Abstract
There is evidence that the total cellular content of placental cord blood (PCB) grafts is related to the speed of engraftment, though the total nucleated cell (TNC) dose is not a precise predictor of the time of neutrophil or platelet engraftment. It is important to understand the reasons for the quantitative association and to improve the criteria for selecting PCB grafts by using indices more precisely predictive of engraftment. The posttransplant course of 204 patients who received grafts evaluated for hematopoietic colony-forming cell (CFC) content among 562 patients reported previously were analyzed using univariate and multivariate life-table techniques to determine whether CFC doses predicted hematopoietic engraftment speed and risk for transplant-related events more accurately than the TNC dose. Actuarial times to neutrophil and platelet engraftment were shown to correlate with the cell dose, whether estimated as TNC or CFC per kilogram of recipient's weight. CFC association with the day of recovery of 500 neutrophils/μL, measured as the coefficient of correlation, was stronger than that of the TNC (R = −0.46 and −0.413, respectively). In multivariate tests of speed of platelet and neutrophil engraftment and of probability of posttransplantation events, the inclusion of CFC in the model displaced the significance of the high relative risks associated with TNC. The CFC content of PCB units is associated more rigorously with the major covariates of posttransplantation survival than is the TNC and is, therefore, a better index of the hematopoietic content of PCB grafts.
Introduction
Placental/umbilical cord blood (PCB) transplantation between related and unrelated donor–recipient pairs is used increasingly in the clinical replacement of bone marrow. Unrelated PCB-derived stem cell grafts have become a realistic option for a large number of patients within a wide range of age and size, largely by reducing the histocompatibility matching requirements and the wait from search to transplantation. Many variables—mostly dependent on patient, diagnosis, and stage of disease—influence the outcome of PCB transplantation, among which the nucleated cell dose has been routinely the variable most clearly associated with the pace of engraftment and the risk for transplant-related events (TRE).1-3 The importance of the cell dose underscores widely held concerns about the clinical adequacy of the hematopoietic stem and progenitor cell numbers in PCB collections.4 For this reason, estimates of the hematopoietic potential of PCB grafts are preponderant criteria for donor selection.
As is the accepted practice in bone marrow transplantation, the hematopoietic potential of PCB collections is estimated as proportional to the total nucleated cell (TNC) number. The graft's TNC, in relation to the recipient's body weight in kilograms, constitutes the “cell dose” and correlates empirically with transplantation endpoints, especially engraftment speed and recipient survival.1-3,5,6 Although the association is highly significant, the TNC dose is only one of the important variables,7 8 and its ability to anticipate the pace of engraftment and the incidence of TRE accounts for a relatively small fraction of the variance of the respective endpoints.
Possibly more informative surrogate indices of the hematopoietic potential of individual PCB collections have been proposed, therefore. Enumeration of hematopoietic colony-forming progenitor cells (CFC) was used initially in PCB collections,9,10 as it had been in other sources.11-14 Inverse correlations between the dose of granulocyte-macrophage colony-forming cells (GM-CFC) and time to autologous or allogeneic engraftment were reported for bone marrow and peripheral blood stem cell transplants.11-17
These correlations were not always reproducible, however,18-24 and an association with better survival but not with the tempo of hematopoietic recovery was also reported.25 More recent reports favor CD34+cell enumeration over CFC assays,26,27 and a threshold of 2 to 5 × 106 CD34+ cells per kilogram was proposed by several investigators (eg, Mavroudis et al28). In a single-institution study of 28 human leukocyte antigen (HLA)-identical sibling donor T-cell–depleted bone marrow transplantations, the threshold applied to mononuclear cell engraftment and transplant-related mortality (TRM) and, thus, to overall survival, though not to neutrophil engraftment. A similar correlation between CD34+ cell dose and time to neutrophil engraftment held when the grafts were collections of mobilized peripheral blood stem cells.26 27
We now report data from the first 562 patients who underwent transplantation with PCB from our Placental Blood Program, approximately one third of whom had been evaluated for their content of CFC. These data indicate that the CFC dose in PCB is more closely correlated with neutrophil and platelet engraftment than the TNC dose. (A preliminary analysis29 was reported in 1997.)
