Abstract
An analysis was performed of the response to treatment with donor lymphocyte infusions (DLI) and the survival in 66 consecutive patients who relapsed after primary treatment by allogeneic stem cell transplantation for BCR-ABL–positive chronic myeloid leukemia. The transplant donor was an HLA-identical sibling (n = 35) or a “matched” unrelated volunteer (n = 31). Fifty-seven patients were transplanted in chronic phase, eight in accelerated phase, and one in second chronic phase. The recognition of relapse was based on precise molecular, cytogenetic, or hematologic criteria. The median interval from transplant to relapse was 12 months (range 3-85). The median interval from relapse to initiation of DLI was 9.4 months (range 1-70). Patients received DLI from their original transplant donors on a bulk-dose (n = 34) or on an escalating-dose (n = 32) regimen. Patients were monitored serially by hematologic, cytogenetic, and molecular criteria. Molecular remission was defined by the finding of negative results by nested primer reverse transcriptase polymerase chain reaction (RT-PCR) for BCR-ABL transcripts on two consecutive occasions, subject to satisfactory controls. Forty-four patients (67%) achieved molecular remission. Patients who had relapsed to advanced phase disease and patients with short intervals between transplant and relapse had significantly lower probabilities of achieving molecular remission. Of the 44 patients who achieved molecular remission, 4 reverted to a PCR-positive status at 15, 18, 37, and 87 weeks after remission. The probability of survival for patients who achieved molecular remission was significantly better than for those who failed to do so (95% versus 53% at 3 years post-DLI,P = .0001). We conclude that the majority of molecular remissions after DLI are durable, and thus the majority of responding patients may prove to have been cured.
Introduction
Allogeneic hematopoietic stem cell transplantation (SCT) is currently the only procedure capable of curing patients with chronic myeloid leukemia (CML)1,2. One of the major causes of treatment failure after allogeneic SCT is relapse. The actuarial probability of relapse is very low for patients allografted in chronic phase with unmanipulated marrow cells and the use of cyclosporine alone as graft-versus-host disease (GVHD) prophylaxis. If, however, the prophylaxis for GVHD is more intensive, as for example when donor marrow cells are T-cell depleted3 or when methotrexate is added to cyclosporine,4 the incidence of relapse is much higher. The incidence of relapse is also higher when the transplant is performed in patients with the advanced phase of CML.5
A number of approaches can be considered to treat patients with CML in relapse after allografting. Although a second allograft produces sustained molecular remission in a proportion of patients, it is associated with a substantial mortality.6 Administration of interferon-α may produce good hematologic control, but the molecular response is rarely sustained.7 Kolb and colleagues8 first exploited the graft-versus-leukemia effect of lymphocytes from the original stem cell donor (donor lymphocyte infusions, DLI) to treat CML in relapse after allogeneic SCT. They used DLI in conjunction with interferon-α but inclusion of interferon-α may not be required.9 Numerous studies have subsequently confirmed that DLI can restore remission in a high percentage of patients with CML in relapse after allografting.10 Thus, this approach has become the treatment of choice in several transplant centers. However, treatment efficacy does not depend exclusively on the response rate; an important additional question relates to the durability of DLI-induced remissions. Because the use of DLI was introduced only relatively recently, the follow-up for DLI responders in published papers has been short; moreover, the definition of remission has not been consistent in the various studies.11-14
We have shown that quantitative reverse transcriptase polymerase chain reaction (RT-PCR) for BCR-ABL transcripts is useful for monitoring patients after allogeneic SCT. A persistently negative RT-PCR finding after transplant predicts a durable remission, whereas rising levels of BCR-ABL transcripts usually precede cytogenetic relapse.15 Although the clinical value of RT-PCR data after SCT for CML has been questioned by some,16 we have in practice used these data to define relapse and to indicate the need for further treatment on a routine basis. Thus, we decided to apply the same approach to monitor the DLI treatment. We report here results of treating by DLI 66 consecutive patients who relapsed after allogeneic SCT. We assessed the response primarily on the basis of molecular studies for BCR-ABL transcripts and correlated results with incidence and durability of molecular remission.
