Allogeneic peripheral blood stem cell grafts contain about 10 times more T and B cells than marrow grafts. Because these cells may survive in transplant recipients for a long time, recipients of blood stem cells may be less immunocompromised than recipients of marrow. Immune reconstitution was studied in 115 patients randomly assigned to receive either allogeneic marrow or filgrastim-mobilized blood stem cell transplantation. Between day 30 and 365 after transplantation, counts of most lymphocyte subsets were higher in the blood stem cell recipients. The difference was most striking for CD4 T cells (about 4-fold higher counts for CD45RAhigh CD4 T cells and about 2-fold higher counts for CD45RAlow/−CD4 T cells;P < .05). On assessment using phytohemagglutinin and herpesvirus antigen-stimulated proliferation, T cells in the 2 groups of patients appeared equally functional. Median serum IgG levels were similar in the 2 groups. The rate of definite infections after engraftment was 1.7-fold higher in marrow recipients (P = .001). The rate of severe (inpatient treatment required) definite infections after engraftment was 2.4-fold higher in marrow recipients (P = .002). The difference in the rates of definite infections was greatest for fungal infections, intermediate for bacterial infections, and lowest for viral infections. Death associated with a fungal or bacterial infection occurred between day 30 and day 365 after transplantation in 9 marrow recipients and no blood stem cell recipients (P = .008). In conclusion, blood stem cell recipients have higher lymphocyte-subset counts and this appears to result in fewer infections.
Introduction
Allogeneic bone marrow transplantation is a recognized treatment for certain hematologic malignant diseases, aplastic anemia, and inborn errors of cells originating from hematopoietic stem cells.1,2 Immune deficiency leading to increased susceptibility to infections follows transplantation and lasts for more than a year.3-8 Although infections that occur in the first month after grafting likely result from a deficiency of both granulocytes and mononuclear cell (MNC) subsets, postengraftment infections are probably due to a deficiency in MNC subsets, primarily CD4 T cells and B cells.9-11Postengraftment infections cause substantial morbidity and mortality. Atkinson et al3 showed that 87% of allograft recipients had at least one postengraftment infection and 48% had at least 3 (excluding upper respiratory tract infections) during a 2-year period. A similar incidence of postengraftment infections was observed by Ochs et al,7 who also noted that occurrence of postengraftment infection was the dominant independent factor associated with increased nonrelapse mortality (relative risk = 5.5;P = .0001).
Recipients of blood stem cells receive at least 10 times more lymphocytes than recipients of marrow.12,13 The lifespan of human T cells is months to years.14-16 Therefore, we hypothesized that the large lymphocyte inoculum given to blood stem cell recipients might result in higher T-cell counts in the first year after transplantation. Results of small nonrandomized studies of blood stem cell recipients compared with marrow recipients support this hypothesis.12 13 We also hypothesized that higher lymphocyte counts in blood stem cell recipients could lead to lower infection rates. Thus, the purpose of this randomized study was to analyze MNC-subset recovery and postengraftment infection rates in marrow grafting compared with blood stem cell grafting.
Patients, materials, and methods
Patients
Of 140 patients who were randomly assigned to undergo either marrow or filgrastim-mobilized blood stem cell allografting, treated at the Fred Hutchinson Cancer Research Center (FHCRC) as reported previously,17 and followed for at least a year, 128 patients agreed to participate in immune reconstitution studies approved by the institutional review board of the FHCRC. One of these patients received marrow instead of blood stem cells, and 12 patients (7 marrow recipients and 5 blood stem cell recipients) died by day 30 after transplantation. Thus, 115 patients were available for MNC-subset testing between day 30 and 365 and could be evaluated for postengraftment infections. Their demographic and clinical characteristics, including infection prophylaxis given, are shown in Table 1. Because the goal of the study was to evaluate transplantation-associated immune deficiency and not malignancy-associated immune deficiency, patients who had a relapse after transplantation were studied only before the diagnosis of relapse. The interval between day 30 and day 365 or the day of death or relapse is referred to in this paper as the interval between “day 30 and day 365.”
Characteristics of patients given marrow as compared with those given blood as the source of hematopoietic cells for transplantation
| . | Patients given marrow (n = 60) . | Patients given blood (n = 55) . | P . |
|---|---|---|---|
| Patients' median (range) age at Tx, y | 43 (22-56) | 43 (15-56) | .57 |
| Donors' median (range) age at Tx, y | 41 (18-65) | 40 (13-63) | .34 |
| Patients' sex (M/F) | 39/21 | 35/20 | .97 |
| Donors' sex (M/F) | 32/28 | 31/24 | .89 |
| Donor-patient histocompatibility | |||
| HLA-A, B, and DR-matched sibling | 60 (100) | 54 (98) | .97 |
| HLA-A, B, and DR-matched child | 0 (0) | 1 (2) | |
| Disease/disease stage at Tx* | |||
| Good risk | 34 (57) | 31 (56) | .88 |
| Poor risk | 26 (43) | 24 (44) | |
| First Tx | 60 (100) | 55 (100) | 1.00 |
| CMV serostatus before Tx | |||
| Donor positive/recipient positive | 22 (37) | 15 (27) | .63 |
| Donor negative/recipient positive | 11 (18) | 14 (25) | |
| Donor positive/recipient negative | 10 (17) | 11 (20) | |
| Donor negative/recipient negative | 17 (28) | 14 (25) | |
| Unknown or equivocal | 0 (0) | 1 (2) | |
| HSV serostatus before Tx | |||
| Patient positive | 44 (73) | 35 (64) | .73 |
| Patient negative | 11 (18) | 14 (25) | |
| Unknown or equivocal | 5 (8) | 6 (11) | |
| VZV serostatus before Tx | |||
| Patient positive | 58 (97) | 53 (96) | .45 |
| Patient negative | 0 (0) | 2 (4) | |
| Unknown or equivocal | 2 (3) | 0 (0) | |
| Patients with splenectomy | 3 (5) | 1 (2) | .67 |
| Myeloablative conditioning | |||
| Chemotherapy only | 30 (50) | 27 (49) | .33 |
| Chemotherapy and total-body irradiation | 29 (48) | 24 (44) | |
| Chemotherapy and total-marrow irradiation | 1 (2) | 4 (7) | |
| GVHD prophylaxis with methotrexate (day 1, 3, 6, and 11) and cyclosporine (daily for 6 months)58 | 60 (100) | 55 (100) | 1.00 |
| Acute GVHD | |||
| Grade 0-1 | 16 (27) | 16 (29) | .94 |
| Grade 2-4† | 44 (73) | 39 (71) | |
| Chronic GVHD diagnosed by day 365 | |||
| None or clinically limited | 25 (42) | 18 (33) | .16 |
| Clinically extensive‡ | 23 (38) | 32 (58) | |
| Not applicable (relapse or death before day 100) | 12 (20) | 5 (9) | |
| Chimerism status | |||
| Full chimera (≥ 90% marrow cells of donor origin by day 80) | 52 (87) | 47 (85) | 1.00 |
| Unknown | 8 (13) | 8 (15) | |
| Median (range) day of neutrophil engraftment (> 0.5 × 109/L) | 20 (18-28) | 16 (11-28) | < .01 |