Patients, materials, and methods
PCB collections
The techniques used to harvest PCB after placental delivery and to obtain maternal informed consent and the testing for various infectious diseases and immunogenetic markers have been described.3,30 31 Partial removal of erythrocytes and plasma to reduce the stored volume was performed on all units collected after November 1994.
PCB testing and typing
Blood cells were counted with a Technicon H1 analyzer (Miles, Tarrytown, NY) according to manufacturer's instructions. Microbiologic and serologic tests for infectious diseases have been described. HLA-A, -B, and -C antigen and DRB1 PCR-SSO phenotypes were determined on PCB and on mothers' samples as described.3Tests for common hematologic genetic diseases were performed, as suggested by the donor family's clinical history and ethnicity.
CFC assays
Testing for hematopoietic CFC was conducted on the first 307 PCB units collected. Thereafter, these tests were conducted on all units with collected volumes of 40 to 60 mL (the lowest collected volume of PCB units stored for transplantation) and on 10% of larger units. In all, CFC had been assayed on 3745 of the 7369 units collected through January 1998. The technique consists of culturing unfractionated 2- and 4-μL PCB samples diluted in 1 mL semisolid medium containing defined concentrations of hematopoietic growth factors.32,33Preliminary experiments indicated that small dish cultures of cord blood samples in these concentrations usually generate between 5 and 75 colonies in a 1-mL dish, allowing reliable microscopic identification and enumeration of clearly distinct colonies. The culture medium was Iscove's modified Dulbecco's medium (IMDM; Gibco, Grand Island, NY) in reverse-osmosis grade pyrogen-free water with 0.8% wt/vol methylcellulose (Eastman-Kodak, Rochester, NY) containing 30% vol/vol fetal bovine serum (Gemini BioProd, Calabasas, CA), 1% vol/vol deionized bovine serum albumin (Sigma, St Louis, MO), 7.5 × 10−5 mol/L β-mercaptoethanol (Sigma), and 1% (vol/vol) antibiotic–antimycotic solution (penicillin, streptomycin, and Fungizone (Gibco).22 23 Purified recombinant human hematopoietic growth factors were added to medium prepared freshly before use, in optimized concentrations. Growth factors included 1.5 U/mL erythropoietin (EPO), 2 × 10−10 mol/L granulocyte colony-stimulating factor (G-CSF), 100 ng/mL stem cell factor (SCF), 4.5 × 10−10 mol/L granulocyte-macrophage colony stimulating factor (GM-CSF), and 2 × 10−10 mol/L interleukin-3 (IL-3). EPO, G-CSF, and SCF were generous gifts from Amgen (Thousand Oaks, CA), and the Genetics Institute (Cambridge, MA) kindly donated both GM-CSF and IL-3. All cultures were established in duplicate dishes, incubated at 37°C in a fully humidified incubator with 5% C02 in air and visually evaluated at 12 to 14 days for the presence of erythroid bursts, GM colonies, and mixed-cell colonies with an inverted microscope. Erythroid bursts contained 200 to 1000 cells, whereas GM- and mixed-cell colonies were usually composed of more than 5000 cells.
Processing and cryoprotection
PCB units were either cryoprotected by the slow addition of an equal volume of 20% dimethyl sulfoxide (DMSO; Fisher Scientific, Pittsburgh, PA) in 0.95% saline for injection or were first volume-reduced to 20 mL by removing erythrocytes and plasma. As previously described,33 after volume reduction, 5 mL cryopreservation solution (0.85 NaCl, 50% DMSO [Cryoserv; Research Industries, Salt Lake City, UT] and 5% Dextran 40 [Baxter Healthcare, Deerfield, IL]) was added to the cell suspension slowly and with continuous mixing. Units were stored in cryogenic tanks within the liquid phase of liquid nitrogen. Selected units were shipped to transplant centers in validated −150°C Dry-Shippers (Custom Biogenics, Shelby, MI).