Patients and methods
Patients and transplant procedure
Sixty-six consecutive patients with BCR-ABL–positive CML who relapsed after allogeneic SCT and were then started on treatment with DLI between January 1, 1990 and May 31, 1998 were included in this study. Informed consent was obtained before patients were enrolled in the study. The original transplant was performed between June 30, 1983 and July 31, 1997. At that time, 57 patients were in chronic phase, 8 were in accelerated phase, and 1 was in second chronic phase of CML. Three patients received DLI for relapse after a second transplant procedure. Thirty-five patients had received bone marrow cells from their respective HLA-identical siblings. Thirty-one patients had received bone marrow cells from volunteer unrelated donors (VUDs). In the latter cases the donor was matched serologically for HLA-A, -B, and -DR antigens; DRB1 identity between donor and recipient was confirmed by molecular methods in 18 cases. Transplant conditioning and GVHD prophylaxis were performed according to our standard procedures as previously described.17 18 Of the 66 patients, 24 had received unmanipulated marrow cells from their respective donor, and 42 had received donor cells that had been treated in vitro with a murine monoclonal antibody of the Campath series (CD52) or had received a Campath monoclonal antibody intravenously.
Post-transplant monitoring
Patients considered to be in remission after allogeneic SCT were monitored at intervals not exceeding 3 months and usually more frequently. When relapse was diagnosed (see below), the frequency with which patients were monitored was increased.
At each clinic visit, full blood counts were performed. For patients transplanted before 1991, samples of bone marrow were aspirated regularly for cytogenetic examination. Cytogenetic studies of bone marrow metaphases were carried out on Giemsa-stained metaphases, using standard techniques. A minimum of 30 marrow metaphases were analyzed whenever possible. Since 1991, peripheral blood samples were studied at intervals of 1 to 3 months after transplant for the presence of BCR-ABL transcripts by a multiplex and/or a two-step RT-PCR. Results were expressed as the ratio between BCR-ABL and ABL transcript numbers (BCR-ABL/ABL ratio).19 20 Patients were judged to be in continuing complete remission post-transplant if RT-PCR studies were negative or were positive at low level and failed to satisfy the criteria for molecular relapse defined below. If BCR-ABL/ABL ratio were raised, cytogenetic studies using standard techniques were performed on bone marrow metaphases. The frequency of cytogenetic and RT-PCR monitoring after SCT was similar for recipients of sibling donor and VUD transplants.
Definitions of relapse
In general, relapse of CML after allogeneic SCT can be recognized first as a result of a progressive rise in the numbers of BCR-ABL transcripts in the blood; subsequently Ph-positive metaphases can be identified in the marrow and eventually patients show features of hematologic relapse. Occasionally molecular or cytogenetic relapses are reversible but such transient relapses are rare.15 The 66 patients in this series satisfied criteria for molecular, cytogenetic, or hematologic relapse at the time when treatment with DLI was initiated (Table 1). A patient was considered to be in molecular relapse if his or her BCR-ABL/ABL ratio remained at or exceeded a level thought to reflect a burden of CML approximately 50-fold less than that seen in early cytogenetic relapse.15,20 In practice, we used the following criteria: a patient was considered to be in molecular relapse if over a minimum of 4 weeks the BCR-ABL/ABL ratio exceeded 0.02% in 3 samples, or exceeded 0.05% in 2 samples, or showed rising levels with the last 2 samples higher than 0.02%. These criteria were adopted to avoid classifying as relapse the occasional patient who had fluctuating low levels of BCR-ABL transcripts. Cytogenetic relapse was defined if one or more Ph-positive metaphase was detected without evidence of hematologic relapse. Hematologic relapse was diagnosed in the presence of peripheral blood leukocytosis, usually with predominance of myelocytes and neutrophils in the differential count, accompanied by a hypercellular bone marrow with Ph chromosome positivity on cytogenetic analysis. In a number of patients transplanted before 1991, serial molecular data were not available, and, therefore, relapse was recognized first by cytogenetic or hematologic criteria. The phase of CML was classified in accordance with criteria proposed by the International Bone Marrow Transplant Registry.21 We used the term advanced phase to include both accelerated and blastic phases. These criteria were used to define the stage of disease both at the time of transplantation and at hematologic relapse.