| Neutropenia (< 0.5 × 109/L) between day 30 day and 3651-153 | 7 (12) | 4 (7) | .63 |
| Relapse between day 30 and day 3651-155 | 8 (13) | 8 (15) | .94 |
| Death without relapse between day 30 and day 365 | 18 (30) | 6 (11) | .02 |
| Glucocorticoid treatment between day 30 and day 365 | 51 (86) | 45 (82) | .84 |
| CMV prophylaxis | |||
| Ganciclovir if pp65-antigenemia positive59 | 53 (88) | 51 (93) | .63 |
| Infusion of ex vivo–expanded CMV-specific T cells601-154 | 7 (12) | 4 (7) | |
| Viral prophylaxis with acyclovir/valacyclovir/famciclovir beyond standard1-159 | |||
| Yes | 22 (37) | 14 (25) | .20 |
| No | 33 (55) | 39 (71) | |
| Unknown | 5 (8) | 2 (4) | |
| Bacterial/Pneumocystis/fungal prophylaxis with sulfamethoxazole and trimethoprim and/or fluconazole/itraconazole beyond standard*,* | |||
| Yes | 24 (40) | 29 (53) | .46 |
| No | 19 (32) | 15 (27) | |
| Not applicable (early relapse or death) or unknown | 17 (28) | 11 (20) | |
| Prophylaxis with intravenous immunoglobulin†,† | 8 (13) | 6 (11) | .91 |
| . | Patients given marrow (n = 60) . | Patients given blood (n = 55) . | P . |
|---|---|---|---|
| Patients' median (range) age at Tx, y | 43 (22-56) | 43 (15-56) | .57 |
| Donors' median (range) age at Tx, y | 41 (18-65) | 40 (13-63) | .34 |
| Patients' sex (M/F) | 39/21 | 35/20 | .97 |
| Donors' sex (M/F) | 32/28 | 31/24 | .89 |
| Donor-patient histocompatibility | |||
| HLA-A, B, and DR-matched sibling | 60 (100) | 54 (98) | .97 |
| HLA-A, B, and DR-matched child | 0 (0) | 1 (2) | |
| Disease/disease stage at Tx* | |||
| Good risk | 34 (57) | 31 (56) | .88 |
| Poor risk | 26 (43) | 24 (44) | |
| First Tx | 60 (100) | 55 (100) | 1.00 |
| CMV serostatus before Tx | |||
| Donor positive/recipient positive | 22 (37) | 15 (27) | .63 |
| Donor negative/recipient positive | 11 (18) | 14 (25) | |
| Donor positive/recipient negative | 10 (17) | 11 (20) | |
| Donor negative/recipient negative | 17 (28) | 14 (25) | |
| Unknown or equivocal | 0 (0) | 1 (2) | |
| HSV serostatus before Tx | |||
| Patient positive | 44 (73) | 35 (64) | .73 |
| Patient negative | 11 (18) | 14 (25) | |
| Unknown or equivocal | 5 (8) | 6 (11) | |
| VZV serostatus before Tx | |||
| Patient positive | 58 (97) | 53 (96) | .45 |
| Patient negative | 0 (0) | 2 (4) | |
| Unknown or equivocal | 2 (3) | 0 (0) | |
| Patients with splenectomy | 3 (5) | 1 (2) | .67 |
| Myeloablative conditioning | |||
| Chemotherapy only | 30 (50) | 27 (49) | .33 |
| Chemotherapy and total-body irradiation | 29 (48) | 24 (44) | |
| Chemotherapy and total-marrow irradiation | 1 (2) | 4 (7) | |
| GVHD prophylaxis with methotrexate (day 1, 3, 6, and 11) and cyclosporine (daily for 6 months)58 | 60 (100) | 55 (100) | 1.00 |
| Acute GVHD | |||
| Grade 0-1 | 16 (27) | 16 (29) | .94 |
| Grade 2-4† | 44 (73) | 39 (71) | |
| Chronic GVHD diagnosed by day 365 | |||
| None or clinically limited | 25 (42) | 18 (33) | .16 |
| Clinically extensive‡ | 23 (38) | 32 (58) | |
| Not applicable (relapse or death before day 100) | 12 (20) | 5 (9) | |
| Chimerism status | |||
| Full chimera (≥ 90% marrow cells of donor origin by day 80) | 52 (87) | 47 (85) | 1.00 |
| Unknown | 8 (13) | 8 (15) | |
| Median (range) day of neutrophil engraftment (> 0.5 × 109/L) | 20 (18-28) | 16 (11-28) | < .01 |
| Neutropenia (< 0.5 × 109/L) between day 30 day and 3651-153 | 7 (12) | 4 (7) | .63 |
| Relapse between day 30 and day 3651-155 | 8 (13) | 8 (15) | .94 |
| Death without relapse between day 30 and day 365 | 18 (30) | 6 (11) | .02 |
| Glucocorticoid treatment between day 30 and day 365 | 51 (86) | 45 (82) | .84 |
| CMV prophylaxis | |||
| Ganciclovir if pp65-antigenemia positive59 | 53 (88) | 51 (93) | .63 |
| Infusion of ex vivo–expanded CMV-specific T cells601-154 | 7 (12) | 4 (7) | |
| Viral prophylaxis with acyclovir/valacyclovir/famciclovir beyond standard1-159 | |||
| Yes | 22 (37) | 14 (25) | .20 |
| No | 33 (55) | 39 (71) | |
| Unknown | 5 (8) | 2 (4) | |
| Bacterial/Pneumocystis/fungal prophylaxis with sulfamethoxazole and trimethoprim and/or fluconazole/itraconazole beyond standard*,* | |||
| Yes | 24 (40) | 29 (53) | .46 |
| No | 19 (32) | 15 (27) | |
| Not applicable (early relapse or death) or unknown | 17 (28) | 11 (20) | |
| Prophylaxis with intravenous immunoglobulin†,† | 8 (13) | 6 (11) | .91 |
Values are numbers (percentages) of patients unless otherwise indicated. The significance of differences between patients given marrow and those given blood stem cells was assessed by eithert test (age and neutrophil engraftment) or χ2 square test (all other variables).
Tx indicates transplantation; CMV, cytomegalovirus; HSV, herpes simplex virus; VZV, varicella zoster virus; GVHD, graft-versus-host disease.
Good risk was defined as the presence of chronic myelogenous leukemia (CML) in first chronic or accelerated phase, acute leukemia in first remission, refractory anemia, or myelofibrosis. Poor risk was defined as the presence of CML in blast crisis, acute leukemia beyond first remission, refractory anemia with excess blasts, lymphoma, or multiple myeloma.
Typically treated with prednisone (1-2 mg/kg per day given orally for 10 to 14 days with subsequent tapering during 50 days).
Typically treated with prednisone (0.5-1.0 mg/kg given orally every other day) and cyclosporine (approximately 6 mg/kg given orally every other day) for at least 9 months.
Values are numbers of episodes. There was one episode per patient. No episode was due to graft rejection. Episodes were typically associated with a viral infection and lasted for several days.
For patients with CML, detection of bcr/abl transcript by polymerase chain reaction in the absence of cytogenetic or hematologic relapse was not considered a relapse.
Values were typically 1 × 109 CD4 cells/m2 and 2 × 109 CD8 cells/m2 of body-surface area.
According to standard practice, all patients seropositive for herpes simplex virus received acyclovir (800 mg twice a day given orally) during the first month only. Some patients or physicians chose prophylaxis with acyclovir, valacyclovir, or famciclovir between day 30 and day 365 after transplantation; the patients are included here.
According to standard practice, all patients received prophylactic sulfamethoxazole and trimethoprim until day 180 after transplantation (800 mg sulfamethoxazole and 160 mg trimethoprim given orally twice a day every Monday and Tuesday) and fluconazole until day 75 (400 mg given orally every day). Itraconazole was given instead of fluconazole to 5 marrow recipients and 2 blood stem cell recipients. After the end of the routine bacterial/Pneumocystis/fungal prophylaxis, some patients or physicians chose to continue prophylactic therapy; the patients are counted here. Typically, these were patients with clinically extensive chronic GVHD who were treated with immunosuppressive drugs, sulfamethoxazole and trimethoprim (800 mg and 160 mg, respectively, given orally twice a day every Monday and Tuesday), penicillin (500 mg given orally twice a day every day), and rarely, fluconazole or itraconazole.
Typically, 200 mg/kg was given weekly before day 100 after transplantation, and 500 mg/kg was given monthly after day 100.