Patients
This report includes 204 of the 562 patients reported to have undergone PCB transplantation between August 1993 and February 1998.3 These 204 patients received PCB grafts that had been assayed for CFC before freezing, and their overall demographic characteristics are summarized in Table1. Transplant recipients were followed as described for the cohort of 562 patients as a whole, and the definitions of clinical endpoints and the statistical methods used were the same.3
Demographic and clinical characteristics of the first 204 patients who underwent transplantation with PCB grafts from unrelated donors with colony-forming cell counts from the New York Blood Center
| Characteristic . | Number (%) . |
|---|---|
| Male | 130 (64) |
| Female | 74 (36) |
| Age at transplantation (y) | |
| <2 | 57 (28) |
| 2-5 | 49 (24) |
| 6-11 | 46 (23) |
| 12-17 | 28 (14) |
| ≥18 | 24 (12) |
| Diagnosis | |
| Leukemia/lymphoma | 125 (61) |
| Acute lymphoblastic leukemia | 58 (46) |
| Acute myeloblastic leukemia | 46 (37) |
| Chronic myelogenous leukemia | 10 (8) |
| Juvenile chronic myelogenous leukemia | 7 (6) |
| Lymphoma | 4 (3) |
| Genetic disease | 62 (30) |
| Fanconi anemia | 19 (31) |
| Severe combined immunodeficiency | 9 (15) |
| Osteopetrosis | 4 (7) |
| Hurler syndrome | 3 (5) |
| Wiskott-Aldrich syndrome | 3 (5) |
| Adrenoleukodystrophy | 3 (5) |
| Blackfan-Diamond | 3 (5) |
| Other genetic | 18 (29) |
| Acquired diseases | 17 (8) |
| Myelodysplastic disease | 6 (35) |
| Severe aplastic anemia | 8 (47) |
| Other cancer | 3 (18) |
| Number of HLA-A, -B, -DR mismatches (including high-resolution DR*) | |
| 0 | 18 (9) |
| 1 | 89 (44) |
| ≥2 | 96 (47) |
| Location of transplantation center | |
| United States | 177 (87) |
| Outside United States | 27 (13) |
| Characteristic . | Number (%) . |
|---|---|
| Male | 130 (64) |
| Female | 74 (36) |
| Age at transplantation (y) | |
| <2 | 57 (28) |
| 2-5 | 49 (24) |
| 6-11 | 46 (23) |
| 12-17 | 28 (14) |
| ≥18 | 24 (12) |
| Diagnosis | |
| Leukemia/lymphoma | 125 (61) |
| Acute lymphoblastic leukemia | 58 (46) |
| Acute myeloblastic leukemia | 46 (37) |
| Chronic myelogenous leukemia | 10 (8) |
| Juvenile chronic myelogenous leukemia | 7 (6) |
| Lymphoma | 4 (3) |
| Genetic disease | 62 (30) |
| Fanconi anemia | 19 (31) |
| Severe combined immunodeficiency | 9 (15) |
| Osteopetrosis | 4 (7) |
| Hurler syndrome | 3 (5) |
| Wiskott-Aldrich syndrome | 3 (5) |
| Adrenoleukodystrophy | 3 (5) |
| Blackfan-Diamond | 3 (5) |
| Other genetic | 18 (29) |
| Acquired diseases | 17 (8) |
| Myelodysplastic disease | 6 (35) |
| Severe aplastic anemia | 8 (47) |
| Other cancer | 3 (18) |
| Number of HLA-A, -B, -DR mismatches (including high-resolution DR*) | |
| 0 | 18 (9) |
| 1 | 89 (44) |
| ≥2 | 96 (47) |
| Location of transplantation center | |
| United States | 177 (87) |
| Outside United States | 27 (13) |
HLA indicates human leukocyte antigen.
One patient was not typed for high-resolution DRB1.