Probabilities of molecular remission at 2 years post-initial DLI for 66 patients treated for relapse after allogeneic SCT for CML
| Variable . | No. of patients . | 2-yr probability of molecular remission % (95% CI) . | P value . |
|---|---|---|---|
| Type of relapse at time of DLI | |||
| Molecular | 8 | 100 | |
| Cytogenetic | 19 | 84 (62-94) | .0003 |
| Hematologic, CP | 30 | 55 (38-78) | |
| Hematologic, AP | 9 | 29 (8-64) | |
| Interval SCT to relapse | |||
| <9 months | 25 | 56 (33-76) | .08 |
| ≥9 months | 41 | 76 (61-89) | |
| Interval relapse to DLI | |||
| <9 months | 28 | 73 (53-87) | .31 |
| ≥9 months | 38 | 66 (49-79) | |
| GVHD prophylaxis | |||
| Non-TCD | 22 | 55 (34-74) | .19 |
| TCD | 44 | 76 (58-83) | |
| Patient sex | |||
| Male | 31 | 72 (52-86) | .87 |
| Female | 35 | 66 (47-80) | |
| Donor sex | |||
| Male | 46 | 72 (57-84) | .19 |
| Female | 19 | 58 (32-80) | |
| Donor match | |||
| Identical sibling | 35 | 65 (47-80) | .20 |
| Matched unrelated | 31 | 75 (55-88) | |
| AGVHD grade post SCT | |||
| 0-1 | 41 | 67 (50-80) | .53 |
| 2-4 | 25 | 63 (43-80) | |
| CGVHD post-SCT | |||
| Nil | 33 | 70 (51-84) | .66 |
| Limited/extensive | 31 | 67 (48-82) |
| Variable . | No. of patients . | 2-yr probability of molecular remission % (95% CI) . | P value . |
|---|---|---|---|
| Type of relapse at time of DLI | |||
| Molecular | 8 | 100 | |
| Cytogenetic | 19 | 84 (62-94) | .0003 |
| Hematologic, CP | 30 | 55 (38-78) | |
| Hematologic, AP | 9 | 29 (8-64) | |
| Interval SCT to relapse | |||
| <9 months | 25 | 56 (33-76) | .08 |
| ≥9 months | 41 | 76 (61-89) | |
| Interval relapse to DLI | |||
| <9 months | 28 | 73 (53-87) | .31 |
| ≥9 months | 38 | 66 (49-79) | |
| GVHD prophylaxis | |||
| Non-TCD | 22 | 55 (34-74) | .19 |
| TCD | 44 | 76 (58-83) | |
| Patient sex | |||
| Male | 31 | 72 (52-86) | .87 |
| Female | 35 | 66 (47-80) | |
| Donor sex | |||
| Male | 46 | 72 (57-84) | .19 |
| Female | 19 | 58 (32-80) | |
| Donor match | |||
| Identical sibling | 35 | 65 (47-80) | .20 |
| Matched unrelated | 31 | 75 (55-88) | |
| AGVHD grade post SCT | |||
| 0-1 | 41 | 67 (50-80) | .53 |
| 2-4 | 25 | 63 (43-80) | |
| CGVHD post-SCT | |||
| Nil | 33 | 70 (51-84) | .66 |
| Limited/extensive | 31 | 67 (48-82) |
DLI, donor lymphocyte infusion; SCT, stem cell transplantation; CML, chronic myeloid leukemia; CI, confidence interval; CP, chronic phase; AP, advanced phase; GVHD, graft-versus-host disease; TCD, in vitro or in vivo T-cell depletion with Campath (CD52) monoclonal antibodies; AGVHD, acute graft-versus-host disease; CGVHD, chronic graft-versus-host disease.