Enumeration of MNC subsets
Blood specimens were drawn from donors before transplantation (before filgrastim was used) and from recipients on about day 30 after transplantation (median, day 31 for marrow and day 32 for blood stem cell recipients), day 80 (median, day 78 and day 79), day 180 (median, day 182 and day 188), and day 365 (median, day 377 and day 376). The days after transplantation on which blood was drawn were not significantly different between the 2 groups of recipients (byt test). With use of density gradient (Ficoll) centrifugation, MNCs were separated from blood specimens and not separated from marrow or blood stem cell grafts. Cells were stained with fluorochrome-conjugated monoclonal antibodies and analyzed by using 3-color flow cytometry as described previously.18,19The antigenic phenotypes defining each MNC subset are detailed in Table2. The rationales for defining the B- and T-cell subsets in Table 2 were as follows. Naive B cells are represented by IgD+ B cells, since most IgD+ B cells lack somatic mutations.20-22 Naive CD4 T cells were defined as CD45RAhigh CD4 T cells, since this subset contains thymic emigrants and nearly all cord blood CD4 T cells are CD45RAhigh.23-25 Naive CD8 T cells were defined as CD11alow CD8 T cells because virtually all cord blood CD8 T cells are CD11alow and become CD11ahigh after activation.26,27CD28+ T cells represent cells that can receive both the signal mediated by the T-cell receptor and the CD28-mediated costimulatory signal.28 Each absolute MNC-subset count was calculated as the absolute MNC count multiplied by the percentage of the MNC subset divided by 100. The absolute MNC count represented the sum of the absolute lymphocyte count and the absolute monocyte count determined by a clinical hematology laboratory. For grafts, total nucleated cells were gated on forward x side-scatter plots and each absolute cell-subset count was calculated as the absolute nucleated-cell count multiplied by the percentage of the cell subset among total nucleated cells divided by 100.
Definitions of subsets of mononuclear cells
| MNC subset . | Definition . |
|---|---|
| B cells | MNCs expressing CD19 or CD20 and not brightly expressing CD3, CD13, CD14, CD16, CD56, CD10, or CD34 |
| Naive (IgD+) | B cells (defined as above) expressing surface IgD |
| Memory (IgD−) | B cells (defined as above) not expressing surface IgD |
| CD4 T cells (total) | CD3+ CD4+ CD8− MNCs |
| Naive (CD45RAhigh) | MNCs expressing CD4, brightly expressing CD45RA, and not expressing CD8, CD13, CD14, or CD16 |
| Memory or effector (CD45RAlow/−) | MNCs expressing CD4, dimly expressing or not expressing CD45RA, and not expressing CD8, CD13, CD14, or CD16 |
| Costimulation competent (CD28+) | MNCs expressing CD4, expressing CD28, and not expressing CD8, CD13, CD14, or CD16 |
| Costimulation incompetent (CD28−) | MNCs expressing CD4, not expressing CD28, and not expressing CD8, CD13, CD14, or CD16 |
| CD8 T cells (total) | CD3+ CD4− CD8+ MNCs |
| Naive (CD11alow) | MNCs brightly expressing CD8, dimly expressing CD11a, and not expressing CD4, CD13, CD14, CD16, or CD19 |
| Memory or effector (CD11ahigh) | MNCs brightly expressing CD8, brightly expressing CD11a, and not expressing CD4, CD13, CD14, CD16, or CD19 |
| Costimulation competent (CD28+) | MNCs brightly expressing CD8, expressing CD28, and not expressing CD4, CD13, CD14, or CD16 |
| Costimulation incompetent (CD28−) | MNCs brightly expressing CD8, not expressing CD28, and not expressing CD4, CD13, CD14, or CD16 |
| CD4− CD8− T cells | CD3+CD4− CD8− MNCs |
| CD8+CD8+ T cells | CD3+ CD4+CD8+ MNCs |
| Natural killer cells | MNCs expressing CD16 or CD56 and not expressing CD3 or CD14 |
| Monocytes | CD14+ MNCs |
| “Stem cells” | CD34+ CD14− cells |
| B-cell progenitors | Cells expressing CD19 or CD20 together with CD10 or CD34 and not brightly expressing CD3, CD13, CD14, CD16, or CD56 |
| Plasma cells | Cells expressing CD38 very brightly and not expressing CD3, CD10, CD13, CD14, CD16, CD34, or CD56 |
| MNC subset . | Definition . |
|---|---|
| B cells | MNCs expressing CD19 or CD20 and not brightly expressing CD3, CD13, CD14, CD16, CD56, CD10, or CD34 |
| Naive (IgD+) | B cells (defined as above) expressing surface IgD |
| Memory (IgD−) | B cells (defined as above) not expressing surface IgD |
| CD4 T cells (total) | CD3+ CD4+ CD8− MNCs |
| Naive (CD45RAhigh) | MNCs expressing CD4, brightly expressing CD45RA, and not expressing CD8, CD13, CD14, or CD16 |
| Memory or effector (CD45RAlow/−) | MNCs expressing CD4, dimly expressing or not expressing CD45RA, and not expressing CD8, CD13, CD14, or CD16 |
| Costimulation competent (CD28+) | MNCs expressing CD4, expressing CD28, and not expressing CD8, CD13, CD14, or CD16 |
| Costimulation incompetent (CD28−) | MNCs expressing CD4, not expressing CD28, and not expressing CD8, CD13, CD14, or CD16 |
| CD8 T cells (total) | CD3+ CD4− CD8+ MNCs |
| Naive (CD11alow) | MNCs brightly expressing CD8, dimly expressing CD11a, and not expressing CD4, CD13, CD14, CD16, or CD19 |
| Memory or effector (CD11ahigh) | MNCs brightly expressing CD8, brightly expressing CD11a, and not expressing CD4, CD13, CD14, CD16, or CD19 |
| Costimulation competent (CD28+) | MNCs brightly expressing CD8, expressing CD28, and not expressing CD4, CD13, CD14, or CD16 |
| Costimulation incompetent (CD28−) | MNCs brightly expressing CD8, not expressing CD28, and not expressing CD4, CD13, CD14, or CD16 |
| CD4− CD8− T cells | CD3+CD4− CD8− MNCs |
| CD8+CD8+ T cells | CD3+ CD4+CD8+ MNCs |
| Natural killer cells | MNCs expressing CD16 or CD56 and not expressing CD3 or CD14 |
| Monocytes | CD14+ MNCs |
| “Stem cells” | CD34+ CD14− cells |
| B-cell progenitors | Cells expressing CD19 or CD20 together with CD10 or CD34 and not brightly expressing CD3, CD13, CD14, CD16, or CD56 |
| Plasma cells | Cells expressing CD38 very brightly and not expressing CD3, CD10, CD13, CD14, CD16, CD34, or CD56 |
Cells with forward-scatter compared with side-scatter characteristics of lymphocytes and monocytes.
MNC indicates mononuclear cell; IgD, immunoglobulin D.
Lymphoproliferation assays
Herpes simplex virus (HSV)–specific, varicella zoster virus (VZV)–specific T-helper-cell function and phytohemagglutinin (PHA)-stimulated T-cell proliferation were assessed as described previously.29 30 Briefly, MNCs separated by a Ficoll device were suspended at a concentration of 2 × 106cells/mL in RPMI with 10% human AB serum, glutamine (4 mM), penicillin (100 U/mL), streptomycin (0.1 mg/mL), and amphotericin B (250 ng/mL). One hundred microliters of the peripheral blood mononuclear cell suspension was dispensed into wells of 96 round-bottomed plates. VZV and HSV antigens were prepared by sonication and heat inactivation of virus grown on foreskin fibroblasts. HSV or VZV antigen or PHA (final concentration, 10 μg/mL) was added to triplicate wells containing MNCs on day zero. The plates were incubated at 37°C in a humidified 5% carbon dioxide atmosphere for 4 days. Eighteen to 24 hours before harvest, tritium thymidine (0.074 MBq [2 μCi]) was added to each well. Cells were harvested by using a semiautomated harvester, and counts per minute (cpm) were determined in a β-scintillation counter. The mean cpm of cells exposed to the antigen minus the mean cpm of cells incubated with medium alone (Δ cpm) was calculated. Because the percentage of CD4 cells, as well as total T cells, among MNCs was higher in the blood stem cell recipients than in the marrow recipients, results were expressed as Δ cpm per 1000 CD4 T cells (HSV and VZV assessment) or per 1000 T cells (PHA assessment).