Selection of HLA-matched units
Units were considered initially matched if their HLA-A, -B, and -DR phenotypes matched at least 4 of the patient's submitted 6 antigen HLA phenotypes at a serologic or intermediate-DNA level of resolution. Searches for 3 antigen matches were performed if requested. Patient and donor HLA types were confirmed by the Placental Blood Program and, usually, also by the respective transplantation center. Confirmatory typing of grafts was usually made on cryoprotected cells kept in a segment of tubing integral to the unit's freezing bag. High-resolution DRB1 alleles were determined after transplantation until September 1994 and prospectively thereafter.
Transplantation and posttransplantation follow-up
Protocols for pretransplant conditioning and graft-versus-host disease prophylaxis were chosen by the transplantation centers and varied widely. Transplantation centers reported on the diagnosis and stage of primary disease at transplantation, on the post-thaw leukocyte number and viability, on the results of bacteriologic cultures, on the clinical complications, and on the various endpoints.
Endpoints
Data on clinical outcome were available on all 204 patients receiving PCB grafts assayed for CFC content for at least the first 100 days after transplantation. This report presents their status at the time of the last report to us (as of January 2000). Myeloid engraftment was defined as the achievement of a neutrophil count of 500/μL (ANC 500), and platelet engraftment was defined as the achievement of counts of 50 000/μL without transfusion support and were taken as the first of 3 and 7 days with the counts mentioned, respectively. Acute and chronic graft-versus-host disease were diagnosed and graded by each transplantation center. Event-free survival (EFS) denotes the length of the posttransplantation period in which the patient was alive without requiring a backup marrow or a second PCB transplant and without autologous reconstitution or relapse. The first event defined the endpoint for survival analyses, when more than one befell the same patient. There were 3 distinct endpoints classified as TRE: autologous reconstitution, backup autograft or allograft, and death. For this endpoint, patients with leukemia who had relapses were censored at the time of relapse.
Statistical analysis
Times to myeloid and platelet engraftment were analyzed by life-table methods (Kaplan-Meier). Multivariate comparisons used Cox logistic regression under an assumption of proportional hazards with all analyzed variables entered into the model. When analyzed as continuous variables, to make their variances more similar, TNC dose was rounded to the nearest 107 cells/kg and CFC dose to the nearest 104 cells/kg. All statistical analyses were performed with the Statistical Program for the Social Sciences (SPSS, Chicago, IL).
Results
Demographics
General features of the patients receiving grafts tested for CFC content are summarized in Table 1. Although not selected on this basis, this subset differed from the entire set of patients by having significantly more of the younger (P = .001) and smaller (P = .002) patients, higher frequency of genetic disease, and lower frequency of leukemia/lymphoma (P = .041). This subset of patients also included higher frequencies of males (P = .027) and of patients who underwent transplantation in the United States (P = .003). These differences reflected the criterion used for testing units for CFC (small volume) and, consequently, their being preferentially selected for smaller, younger patients.
CFC counts
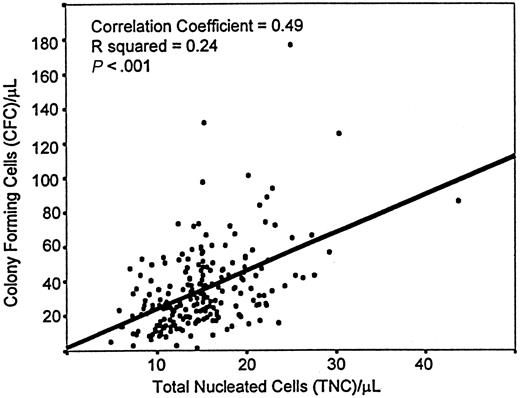
Overall analysis of the 3745 PCB samples tested for CFC content showed that the reproducibility of the duplicate counts was higher when measured as total CFC numbers (Pearson's correlation coefficient,R = 0.95) than as individual types of colonies (not shown). The mean concentration of total CFC in PCB was 29.1/μL ± 0.31/μL (SEM), and the median concentration was 24.7/μL. Ninety-five percent of all samples had between 7 and 65 CFC/μL. The total CFC counts were significantly associated with the TNC counts (R = 0.492; P > .001) (Figure1).