Management of relapse with DLI
Sixty-six patients relapsed after allogeneic SCT at a median time of 12 months (range 3-85). The diagnosis of relapse was based on the molecular (n = 33), cytogenetic (n = 28), or hematologic (n = 5) criteria defined above. At diagnosis of relapse, the only patient who was still receiving immunosuppression had the drugs discontinued. In general, preparations were made for administration of DLI as soon as this could conveniently be scheduled. The median time between relapse and DLI was 10 months (range 1-70). In some cases the stage of relapse had progressed during this interval. Thus, at the time of DLI, 8 patients were in molecular relapse, 19 were in cytogenetic relapse, and in 39 the disease was detectable at the hematologic level (30 in chronic phase and 9 in advanced phase). Thirty-four of the patients received a single infusion of donor lymphocytes from their original transplant donor (bulk-dose regimen). The median dose of lymphocytes transfused was 1.5 × 108/kg (range 0.4-5.3). Thirty-two patients received lymphocytes from their original donor, administered according to an escalating-dose schedule as previously described (escalating-dose regimen).22 Briefly, for sibling transplant recipients the target doses of CD3+ cells/kg were sequentially 107, 5 × 107, and 108, whereas for VUD recipients the target doses were 106, 107, 5 × 107, and 108. Each patient was assessed with RT-PCR and/or cytogenetic studies 12 weeks after the preceding dose, and a further dose of lymphocytes was planned if there was no clear evidence of response; in practice the median interval between doses was 20 weeks (range 12-33 weeks). All patients included in this report completed the full protocol, that is, they were treated until they responded or until they were deemed refractory to DLI. The management of patients pre- and post-BDR DLI has been described elsewhere.22 23
Definition of molecular remission post-DLI
RT-PCR was performed at 4-week intervals or more frequently after initiation of DLI. Molecular remission was defined as the absence of detectable BCR-ABL transcripts by RT-PCR analysis of peripheral blood on 2 consecutive occasions. To control for sample quality in BCR-ABL–negative specimens, complementary DNA (cDNA) was considered evaluable only if it contained at least 2 × 104 normal ABL transcripts/5 μL cDNA. This ensured that the absence of BCR-ABL transcripts could be excluded at a sensitivity of at least 5 × 10−5.24
Statistical methods
The probabilities of survival, achieving molecular remission, and relapse post-DLI were calculated, using the methods of Kaplan and Meier25 and cumulative incidence.26 An initial analysis comparing prognostic groups for molecular remission was carried out using the log-rank test. Those variables significant at theP < .2 level in univariate analyses were further examined by using proportional hazards regression analysis employing a backward-stepping procedure to identify the most statistically significant model. All P values are 2-sided. Confidence intervals refer to 95% boundaries.
Results
Molecular response and durability of remission after DLI
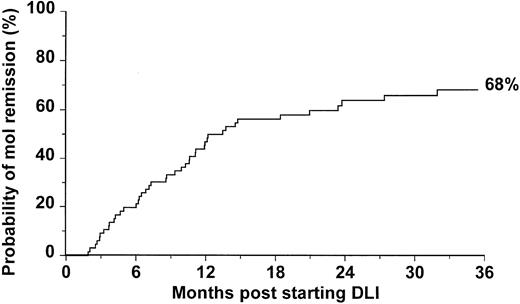
Forty-four patients had a negative result RT-PCR on at least 2 occasions after administration of DLI and thus satisfied our criteria for molecular remission; the probability of achieving molecular remission at 3 years post-DLI was 68% (Figure1). The median follow-up after molecular remission was 29 months (range 5-89). Ten patients had one BCR/ABL negative result, followed by BCR/ABL positivity, and later satisfied our criteria for molecular remission. The intervals between this initial negative result and subsequent molecular remission were 21, 34, 46, 62, 70, 92, 143, 154, 441, and 1069 days, respectively.
Probability of achieving molecular remission after treatment with DLI for 66 patients with CML in relapse after allogeneic SCT.
Probability of achieving molecular remission after treatment with DLI for 66 patients with CML in relapse after allogeneic SCT.
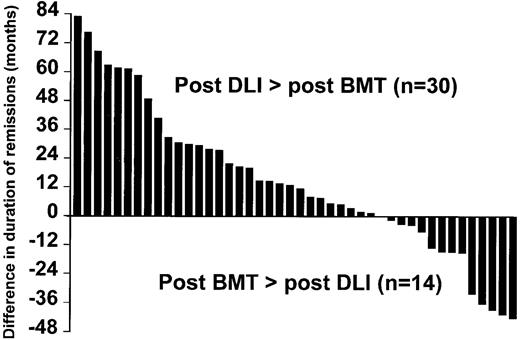
In 30 patients (68%), the duration of molecular remission post-DLI exceeded the duration of molecular remission post-SCT (Figure 2).
Comparison between the duration of remission after DLI and after the original transplant.
Comparison between the duration of remission after DLI and after the original transplant.
