dexamethasone; DHAP, dexamethasone, cytosine arabinoside, cisplatin; E, etoposide; F, fludarabine; FLAG, fludarabine, cytosine arabinoside, granulocyte colony-stimulating factor; FMD, fludarabine, mitoxantrone, dexamethasone; Gc, gemcitabine; HAM, high-dose cytosine arabinoside, mitoxantrone; HDAC, high-dose cytosine arabinoside; HDCy, high-dose cyclophosphamide; HDT, high-dose chemotherapy and autologous transplantation; Hh, homoharringtonine; Hu, hydroxyurea; Ida, idarubicin; IFN, alpha-interferon; M, melphalan; MCE, mitoxantone, cytosine arabinoside, etoposide; Mi, mitoxantrone; MOPP, nitrogen mustard, vincristine, procarbazine, prednisone; P, prednisone; TAD, thioguanine, cytosine arabinoside, daunorubicin; TG, 6-thioguanine; V, vincristine; VAD, vincristine, adriamycin, dexamethasone; Vb, vinblastine; XRT, local radiation therapy.
Supported in part by grants CA78902, CA49605, HL36444, CA18221, CA15704, and HL03701 awarded by the National Institutes of Health, Department of Health and Human Services, Bethesda, MD. P.A.M. and D.G.M. were also supported by a grant from the Gabrielle Rich Leukemia Foundation. R.F.S. also received support from the Laura Landro Salomon Endowment Fund.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
References
Author notes
Peter A. McSweeney, Bone Marrow Transplant Program, University of Colorado Health Sciences Center, 4200 East 9th Avenue, Campus Box B-190, Denver, CO 80262; e-mail:peter.mcsweeney@uchsc.edu.


This feature is available to Subscribers Only
Sign In or Create an Account Close Modal