IgG levels
Serum IgG levels were determined by standard rate nephelometry.
Enumeration of infections
The goal of this study was to determine whether higher lymphocyte counts after blood stem cell grafting would be associated with an infection rate lower than that after marrow grafting. Because blood stem cell grafting is associated with earlier neutrophil engraftment,17 only postengraftment infections were counted. These were defined as infections diagnosed between day 30 and day 365, since both marrow recipients and blood stem cell recipients achieved neutrophil engraftment by day 30 (Table 1) and the minimum follow-up period was 365 days.
Definite infection was defined as an illness with symptoms and signs consistent with an infection and microbiological documentation of a pathogen, except in cases of dermatomal zoster, for which a clinical diagnosis was considered sufficient to classify an infection as definite. Microbiological documentation of an infection consisted of isolation of the pathogen by culture from a sterile or nonsterile site (if from a nonsterile site, the organism had to be clinically judged to be pathogenic) or histologic or immunohistologic evidence. Culture-documented viremia, bacteremia, or fungemia was considered a definite infection even if the patient had no symptoms or signs of infection. Oral candidiasis was not considered a definite infection because it could not be reliably distinguished from oral graft-versus-host disease (GVHD) with candidal colonization.
Clinical infection (no microorganism identified) was defined as illness with symptoms and signs consistent with an infection. Presumed oral, gastrointestinal, conjunctival, and respiratory tract infections were not included, however, because they could not be reliably distinguished from GVHD or allergy. Hemorrhagic cystitis was not included because it could not be differentiated from conditioning-regimen–induced cystitis. Fever of presumed infectious cause was included only if the patient's body temperature was above 38.5°C and the condition responded to antibiotic therapy within 3 days. Sinusitis and pneumonia were included only if documented radiologically.
A chronic infection was counted as one infection. A recurrent infection was counted as multiple infections only if episodes were clearly separated by an asymptomatic period of longer than 4 weeks. A polymicrobial infection of one organ or several adjacent organs was considered one infection (due to the organism judged to be the major pathogen). Infections with one microorganism in 2 nonadjacent organs were counted as 2 infections. The respiratory tract was considered adjacent to the paranasal sinuses and lungs. Lungs and paranasal sinuses were considered nonadjacent. An organ infection with viremia, bacteremia, or fungemia was counted only as the organ infection. Severe infections were defined as infections treated in a hospital. Nonsevere infections were treated in an outpatient setting.
Death associated with a definite infection was defined as (1) autopsy findings consistent with an infection and detection of the pathogen in an autopsy specimen, or (2) death after a definite infection that was judged to have caused the death either directly (eg, pneumonia) or indirectly (eg, sepsis with subsequent adult respiratory distress syndrome).
Statistical analysis
The significance of differences between marrow recipients and blood stem cell recipients in MNC-subset counts, Δ cpm, and serum IgG levels at each time point was tested by the Mann-Whitney rank sum test. The significance of differences in infection rates was determined by the likelihood ratio test. The number of infections was treated as a Poisson random variable. Regression models were fit with the SAS Genmod (SAS Institute, Cary, NC) procedure using the log of the number of days at risk as a fixed predictor (offset). Days at risk were calculated as 365 or the day after transplantation of death or relapse (whatever occurred first) minus 30.
Results
MNC subsets
Compared with marrow recipients, blood stem cell recipients received significantly higher numbers of all subsets of MNCs with the graft, except for B-cell progenitors and plasma cells (Table3). In the first year after grafting, counts of the following MNC subsets were significantly higher in blood stem cell recipients at 3 or more time points (Figure1): total CD4 T cells and their CD45RAhigh (naive), CD45RAlow/−(memory/effector) and CD28+ subpopulations, CD8 T cells and their CD11alow (naive) and CD28+subpopulations, and CD4−CD8− T cells. Subsets not significantly different at any time point after transplantation included CD28−CD8 T cells and monocytes. MNC subsets with significantly greater numbers in marrow recipients than in blood stem cell recipients early (on day 30 or day 80) but not late (on day 180 or day 365) after transplantation included total B cells and their IgD+ (naive) and IgD− (memory) subsets, CD11ahigh (memory/effector) CD8 T cells, and natural killer (NK) cells. Conversely, CD28−CD4 T-cell counts and CD4+CD8+ T cell counts were similar in the 2 groups of recipients early after transplantation but higher in blood stem cell recipients later. MNC-subset counts in marrow donors and those in blood stem cell donors (before use of filgrastim) were similar (P > 0.1 for all the subsets). The data from donors were pooled and are shown in Figure 1 as the normal reference values.
Quantities of cells infused into patients, according to mononuclear cell subset and source of hematopoietic cells (marrow or blood)
| MNC subset . | Marrow . | Blood3-150 . | Fold increase3-151 . |
|---|---|---|---|
| B cells (total) | 4 (1-25) | 71 (6-141) | 18 |
| Naive (IgD+) | 3 (1-22) | 62 (22-129) | 20 |
| Memory (IgD−) | 0.8 (0.1-3.1) | 9.6 (0.9-33.8) | 12 |
| CD4 T cells (total) | 14 (2-29) | 174 (64-414) | 12 |
| Naive (CD45RAhigh) | 6 (0-16) | 60 (19-233) | 10 |
| Memory or effector (CD45RAlow/−) | 8 (1-25) | 115 (1-204) | 14 |
| Costimulation competent (CD28+) | 14 (1-30) | 156 (57-348) | 11 |
| Costimulation incompetent (CD28−) | 0.4 (0.0-12.8) | 4.0 (0.8-50.7) | 10 |
| CD8 T cells (total) | 10 (1-31) | 82 (22-254) | 8 |
| Naive (CD11alow) | 4 (0-13) | 34 (15-114) | 9 |
| Memory or effector (CD11ahigh) | 7 (1-17) | 34 (7-106) | 5 |
| Costimulation competent (CD28+) | 7 (1-20) | 55 (16-142) | 8 |
| Costimulation incompetent (CD28−) | 3 (0-8) | 11 (2-49) | 4 |
| CD4−CD8− T cells | 0.6 (0.1-2.9) | 4.8 (0.9-19.5) | 8 |
| CD8+CD8+ T cells | 1.0 (0.1-7.9) | 6.5 (0.8-101.6) | 7 |
| Natural killer cells | 3 (0-13) | 30 (13-71) | 10 |
| Monocytes3-152 | 26 (0-111) | 547 (150-2142) | 21 |
| “Stem cells” | 2.5 (1.0-8.0) | 7.4 (1.0-17.5) | 3 |
| B-cell progenitors | 4 (0-22) | 0 (0-2) | 0 |
| Plasma cells | 0.5 (0.0-4.7) | 0.0 (0.0-0.7) | 0 |
| MNC subset . | Marrow . | Blood3-150 . | Fold increase3-151 . |
|---|---|---|---|
| B cells (total) | 4 (1-25) | 71 (6-141) | 18 |
| Naive (IgD+) | 3 (1-22) | 62 (22-129) | 20 |
| Memory (IgD−) | 0.8 (0.1-3.1) | 9.6 (0.9-33.8) | 12 |
| CD4 T cells (total) | 14 (2-29) | 174 (64-414) | 12 |
| Naive (CD45RAhigh) | 6 (0-16) | 60 (19-233) | 10 |
| Memory or effector (CD45RAlow/−) | 8 (1-25) | 115 (1-204) | 14 |
| Costimulation competent (CD28+) | 14 (1-30) | 156 (57-348) | 11 |
| Costimulation incompetent (CD28−) | 0.4 (0.0-12.8) | 4.0 (0.8-50.7) | 10 |
| CD8 T cells (total) | 10 (1-31) | 82 (22-254) | 8 |
| Naive (CD11alow) | 4 (0-13) | 34 (15-114) | 9 |
| Memory or effector (CD11ahigh) | 7 (1-17) | 34 (7-106) | 5 |
| Costimulation competent (CD28+) | 7 (1-20) | 55 (16-142) | 8 |
| Costimulation incompetent (CD28−) | 3 (0-8) | 11 (2-49) | 4 |
| CD4−CD8− T cells | 0.6 (0.1-2.9) | 4.8 (0.9-19.5) | 8 |
| CD8+CD8+ T cells | 1.0 (0.1-7.9) | 6.5 (0.8-101.6) | 7 |
| Natural killer cells | 3 (0-13) | 30 (13-71) | 10 |
| Monocytes3-152 | 26 (0-111) | 547 (150-2142) | 21 |
| “Stem cells” | 2.5 (1.0-8.0) | 7.4 (1.0-17.5) | 3 |
| B-cell progenitors | 4 (0-22) | 0 (0-2) | 0 |
| Plasma cells | 0.5 (0.0-4.7) | 0.0 (0.0-0.7) | 0 |
Values are median (range) number of cells × 106/kg of body weight of recipient. The difference between the marrow and the blood stem cell graft was significant for all the cell subsets (P < .001).