Scatterplot of CFC and TNC concentrations in PCB.
This figure describes a highly significant Pearson correlation coefficient (R) and its squared value (R2).
Scatterplot of CFC and TNC concentrations in PCB.
This figure describes a highly significant Pearson correlation coefficient (R) and its squared value (R2).
Effect of volume reduction on the ability of PCB to engraft
The time to engraftment was analyzed separately on 102 patients who underwent transplantation with whole PCB and on 98 recipients of blood cryopreserved and frozen after volume reduction. There were no significant differences in the Kaplan-Meier estimates of time to engraftment of patients receiving whole versus volume-reduced blood (log-rank test; P = .35). Median times to neutrophil and platelet engraftment were 29 and 84 days for whole blood and 28 and 94 days for volume-reduced blood. Because these differences were not statistically significant, the use of volume reduction was not considered in further analyses.
Cell dose and engraftment
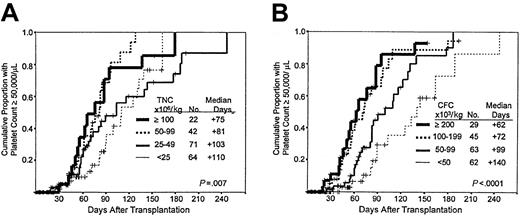
Times to neutrophil and platelet engraftment are available for 200 and 199, respectively, of the 204 patients. Times to neutrophil and platelet engraftment were significantly associated with the number of cells in the grafts per kilogram of recipient's weight, categorized as shown in Figure 2, panels A and B and Figure 3, panels A and B, respectively. The Kaplan-Meier associations with TNC and CFC dose show progressively faster engraftment with higher cell doses, which may be summarized by the median time in days required to reach 500 neutrophils and 50 000 platelets/L (Figures 2, 3). Among the patients with myeloid engraftment, Pearson's coefficient of correlation of neutrophil engraftment with CFC dose was −0.462, and with TNC dose it was −0.413. The R values for platelet engraftment were 0.234 and −0.078 for CFC and TNC dose, respectively. Multivariate analysis of engraftment endpoints showed that inclusion of the CFC dose in the model eliminated the significance of the relative risks associated with the TNC dose (dose as a categorical variable in both cases) (Table2). When dose is included as a continuous variable, the CFC dose remains predominant in predicting the time to engraftment (data not shown).
Speed of myeloid engraftment after PCB transplantation.
(A) Influence of TNC dose. Kaplan-Meier plot of neutrophil recovery in patients receiving PCB grafts with the cell dose ranges (groups) shown. The negative association is significant at the 0.0001 level. (B) Influence of CFC dose. Kaplan-Meier plot of neutrophil recovery in patients receiving PCB grafts with the cell dose ranges (groups) shown. The negative association is significant at the 0.0001 level.
Speed of myeloid engraftment after PCB transplantation.
(A) Influence of TNC dose. Kaplan-Meier plot of neutrophil recovery in patients receiving PCB grafts with the cell dose ranges (groups) shown. The negative association is significant at the 0.0001 level. (B) Influence of CFC dose. Kaplan-Meier plot of neutrophil recovery in patients receiving PCB grafts with the cell dose ranges (groups) shown. The negative association is significant at the 0.0001 level.
Speed of platelet engraftment after PCB transplantation.
(A) Influence of TNC dose. Kaplan-Meier plot of platelet recovery in patients receiving PCB grafts with the cell dose ranges (groups) shown. The negative association is significant at the 0.007 level. (B) Influence of CFC dose. Kaplan-Meier plot of platelet recovery in patients receiving PCB grafts with the cell dose ranges (groups) shown. The negative association is significant at the 0.0001 level.
Speed of platelet engraftment after PCB transplantation.
(A) Influence of TNC dose. Kaplan-Meier plot of platelet recovery in patients receiving PCB grafts with the cell dose ranges (groups) shown. The negative association is significant at the 0.007 level. (B) Influence of CFC dose. Kaplan-Meier plot of platelet recovery in patients receiving PCB grafts with the cell dose ranges (groups) shown. The negative association is significant at the 0.0001 level.