Survival post-DLI
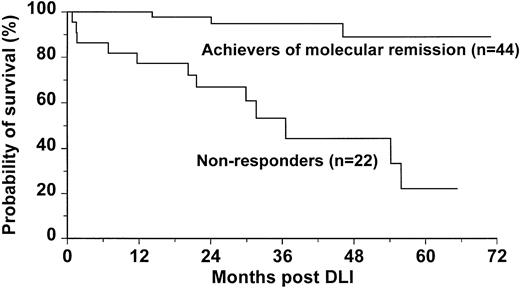
Patients who achieved molecular remission post-DLI had significantly improved survival when compared with those who failed to achieve molecular remission (95% versus 53% at 3 years post-DLI,P = .0001; Figure 3). Of the 3 patients in molecular remission who died, one had relapsed in blastic phase, one died of infectious complications during chronic GVHD, and one died of unknown causes. Three patients treated with DLI in accelerated phase died early after treatment (days 24, 46, and 49).
Probability of survival for 44 patients who achieved molecular remission compared with survival for 22 patients who failed to achieve molecular remission dated from initiation of DLI.
Probability of survival for 44 patients who achieved molecular remission compared with survival for 22 patients who failed to achieve molecular remission dated from initiation of DLI.
Prognostic factors for molecular remission post-DLI
Previous studies have shown that disease stage at time of DLI, donor match, and choice of GVHD prophylaxis are important in determining the efficacy of DLI in inducing molecular remission. Univariate analyses performed in this study confirmed the importance of the above factors (Table 1). However, on further examination with multivariate methods, the only adverse effects were advanced phase disease at the time of starting DLI and a relatively short interval between SCT and relapse (Table 2). Our data suggest that disease recurrence early after SCT (< 9 months) was less likely to respond to DLI. In patients relapsing less than 9 months post-SCT, failure to respond to DLI correlated with disease stage at relapse (Table 3).
Results of a multivariate analysis to find prognostic factors for molecular remission post-DLI in 66 patients who relapsed post-allogeneic SCT for CML
| Variable . | No. of patients . | RR . | 95% CI . | P value . |
|---|---|---|---|---|
| Type of relapse at time of DLI | ||||
| Molecular | 8 | 1.00 | ||
| Cytogenetic | 19 | 0.55 | 0.2-1.4 | .21 |
| Hematologic, CP | 30 | 0.24 | 0.1-0.6 | .002 |
| Hematologic, AP | 9 | 0.07 | 0.02-0.44 | .001 |
| Interval SCT to relapse | ||||
| <9 months | 25 | 1.00 | ||
| ≥9 months | 41 | 2.82 | 1.35-5.92 | .006 |
| Donor match | ||||
| HLA-identical sibling | 35 | 1.00 | ||
| Matched unrelated | 31 | 1.91 | 0.78-4.70 | .16 |
| GVHD prophylaxis | ||||
| Non-TCD | 22 | 1.00 | ||
| TCD | 44 | 1.06 | 0.44-2.56 | .89 |
| Variable . | No. of patients . | RR . | 95% CI . | P value . |
|---|---|---|---|---|
| Type of relapse at time of DLI | ||||
| Molecular | 8 | 1.00 | ||
| Cytogenetic | 19 | 0.55 | 0.2-1.4 | .21 |
| Hematologic, CP | 30 | 0.24 | 0.1-0.6 | .002 |
| Hematologic, AP | 9 | 0.07 | 0.02-0.44 | .001 |
| Interval SCT to relapse | ||||
| <9 months | 25 | 1.00 | ||
| ≥9 months | 41 | 2.82 | 1.35-5.92 | .006 |
| Donor match | ||||
| HLA-identical sibling | 35 | 1.00 | ||
| Matched unrelated | 31 | 1.91 | 0.78-4.70 | .16 |
| GVHD prophylaxis | ||||
| Non-TCD | 22 | 1.00 | ||
| TCD | 44 | 1.06 | 0.44-2.56 | .89 |
DLI, donor lymphocyte infusion; SCT, stem cell transplantation; CML, chronic myeloid leukemia; RR, relative risk; CI, confidence interval; CP, chronic phase; AP, advanced phase; GVHD, graft-versus-host disease; TCD, in vitro or in vivo T-cell depletion with Campath (CD52) monoclonal antibodies.