For abbreviations, see Table 2.
Eighteen recipients of blood stem cells received 2 MNC apheresis products. The values given represent the sum of the 2 products.
Median for blood stem cell grafts compared with median for marrow grafts.
When monocytes were defined as CD14highinstead of CD14+ MNCs, median values were 2 × 106/kg for marrow cells and 221 × 106/kg for blood stem cells (a 111-fold increase).
Median MNC-subset counts in recipients of marrow and blood stem cells on about day 30, 80, 180, and 365 after transplantation.
Gray circles indicate marrow; black triangles, stem cells. Error bars indicate the 25th to 75th percentiles. Stars indicate a significant difference (P < .05). Normal values are shown as horizontal lines (thick solid line for the 10th and 90th percentiles and broken line for the median). Days after transplantation are shown on all x-axes. On all y-axes, values for cell counts are per microliter. The numbers of marrow recipients and blood stem cell recipients studied were 43 and 45, respectively, on day 30; 40 and 40 on day 80; 29 and 26 on day 180; and 25 and 30 on day 365.
Median MNC-subset counts in recipients of marrow and blood stem cells on about day 30, 80, 180, and 365 after transplantation.
Gray circles indicate marrow; black triangles, stem cells. Error bars indicate the 25th to 75th percentiles. Stars indicate a significant difference (P < .05). Normal values are shown as horizontal lines (thick solid line for the 10th and 90th percentiles and broken line for the median). Days after transplantation are shown on all x-axes. On all y-axes, values for cell counts are per microliter. The numbers of marrow recipients and blood stem cell recipients studied were 43 and 45, respectively, on day 30; 40 and 40 on day 80; 29 and 26 on day 180; and 25 and 30 on day 365.
Lymphoproliferation in vitro
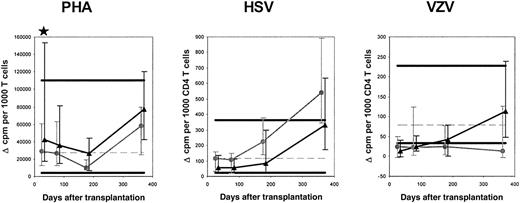
To assess whether the functional capacity of T cells was different in blood stem cell recipients compared with marrow recipients, PHA-stimulated proliferation of MNCs corrected for the percentage of T cells among MNCs was evaluated. The median corrected Δ cpm was 1.5 times higher in blood stem cell recipients on day 30 (P = .04) and not significantly different on day 80, 180, or 365 (Figure 2). This suggests that during most of the first year after transplantation, the functional capacity of single T cells is similar in marrow recipients and blood stem cell recipients.
Results of lymphoproliferation assays using stimulation with PHA, HSV, and VZV.
The symbols are the same as in Figure 1. Only HSV (VZV)–infected patients with HSV (VZV)–infected donors were included in the analysis of HSV (VZV)–induced proliferation. The numbers of marrow recipients and blood stem cell recipients studied with PHA were 41 and 41, respectively, on day 30; 38 and 42 on day 80; 31 and 33 on day 180; and 27 and 30 on day 365. For HSV, the numbers of marrow recipients and blood stem cell recipients studied were 13 and 22, respectively, on day 30; 13 and 20 on day 80; 8 and 16 on day 180; and 6 and 14 on day 365. For VZV, the numbers of marrow recipients and blood stem cell recipients studied were 16 and 19, respectively, on day 30; 15 and 17 on day 80; 11 and 14 on day 180; and 10 and 12 on day 365.
Results of lymphoproliferation assays using stimulation with PHA, HSV, and VZV.
The symbols are the same as in Figure 1. Only HSV (VZV)–infected patients with HSV (VZV)–infected donors were included in the analysis of HSV (VZV)–induced proliferation. The numbers of marrow recipients and blood stem cell recipients studied with PHA were 41 and 41, respectively, on day 30; 38 and 42 on day 80; 31 and 33 on day 180; and 27 and 30 on day 365. For HSV, the numbers of marrow recipients and blood stem cell recipients studied were 13 and 22, respectively, on day 30; 13 and 20 on day 80; 8 and 16 on day 180; and 6 and 14 on day 365. For VZV, the numbers of marrow recipients and blood stem cell recipients studied were 16 and 19, respectively, on day 30; 15 and 17 on day 80; 11 and 14 on day 180; and 10 and 12 on day 365.
T-helper-cell function was assessed by observing proliferation on stimulation with HSV and VZV proteins. We focused on recipients with a latent or active infection whose donors had an infection because uninfected recipients with uninfected donors are not expected to mount a lymphoproliferative response. Infection can be assessed indirectly by detection of antiviral antibodies or, in healthy persons, detection of antiviral T cells, by lymphoproliferation.31 HSV-specific lymphoproliferation was evaluated in HSV-seropositive patients with HSV-lymphoproliferation–positive donors (13 marrow recipients and 21 blood stem cell recipients), and VZV-specific lymphoproliferation was evaluated in VZV-seropositive patients with VZV-lymphoproliferation–positive donors (17 marrow recipients and 19 blood stem cell recipients). For both HSV and VZV, the median corrected Δ cpm in marrow recipients and blood stem cell recipients at all the time points were not significantly different (Figure 2). Groups of HSV (VZV)-seronegative recipients with HSV (VZV)-lymphoproliferation–positive donors and HSV (VZV)-seropositive recipients with HSV (VZV)-lymphoproliferation–negative donors were too small for a formal statistical analysis; however, there appeared to be no differences between marrow recipients and blood stem cell recipients. The results of the HSV- and VZV-induced proliferation assessment suggest that the functional capacity of single CD4 T cells is similar in marrow recipients and blood stem cell recipients.
Interestingly, when uncorrected Δ cpm in marrow recipients were compared with those in blood stem cell recipients, there were no significant differences in PHA-, HSV-, or VZV-stimulated responses at any time point (data not shown) except day 30, when the median Δ cpm PHA-stimulated response was 1.5 times higher in blood stem cell recipients (P = .03).
Serum IgG
Measurements of total serum IgG levels on day 80 after transplantation were available for 44 marrow recipients and 44 blood stem cell recipients who did not have multiple myeloma and did not receive intravenous immunoglobulin between day 0 and day 80. The levels were similar (median, 5.70 g/L in marrow recipients and 5.45 g/L in blood stem cell recipients; P = .99). Determinations of day 365 IgG levels were available for 31 marrow recipients and 39 blood stem cell recipients who did not have multiple myeloma and had not received intravenous immunoglobulin within 2 months before the evaluation on day 365. Levels were also similar at that time (median, 6.32 g/L in marrow recipients and 5.65 g/L in blood stem cell recipients; P = .56). Our normal range (5th-95th percentile) is 6.94 to 15.18 g/L. Thus, the degree of IgG deficiency was similar in the 2 groups of patients.
Infections
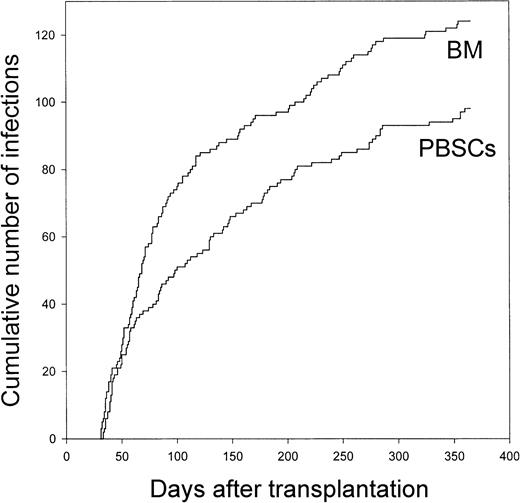
The rate of total infections was 1.4 times higher in marrow recipients than in blood stem cell recipients (P ≤ .01; Table 4). The rate of total definite infections was 1.7 times higher in marrow recipients (P ≤ .001). Importantly, the difference was more striking for severe definite infections (2.4 times higher rate in marrow recipients) than for nonsevere definite infections (1.4 times higher rate in marrow recipients). The difference was more striking for bacterial infections (1.7 times higher rate) and fungal infections (5.5 times higher rate) than for viral infections (1.4 times higher rate). The rate of clinical infections was similar in the 2 groups. Details on the infections are shown in Table 5. There was no difference in the posttransplantation day of diagnosis of the infections (median, day 78 for marrow recipients [25th-75th percentile, day 52-day 168] and day 98 for blood stem cell recipients [25th-75th percentile, day 50-day 182];P = .43; Figure 3).
Infection rates between day 30 and day 365 in patients given marrow compared with those given blood stem cells
| . | Marrow4-150 . | Blood4-150 . | Rate ratio for marrow/blood (95% CI) . | P . | Adjusted rate ratio (95% CI)4-151 . | P . |
|---|---|---|---|---|---|---|
| Total infections (definite and clinical) | 0.88 | 0.62 | 1.42 (1.09-1.85) | .01 | 1.48 (1.13-1.94) | .004 |
| Total definite infections | 0.68 | 0.40 | 1.70 (1.24-2.34) | .001 | 1.79 (1.29-2.47) | .0005 |
| Severe definite infections | 0.30 | 0.13 | 2.36 (1.39-4.02) | .002 | 2.46 (1.44-4.21) | .001 |
| Nonsevere definite infections | 0.38 | 0.27 | 1.39 (0.93-2.08) | .11 | 1.46 (0.97-2.21) | .07 |
| Viral infections (definite) | 0.33 | 0.23 | 1.44 (0.93-2.24) | .10 | 1.59 (1.02-2.48) | .04 |
| Bacterial infections (definite) | 0.28 | 0.16 | 1.76 (1.07-2.90) | .03 | 1.73 (1.04-2.88) | .04 |
| Fungal infections (definite) | 0.071 | 0.013 | 5.50 (1.20-25.1) | .03 | 5.55 (1.20-25.7) | .03 |
| Total clinical infections | 0.21 | 0.23 | 0.91 (0.56-1.49) | .71 | 0.94 (0.57-1.55) | .81 |
| Severe clinical infections | 0.09 | 0.08 | 1.19 (0.54-2.61) | .66 | 1.34 (0.61-2.97) | .47 |
| Nonsevere clinical infections | 0.11 | 0.15 | 0.76 (0.40-1.45) | .41 | 0.74 (0.39-1.41) | .36 |
| . | Marrow4-150 . | Blood4-150 . | Rate ratio for marrow/blood (95% CI) . | P . | Adjusted rate ratio (95% CI)4-151 . | P . |
|---|---|---|---|---|---|---|
| Total infections (definite and clinical) | 0.88 | 0.62 | 1.42 (1.09-1.85) | .01 | 1.48 (1.13-1.94) | .004 |
| Total definite infections | 0.68 | 0.40 | 1.70 (1.24-2.34) | .001 | 1.79 (1.29-2.47) | .0005 |
| Severe definite infections | 0.30 | 0.13 | 2.36 (1.39-4.02) | .002 | 2.46 (1.44-4.21) | .001 |
| Nonsevere definite infections | 0.38 | 0.27 | 1.39 (0.93-2.08) | .11 | 1.46 (0.97-2.21) | .07 |
| Viral infections (definite) | 0.33 | 0.23 | 1.44 (0.93-2.24) | .10 | 1.59 (1.02-2.48) | .04 |
| Bacterial infections (definite) | 0.28 | 0.16 | 1.76 (1.07-2.90) | .03 | 1.73 (1.04-2.88) | .04 |
| Fungal infections (definite) | 0.071 | 0.013 | 5.50 (1.20-25.1) | .03 | 5.55 (1.20-25.7) | .03 |
| Total clinical infections | 0.21 | 0.23 | 0.91 (0.56-1.49) | .71 | 0.94 (0.57-1.55) | .81 |
| Severe clinical infections | 0.09 | 0.08 | 1.19 (0.54-2.61) | .66 | 1.34 (0.61-2.97) | .47 |
| Nonsevere clinical infections | 0.11 | 0.15 | 0.76 (0.40-1.45) | .41 | 0.74 (0.39-1.41) | .36 |
CI indicates confidence interval.
Number of infections/100 patient days, ie, number of infections in all recipients of marrow or blood stem cells divided by the number of days at risk and multiplied by 100. The number of days at risk (day 30 through day 365 or the day of death or relapse) was 14 131 for recipients of marrow and 15 537 for recipients of blood stem cells.
Adjusted for pretransplantation variables known to influence the rate of posttransplantation infections, ie, donor/recipient cytomegalovirus (CMV) serostatus before transplantation (+/+, +/−, −/+, or −/−), disease/disease stage (good risk or poor risk), and splenectomy (yes or no).