Relative risk for engraftment endpoints and transplant-related events
| Variable . | No. patients . | Relative risk (95% CI) . | |
|---|---|---|---|
| Univariate . | Multivariate . | ||
| TNC/kg (before cryopreservation, ×106)* | |||
| ≥100 | 21 | 4.8 (2.8-8.4) P < .001 | 1.5 (0.6-3.9) P = .4 |
| 50-99 | 41 | 1.7 (1.1-2.8) P = .024 | 0.7 (0.4-1.3)P = .3 |
| 25-49 | 67 | 1.8 (1.2-2.8) P = .006 | 1.2 (0.8-1.9) P = .4 |
| <25 | 61 | 1.0 | 1.0 |
| CFC/kg (before cryopreservation, ×103)* | |||
| ≥200 | 28 | 5.2 (3.0-9.0)P < .001 | 4.7 (2.0-11.1) P < .001 |
| 100-199 | 43 | 4.2 (2.5-7.0) P < .001 | 4.7 (2.6-8.8) P < .001 |
| 50-99 | 61 | 2.4 (1.5-3.8)P < .001 | 2.4 (1.5-3.9) P < .001 |
| <50 | 58 | 1.0 | 1.0 |
| TNC/kg (before cryopreservation, ×106)† | |||
| ≥100 | 21 | 2.0 (0.99-4.0)P = .052 | 0.4 (0.1-1.3) P = .12 |
| 50-99 | 40 | 0.9 (1.3-4.6) P = .005 | 1.1 (0.5-2.4)P = .9 |
| 25-49 | 64 | 1.1 (0.6-2.0) P = .8 | 0.8 (0.4-1.5) P = .5 |
| <25 | 55 | 1.0 | 1.0 |
| CFC/kg (before cryopreservation, ×103)† | |||
| ≥200 | 28 | 4.4 (2.1-9.1)P < .001 | 8.1 (2.7-24.2) P < .001 |
| 100-199 | 40 | 3.5 (1.8-6.9) P < .001 | 4.0 (1.8-8.7) P = .001 |
| 50-99 | 59 | 2.0 (1.1-3.9)P = .034 | 2.0 (1.0-3.9) P = .37 |
| <50 | 53 | 1.0 | 1.0 |
| TNC/kg (before cryopreservation, ×106)‡ | |||
| 100 | 22 | 1.0 | 1.0 |
| 50-99 | 42 | 0.9 (0.4-2.0) P = .9 | 0.9 (0.3-2.6)P = .9 |
| 25-49 | 72 | 1.1 (0.6-2.2) P = .8 | 1.0 (0.3-3.0) P = 1 |
| <25 | 66 | 2.0 (1.02-3.8)P = .04 | 1.5 (0.5-4.9) P = .4 |
| CFC/kg (before cryopreservation, ×103)‡ | |||
| 200 | 29 | 1.0 | 1.0 |
| 100-199 | 45 | 1.1 (0.5-2.1) P = .8 | 1.1 (0.4-2.9)P = .9 |
| 50-99 | 64 | 1.0 (0.5-1.9) P = .9 | 0.8 (0.3-2.3) P = .7 |
| <50 | 64 | 2.3 (1.2-4.2)P = .009 | 1.7 (0.6-4.9) P = .4 |
| Variable . | No. patients . | Relative risk (95% CI) . | |
|---|---|---|---|
| Univariate . | Multivariate . | ||
| TNC/kg (before cryopreservation, ×106)* | |||
| ≥100 | 21 | 4.8 (2.8-8.4) P < .001 | 1.5 (0.6-3.9) P = .4 |
| 50-99 | 41 | 1.7 (1.1-2.8) P = .024 | 0.7 (0.4-1.3)P = .3 |
| 25-49 | 67 | 1.8 (1.2-2.8) P = .006 | 1.2 (0.8-1.9) P = .4 |
| <25 | 61 | 1.0 | 1.0 |
| CFC/kg (before cryopreservation, ×103)* | |||
| ≥200 | 28 | 5.2 (3.0-9.0)P < .001 | 4.7 (2.0-11.1) P < .001 |
| 100-199 | 43 | 4.2 (2.5-7.0) P < .001 | 4.7 (2.6-8.8) P < .001 |
| 50-99 | 61 | 2.4 (1.5-3.8)P < .001 | 2.4 (1.5-3.9) P < .001 |
| <50 | 58 | 1.0 | 1.0 |
| TNC/kg (before cryopreservation, ×106)† | |||
| ≥100 | 21 | 2.0 (0.99-4.0)P = .052 | 0.4 (0.1-1.3) P = .12 |
| 50-99 | 40 | 0.9 (1.3-4.6) P = .005 | 1.1 (0.5-2.4)P = .9 |
| 25-49 | 64 | 1.1 (0.6-2.0) P = .8 | 0.8 (0.4-1.5) P = .5 |
| <25 | 55 | 1.0 | 1.0 |
| CFC/kg (before cryopreservation, ×103)† | |||
| ≥200 | 28 | 4.4 (2.1-9.1)P < .001 | 8.1 (2.7-24.2) P < .001 |
| 100-199 | 40 | 3.5 (1.8-6.9) P < .001 | 4.0 (1.8-8.7) P = .001 |
| 50-99 | 59 | 2.0 (1.1-3.9)P = .034 | 2.0 (1.0-3.9) P = .37 |
| <50 | 53 | 1.0 | 1.0 |
| TNC/kg (before cryopreservation, ×106)‡ | |||
| 100 | 22 | 1.0 | 1.0 |
| 50-99 | 42 | 0.9 (0.4-2.0) P = .9 | 0.9 (0.3-2.6)P = .9 |
| 25-49 | 72 | 1.1 (0.6-2.2) P = .8 | 1.0 (0.3-3.0) P = 1 |
| <25 | 66 | 2.0 (1.02-3.8)P = .04 | 1.5 (0.5-4.9) P = .4 |
| CFC/kg (before cryopreservation, ×103)‡ | |||
| 200 | 29 | 1.0 | 1.0 |
| 100-199 | 45 | 1.1 (0.5-2.1) P = .8 | 1.1 (0.4-2.9)P = .9 |
| 50-99 | 64 | 1.0 (0.5-1.9) P = .9 | 0.8 (0.3-2.3) P = .7 |
| <50 | 64 | 2.3 (1.2-4.2)P = .009 | 1.7 (0.6-4.9) P = .4 |
TNC indicates total nucleated cell; CFC, colony-forming cell.
Myeloid engraftment (time to absolute neutrophil count, 500/μL).
Platelet engraftment (time to platelet count 50,000/μL).
Transplant-related events during first 2 years after transplantation (autologous reconstitution, other transplants, and death; relapse censored). Two patients who did not survive after the day of transplantation are excluded.
Cell dose and transplant-related events
The Kaplan-Meier incidence of TRE at 2 years was calculated as a function of the TNC or CFC cell dose categories separately (Figure4A,B). The incidence of TRE appears also to be anticipated just slightly better by CFC than by TNC dose categories. Cox regression univariate analysis shows that both variables result in significant relative risks for the respective lowest dose categories and that neither CFC nor TNC predominates when both are included in a multivariate model.
Probability of TRE after PCB transplantation.
(A) Influence of TNC dose. Kaplan-Meier plots of the proportion of patients in whom TRE developed after they received TNC doses in the ranges (groups) shown. The negative association is significant at the 0.01 level. (B) Influence of CFC dose. Kaplan-Meier plots of the proportion of patients in whom TRE developed after they received CFC doses in the ranges (groups) shown. The negative association is significant at the 0.008 level.
Probability of TRE after PCB transplantation.
(A) Influence of TNC dose. Kaplan-Meier plots of the proportion of patients in whom TRE developed after they received TNC doses in the ranges (groups) shown. The negative association is significant at the 0.01 level. (B) Influence of CFC dose. Kaplan-Meier plots of the proportion of patients in whom TRE developed after they received CFC doses in the ranges (groups) shown. The negative association is significant at the 0.008 level.
Discussion
The PCB units transplanted in this study provided roughly one twentieth to one third of the TNC dose and one hundredth to one tenth of the CFC dose when compared to the doses recommended for unrelated bone marrow grafts and mobilized peripheral blood hematopoietic stem cell (HSC) collections, respectively. These lower doses reflect the quantitative limitations of PCB collections and underlie the concern that such collections might be occasionally insufficient for clinically appropriate engraftment kinetics.4 Indeed, the TNC dose in PCB grafts is significantly correlated with the speed of myeloid and platelet engraftment and the probability of long-term posttransplantation survival.1-3 Despite the highly significant TNC association with time to engraftment, the correlation is not very strong (R = 0.413); thus its contribution (asR2) to the overall variance in time to ANC 500/μL is only 17% of the total. The CFC dose is a stronger covariate (R = 0.458) and accounts for 21% of the variance. Most important, however, multivariate analysis using Cox regression indicates that the association between TNC dose and time to engraftment is no longer significant if the CFC dose is included in the model. Thus, the association of the speed of engraftment with the TNC dose is overshadowed by its relationship to the CFC dose, which constitutes a more predictive index. In addition to its usefulness in identifying PCB units with adequate HSC content, determining the CFC dose should help elucidate the mechanisms underlying the association between the numbers of more or less differentiated cells and those of “true” HSC in neonatal peripheral blood. The enumeration of CFC is, however, more demanding than the TNC count. CFC cultures must be grown with highly diluted samples to allow colonies to form in numbers adequate for counting, which introduces imprecision and, thus, error. The technique is also subject to substantial quantitative variability between laboratories that may use different methods of cell isolation and culture and growth factor combinations.
Not surprisingly, therefore, discrepant conclusions as to the association between clinical results and CFC determinations have been reached (eg, 11-13 vs 18-25). Such discrepancies, and the long incubation times for CFC cultures, have led to the exploration of the usefulness of the CD34 marker in evaluating HSC grafts. No formal correlation analyses of the dose of human CD34+ cells in PCB transplantation have been reported, though, in the case of other sources of HSC, the results seem encouraging.26-28 Despite its variability, CFC enumeration can be reproducible in duplicate testing within a laboratory (particularly when the assays require no isolation of cellular subsets), and the coefficient of correlation for duplicate tests over time has been consistently greater than 0.94 in our laboratory. This high reproducibility is consistent with the significant relationship demonstrated between the concentrations of TNC and CFC in PCB (Figure1).
It is widely held that, in addition to its association with the speed of neutrophil and platelet engraftment, TNC dose is related to the incidence of graft failure, TRE, and death after transplantation.1-3 6 Our univariate analysis (Table2, Figures 4A,B) confirms this, but the elevated relative risks for TRE are significantly associated only with the lowest cell dose categories (either TNC < 25 × 106/kg or CFC < 50 × 103/kg); higher doses are equivalent in this regard. In the multivariate analysis, Cox regression was unable to discern whether CFC or TNC configured the more accurate TRE predictor.
Taken together, therefore, these results indicate that the quantitation of CFC content more accurately characterizes the ability of these grafts to achieve neutrophil and platelet engraftment in clinically useful time than do the corresponding TNC counts. This conclusion indicates that the mechanisms that maintain the levels of nucleated cells in the peripheral blood of neonates at birth do so in significant and close proportionality to the concentrations in the blood of the progenitor cells and of the stem cells responsible for long-term engraftment.
Supported in part by National Heart, Lung, and Blood Institute grant HL 48031 (1992-1995).
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
References
Author notes
Pablo Rubinstein, Placental Blood Program, New York Blood Center, 310 East 67th Street, New York, New York 10021; e-mail: prubins@nybc.org.



This feature is available to Subscribers Only
Sign In or Create an Account Close Modal