Relationship between response to DLI, disease phase, and interval SCT relapse
| Relapse type . | Interval SCT relapse . | |
|---|---|---|
| <9 months . | >9 months . | |
| Molecular (n = 8)3-150 | 5/5 (100%)3-151 | 3/3 (100%) |
| Cytogenetic (n = 19) | 3/5 (60%) | 14/14 (100%) |
| Hematologic | ||
| CP (n = 30) | 5/11 (45%) | 12/19 (73%) |
| AP (n = 9) | 0/4 (0%) | 2/5 (40%) |
| Relapse type . | Interval SCT relapse . | |
|---|---|---|
| <9 months . | >9 months . | |
| Molecular (n = 8)3-150 | 5/5 (100%)3-151 | 3/3 (100%) |
| Cytogenetic (n = 19) | 3/5 (60%) | 14/14 (100%) |
| Hematologic | ||
| CP (n = 30) | 5/11 (45%) | 12/19 (73%) |
| AP (n = 9) | 0/4 (0%) | 2/5 (40%) |
DLI, donor lymphocyte infusion; SCT, stem cell transplantation; CP, chronic phase; AP, advanced phase.
Total number of patients at each disease stage at DLI.
Number of responder/number of patients (percentage).
Probability of PCR positivity after molecular remission
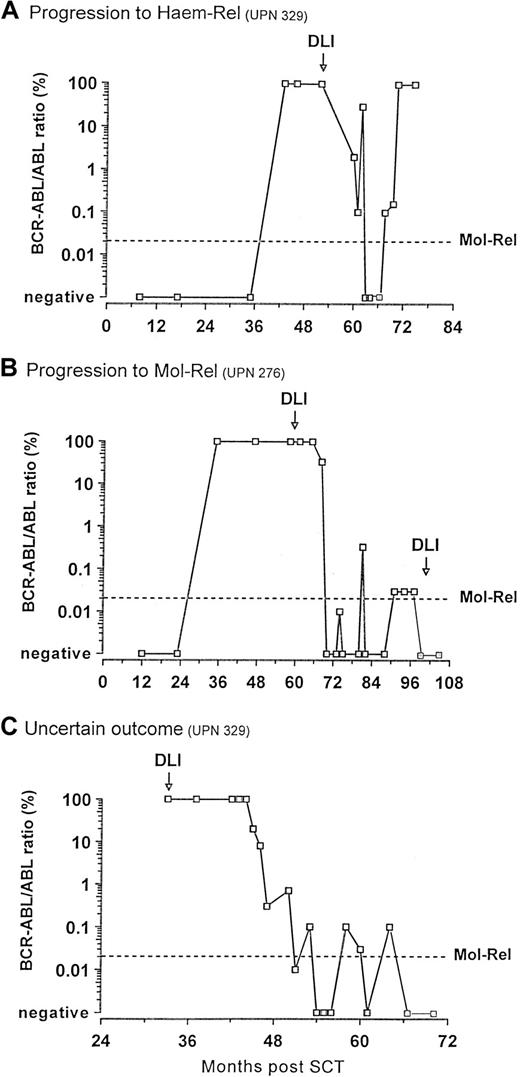
Of the 44 patients achieving molecular remission, 4 subsequently became PCR positive (Figure 4A-C). Their PCR negativity lasted 15, 18, 37, and 87 weeks. Their median follow-up was 29 months (range 5-89). Noteworthy, all these patients had received DLI according to the escalating-dose regimen. One patient progressed to a blastic phase and died (Figure 4A). The other 3 patients showed fluctuating levels of BCR-ABL transcripts; of these, 2 patients fulfilled the criteria for molecular relapse and received a further dose of donor cells that further restored remission (Figure4B-C).
: Outcome of the patients who became PCR positive after achieving molecular remission post-DLI.
(A) One patient evolved to blastic phase and died. (B) Two patients (only one is represented) fulfilled the criteria for molecular relapse and were treated with a further dose of donor lymphocytes that restored remission. (C) One patient showed persistently fluctuating low levels of BCR-ABL transcripts but did not fulfill the criteria for molecular relapse.
: Outcome of the patients who became PCR positive after achieving molecular remission post-DLI.
(A) One patient evolved to blastic phase and died. (B) Two patients (only one is represented) fulfilled the criteria for molecular relapse and were treated with a further dose of donor lymphocytes that restored remission. (C) One patient showed persistently fluctuating low levels of BCR-ABL transcripts but did not fulfill the criteria for molecular relapse.