Infections occurring between day 30 and day 365, according to source of hematopoietic cells (marrow or blood)
| Type of infection . | Marrow . | Blood . |
|---|---|---|
| Viral (total n) | 46 | 35 |
| Herpes simplex | ||
| Oral/perioral | 7 | 12 |
| Anogenital | 5 | 2 |
| Zoster | 10 | 8 |
| CMV | ||
| Viremia only5-150 | 11 | 5 |
| Pneumonia | 1 | 3 |
| Gastroenteritis | 3 | 0 |
| Respiratory virus (influenza A or B, parainfluenza, or respiratory syncytial virus) | ||
| Upper respiratory tract infection only | 3 | 4 |
| Sinusitis | 2 | 0 |
| Pneumonia | 1 | 1 |
| BK virus cystitis and/or prostatitis | 3 | 1 |
| Bacterial (total n) | 40 | 25 |
| Gram-positive | ||
| Bacteremia only, with or without catheter-site infection | 17 | 10 |
| Sinusitis | 1 | 4 |
| Bronchitis | 1 | 2 |
| Pneumonia | 0 | 1 |
| Esophagitis | 1 | 0 |
| Urinary system infection | 1 | 0 |
| Gram-negative | ||
| Bacteremia only, with or without catheter site infection | 9 | 4 |
| Bronchitis | 2 | 0 |
| Pneumonia | 3 | 0 |
| Urinary system infection | 3 | 3 |
| Clostridium difficile colitis | 1 | 1 |
| Legionellapneumonia | 1 | 0 |
| Fungal (total n) | 10 | 2 |
| Aspergillus pneumonia | 6 | 2 |
| Candidemia only | 3 | 0 |
| Candida esophagitis | 1 | 0 |
| Clinical (total n) | 29 | 34 |
| Brain abscess | 1 | 0 |
| Otitis media | 0 | 1 |
| Parotitis | 1 | 0 |
| Lip infection | 0 | 3 |
| Sinusitis | 4 | 5 |
| Pneumonia | 9 | 85-151 |
| Diverticulitis | 1 | 0 |
| Colitis with pericolic phlegmon/abscess | 0 | 1 |
| Urinary tract infection or prostatitis | 1 | 1 |
| Vaginitis | 1 | 2 |
| Venous catheter site infection without bacteremia/fungemia | 2 | 1 |
| Superficial phlebitis | 0 | 1 |
| Cellulitis | 3 | 1 |
| Warts | 2 | 2 |
| Other skin infections (paronychia, tinea, folliculitis) | 1 | 6 |
| Fever of presumed infectious cause | 3 | 3 |
| Type of infection . | Marrow . | Blood . |
|---|---|---|
| Viral (total n) | 46 | 35 |
| Herpes simplex | ||
| Oral/perioral | 7 | 12 |
| Anogenital | 5 | 2 |
| Zoster | 10 | 8 |
| CMV | ||
| Viremia only5-150 | 11 | 5 |
| Pneumonia | 1 | 3 |
| Gastroenteritis | 3 | 0 |
| Respiratory virus (influenza A or B, parainfluenza, or respiratory syncytial virus) | ||
| Upper respiratory tract infection only | 3 | 4 |
| Sinusitis | 2 | 0 |
| Pneumonia | 1 | 1 |
| BK virus cystitis and/or prostatitis | 3 | 1 |
| Bacterial (total n) | 40 | 25 |
| Gram-positive | ||
| Bacteremia only, with or without catheter-site infection | 17 | 10 |
| Sinusitis | 1 | 4 |
| Bronchitis | 1 | 2 |
| Pneumonia | 0 | 1 |
| Esophagitis | 1 | 0 |
| Urinary system infection | 1 | 0 |
| Gram-negative | ||
| Bacteremia only, with or without catheter site infection | 9 | 4 |
| Bronchitis | 2 | 0 |
| Pneumonia | 3 | 0 |
| Urinary system infection | 3 | 3 |
| Clostridium difficile colitis | 1 | 1 |
| Legionellapneumonia | 1 | 0 |
| Fungal (total n) | 10 | 2 |
| Aspergillus pneumonia | 6 | 2 |
| Candidemia only | 3 | 0 |
| Candida esophagitis | 1 | 0 |
| Clinical (total n) | 29 | 34 |
| Brain abscess | 1 | 0 |
| Otitis media | 0 | 1 |
| Parotitis | 1 | 0 |
| Lip infection | 0 | 3 |
| Sinusitis | 4 | 5 |
| Pneumonia | 9 | 85-151 |
| Diverticulitis | 1 | 0 |
| Colitis with pericolic phlegmon/abscess | 0 | 1 |
| Urinary tract infection or prostatitis | 1 | 1 |
| Vaginitis | 1 | 2 |
| Venous catheter site infection without bacteremia/fungemia | 2 | 1 |
| Superficial phlebitis | 0 | 1 |
| Cellulitis | 3 | 1 |
| Warts | 2 | 2 |
| Other skin infections (paronychia, tinea, folliculitis) | 1 | 6 |
| Fever of presumed infectious cause | 3 | 3 |
CMV indicates cytomegalovirus.
On culture (not by pp65-antigen detection only).
In one case, pneumonia was reported to be due toTrichomonas, but no written confirmation or clinical details were obtained.
Cumulative number of day 30 to day 365 infections after allogeneic transplantation of bone marrow or peripheral blood stem cells.
Both definite and clinical infections are included. BM, bone marrow; PBSCs, peripheral blood stem cells.
Cumulative number of day 30 to day 365 infections after allogeneic transplantation of bone marrow or peripheral blood stem cells.
Both definite and clinical infections are included. BM, bone marrow; PBSCs, peripheral blood stem cells.
Although all patients had a sustained absolute neutrophil count of 0.5 × 109/L by day 28 after transplantation (Table 1), absolute neutrophil counts were significantly higher in blood stem cell recipients compared with marrow recipients until day 48. To eliminate any influence of the different neutrophil counts, we compared infection rates between day 60 and day 365. The rate of total infections was significantly higher in marrow recipients (unadjusted rate ratio, 1.48 [P = .02], and adjusted rate ratio, 1.53 [P = .01]). The rate of total definite infections was also significantly higher in marrow recipients (unadjusted rate ratio, 1.90 [P = .002], and adjusted rate ratio, 1.99 [P = .001]). Therefore, the differences in infection rates between day 30 and day 365 in the 2 groups of patients were most likely due to the differences in lymphocyte-subset counts and not neutrophil counts.
Death associated with a definite infection diagnosed between day 30 and day 365 occurred between day 30 and day 365 in 9 marrow recipients and 3 blood stem cell recipients (P = .17 by Χ2test). Details on these infections are provided in Table6. There were 9 deaths associated with a bacterial or fungal infection among marrow recipients and none among blood stem cell recipients (P = .008 by Χ2test).
Deaths (occurring between day 30 and day 365 after transplantation) associated with a definite infection diagnosed between day 30 and day 365
| Tx type/infection . | Other conditions contributing to death . | Post-Tx day of diagnosis of infection/death . |
|---|---|---|
| Marrow | ||
| Haemophilus influenzaepneumonia | None | 156/210 |
| Enterobacter cloacae pneumonia | Chronic GVHD | 145/153 |
| Sepsis due to gram-negative rod (unspeciated) leading to ARDS | None | 221/234 |
| Legionella micdadei/CMV pneumonia | Chronic GVHD | 65/108 |
| Staphylococcus epidermidis sepsis leading to ARDS | Chronic GVHD | 224/241 |
| Staphylococcus epidermidis sepsis leading to ARDS | None | 31/37 |
| Aspergillus fumigatus pneumonia | Acute GVHD, VOD | 38/38 |
| Aspergillus flavus pneumonia | Chronic GVHD | 68/121 |
| Aspergillus fumigatus/CMV pneumonia | Chronic GVHD | 253/272 |
| Blood stem cells | ||
| RSV pneumonia | Acute GVHD, VOD | 33/34 |
| CMV pneumonia | Acute GVHD, MOF | 34/34 |
| CMV pneumonia | Chronic GVHD | 130/158 |
| Tx type/infection . | Other conditions contributing to death . | Post-Tx day of diagnosis of infection/death . |
|---|---|---|
| Marrow | ||
| Haemophilus influenzaepneumonia | None | 156/210 |
| Enterobacter cloacae pneumonia | Chronic GVHD | 145/153 |
| Sepsis due to gram-negative rod (unspeciated) leading to ARDS | None | 221/234 |
| Legionella micdadei/CMV pneumonia | Chronic GVHD | 65/108 |
| Staphylococcus epidermidis sepsis leading to ARDS | Chronic GVHD | 224/241 |
| Staphylococcus epidermidis sepsis leading to ARDS | None | 31/37 |
| Aspergillus fumigatus pneumonia | Acute GVHD, VOD | 38/38 |
| Aspergillus flavus pneumonia | Chronic GVHD | 68/121 |
| Aspergillus fumigatus/CMV pneumonia | Chronic GVHD | 253/272 |
| Blood stem cells | ||
| RSV pneumonia | Acute GVHD, VOD | 33/34 |
| CMV pneumonia | Acute GVHD, MOF | 34/34 |
| CMV pneumonia | Chronic GVHD | 130/158 |
ARDS indicates adult respiratory distress syndrome; VOD, venocclusive disease of the liver; RSV, respiratory syncytial virus; MOF, multiorgan failure; for other abbreviations, see Table 1.
Discussion
The important findings of this study were that (1) compared with marrow recipients, blood stem cell recipients had higher counts of T cells (particularly CD4 T cells); (2) T cells were equally functional in the 2 patient groups; and (3) improving the quantitative lymphocyte deficiency in recipients of hematopoietic transplants may result in fewer infections.
The higher counts of certain lymphocyte subsets in blood stem cell recipients were anticipated because of the results of smaller nonrandomized studies and have been attributed to the larger lymphocyte inoculum.12,13 It is reasonable to attribute the higher posttransplantation counts of T cells, particularly naive T cells, to the large inoculum because T cells, especially naive T cells, are extremely long-lived.14-16 Also, few T cells are produced from stem cells in adult transplant recipients in the first year after transplantation because of age- and allografting-associated thymic atrophy; therefore, most T cells in patients in this year originate from the T cells infused with the graft.8 32-35 This idea is in agreement with the observation that naive T-cell counts but not CD34 cell counts in the grafts correlate with naive T-cell counts after transplantation (J. S. et al, unpublished data, March 2001). We found that even though blood stem cell grafts contained more monocytes, NK cells, and B cells than marrow grafts, reconstitution of these cell populations was similar in marrow recipients and blood stem cell recipients. The numbers of monocytes and NK cells had already reached the normal range by day 30, suggesting that reconstitution of these populations occurs rapidly and is not limited by the number of cells in the graft. B-cell counts were higher during the first 3 months after blood stem cell transplantation compared with marrow transplantation. This finding is consistent with the presence of much higher numbers of naive and memory B cells in blood stem cell grafts than in marrow grafts. However, the rate of B-cell recovery was faster in marrow recipients, as indicated by the steeper slope during the first 3 months after grafting (Figure 1). This might have been due to the higher number of B-cell progenitors in the marrow grafts (Table 3).
Total IgG levels were similar in the 2 patient groups, suggesting that humoral immunity is not improved after blood stem cell grafting compared with marrow grafting. After marrow grafting, IgG is frequently composed of monoclonal or oligoclonal immunoglobulins of unclear specificity (not against infectious agents)36-50 and therefore the protective value of these immunoglobulins in marrow recipients is low. Qualitative comparisons of immunoglobulins will be needed to assess the contribution of humoral immunity to preventing infection after blood stem cell transplantation. The presence of higher numbers of plasma cells in the marrow inoculum (Table 3) raises the question of whether the immunoglobulins produced by the infused plasma cells markedly contribute to the total IgG levels. If they do, this could be why IgG levels are not higher in blood stem cell recipients than in marrow recipients, even though B-cell counts are higher.
The difference in the rates of total definite infections cannot be attributed to differences in clinical patient characteristics because those were balanced between the 2 patient groups (Table 1). Adjustment for minor imbalances in pretransplantation characteristics (splenectomy, disease/disease stage, and CMV serostatus) only strengthened the significance of the differences (Table 4). We did not adjust for minor imbalances in posttransplantation characteristics because those may have been associated with the main treatment (blood stem cell grafting or marrow grafting). Nevertheless, the higher infection rates in the marrow recipients cannot be attributed to a weaker graft, since the incidence of severe neutropenia between day 30 and day 365 was not significantly higher in those patients (Table 1). Also, day 60 to day 365 infection rates, which were not influenced by the higher neutrophil counts in blood stem cell recipients up to day 48, were significantly higher in marrow recipients. Use of glucocorticoids is unlikely to have been responsible for the observed differences in infection rates because the number of patients treated with glucocorticoids was similar in the 2 groups. The differences also cannot be attributed to GVHD, since the incidence of grade 2 to 4 acute GVHD was similar in the 2 patient groups and there was a trend toward a lower incidence of clinical extensive chronic GVHD among the marrow recipients. Infection prophylaxis strategies were also similar in the 2 groups. The only difference was a trend toward a greater use of prophylactic acyclovir in marrow recipients (Table 1), a finding that may explain why the incidence of HSV and VZV infections among blood stem cell recipients was not lower than that among marrow recipients.
The difference in total definite infection rates was attributable primarily to differences in the rates of bacterial and fungal infections. Also, blood stem cell recipients had fewer fatal bacterial and fungal infections. This may seem puzzling because the major difference between the marrow recipients and the blood stem cell recipients appeared to be in their T-cell counts and T cells have traditionally been considered to play a much less important role than neutrophils in host defense against extracellular bacteria and fungi (particularly molds). Perhaps the importance of T cells in host defense against extracellular bacteria and fungi (particularly molds) has been underestimated. Adoptively transferred T cells from immune donors to naive hosts have been found to protect rodents against infection with Bacteroides fragilis, Pseudomonas aeruginosa, Candida albicans, or Aspergillus fumigatus.51-54
The concurrence of higher T-cell counts and, in part, B-cell and NK cell counts and the lower infection rates in the blood stem cell recipients suggests but does not prove that the higher lymphocyte-subset counts resulted in lower infection rates. However, this is likely because previous studies showed inverse correlations between CD4 T-cell or B-cell counts and postengraftment infection rates.9-11 Moreover, in the marrow recipients in our study, there was a trend toward inverse correlations between day 30, 80, 180, and 365 total CD4 T-cell counts and day 30 to day 365 infection rates; between day 30, 80, 180, and 365 total B-cell counts and day 30 to day 365 infection rates; and between day 30, 80, and 180 NK cell counts and day 30 to day 365 infection rates (by Spearman rank correlation; data not shown). The most striking correlations in marrow recipients were those between day 80 total B-cell counts and total definite infections (r = −0.43; P = .006), day 80 IgD+ B-cell counts and total definite infections (r = −0.50; P = .001), and day 30 IgD−B-cell counts and total definite infections (r = −0.42;P = .005). Also, there was a significant correlation in blood stem cell recipients between day 30 NK cell counts and day 30 to day 365 total infection rates (r = −0.38; P = .01). These analyses support the idea that the lower infection rates in blood stem cell recipients compared with marrow recipients probably resulted from the increased counts of T cells, B cells, and possibly NK cells.
It would not be prudent to interpret the finding of lower day 30 to day 365 infection rates in blood stem cell recipients as a recommendation for discontinuing marrow transplantation. Other end points, such as relapse and survival, must also be taken into consideration. Moreover, if chronic GVHD is more prevalent or more severe (requiring more immunosuppressive treatment) after blood stem cell grafting than after marrow grafting,55-57 it is possible that infection rates after day 365 in blood stem cell recipients will be equal to or even higher than those in marrow recipients.
In summary, we found that blood stem cell recipients have fewer day 30 to day 365 infections than marrow recipients. This might be because of improved lymphocyte-subset counts after blood stem cell grafting, an improvement that may result from the high numbers of lymphocytes in blood stem cell grafts.
We thank Patrick Sudour, Kristen White, Erica Ryberg, Amber Wyman, Hana Gage, and the dedicated staff of the FHCRC Cryobiology Laboratory for excellent technical assistance; the study nurse, Terri Cunningham; the patients who agreed to participate in the study; and the staff of the FHCRC Long- Term Follow-Up Department, who diligently gathered clinical information.
Supported by National Institutes of Health grants CA68496, CA18221, CA18029, CA15704, HL36444, and AI46108.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
References
Author notes
Jan Storek, FHCRC, D1-100, 1100 Fairview Ave N, Seattle, WA 98109-1024; e-mail: jstorek@fhcrc.org.


This feature is available to Subscribers Only
Sign In or Create an Account Close Modal