Discussion
Although DLI is highly effective in restoring remission in patients who relapse after allogeneic SCT for CML, the durability of these remissions is not yet known. The very few data generated so far are inconclusive because the definition of remission has not been consistent, and, therefore, the final outcome for patients achieving different levels of remission can be significantly different. We have demonstrated that, after transplant, only patients who do not show or show very low levels of BCR-ABL transcripts for a prolonged period of time experience long-term remissions.12,13 27 On the basis of such evidence, we decided to adopt molecular remission as a reference to assess the long-term outcome of 66 consecutive patients receiving DLI for CML in relapse after allografting. The probability of attaining molecular remission was 68% at 3 years post-DLI (Figure 1). Notably, of all the patients responding to DLI in our study, only 2 achieved a cytogenetic remission but failed to achieve molecular remission. One of these patients progressed to a cytogenetic relapse 46 months after the first evidence of cytogenetic remission; he remains alive, but further DLI could not be given because his sibling donor is no longer available. The other patient has recently received a lymphocyte dose (2 × 108 CD3+ cells/kg) higher than the maximum dose scheduled in our escalating protocol and his BCR-ABL transcripts have been falling.
In this analysis, we have shown that patients who relapse within 9 months after allografting are less likely to respond to DLI than patients who relapse relatively late after allografting (Table1). We have shown previously that patients whose relapse was based on molecular or cytogenetic criteria have a higher rate of response to DLI than those whose relapse was based on hematologic criteria.11 This observation was confirmed in this study, but the superior response rate of patients diagnosed at the molecular and cytogenetic levels was more obvious in patients diagnosed early after allografting than in those diagnosed more than 9 months after the procedure (Table 3). These findings support the notion that the tumor burden at the time of relapse correlates with the probability of response to DLI. However, they may also reflect differing tempos of disease in different patients, whereby patients whose disease progresses relatively rapidly to hematologic relapse may be more resistant to remission induction by DLI than those whose disease relapses only relatively slowly.
A large multicenter study of the results of DLI reported recently from the United States showed that the optimal response to DLI was obtained when the interval from SCT to DLI was less than 2 years.13 However, we were unable to show any difference in response rate when we compared response rates between patients treated within and beyond 2 years from the original transplantation (P = .86). It should be noted that the interval from transplant to DLI comprises the interval from transplant to diagnosis of relapse plus the interval from relapse to initiation of DLI. Thus, these discordant results might be explained if the interval from SCT to diagnosis of relapse was a more important determinant of response than the interval from transplant to initiation of treatment. The patients included in the 2 studies were not in fact comparable with regard to the interval from transplantation to diagnosis of relapse.
The 44 patients who achieved molecular remission showed a better survival than those who failed to achieve molecular remission (Figure3). Of the 3 patients who died after achieving molecular remission, only one had evidence of leukemia at the time of death. It is worth noting that, for 68% of patients who achieved molecular remission, its duration already exceeds the duration of remission after the original transplant procedure (Figure 2). Thus, in these patients the DLI was more effective than the lymphocytes administered at the time of transplant. This benefit may reflect the higher lymphocyte dose at DLI, the lack of post-DLI immunosuppression, and the status of graft-versus-host tolerance induced at the time of the original transplantation.
Four patients who achieved molecular remission subsequently became positive for BCR-ABL transcripts. The one patient who proceeded to frank hematologic relapse had been PCR-negative for only 15 weeks (Figure 4A). Two other patients had levels of BCR-ABL transcripts equivalent to those that we have used to diagnose molecular relapse and to initiate DLI after an allograft. Both received a further dose of DLI: one has regained molecular remission that has lasted thus far 15 weeks (Figure 4B); the molecular status of the other patient is unchanged 8 weeks post-DLI. The fourth patient had persistently fluctuating levels of BCR-ABL transcripts with intermittent negative readings for 10 months (Figure 4C). He received no further treatment, but thereafter his PCR status has been persistently negative for 12 weeks. It is tempting to speculate that donor T cells may have a limited period of activity, but that they can be reactivated in certain circumstances. It should be noted that these four patients all received DLI according to the escalating-dose regimen, which suggests that in these cases the cell dose administered may have reduced the number of residual leukemia cells to a level undetectable by PCR but still sufficient to regenerate the disease.
Our study shows that molecular remissions post-DLI are durable and thus that survival is best for patients in whom molecular evidence of leukemia cannot be detected. Although we cannot exclude the possibility that patients who satisfy less stringent criteria for remission may survive equally well, we suggest that molecular remission should be the aim of DLI treatment.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
References
Author notes
Francesco Dazzi, Department of Haematology, Hammersmith Hospital/ ICSM, Du Cane Rd, London W12 0NN, United Kingdom; e-mail: f.dazzi@ic.ac.uk.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal