Abstract
The low frequency of transplantable hematopoietic stem cells in adult human bone marrow (BM) and other differences from cord blood stem cells have impeded studies to optimize the retroviral transduction of stem cells from adult sources. To address this problem, first a cytokine combination was defined that would both maximize the kinetics of adult BM CD34+CD38− cell mitogenesis and minimize the period of prestimulation required for the transduction of these cells by a MSCV-GFP/neor virus in tissue culture dishes in the absence of fibronectin. Three days of stimulation with flt3-ligand, Steel factor, interleukin (IL)-3, and hyper-IL-6 proved both necessary and sufficient to obtain 83% ± 2% GFP+ CD34+CD38− cells, 75% ± 10% G418-resistant clonogenic progenitors, and 50% ± 20% transduced long-term culture-initiating cells as recovered 48 hours after a single exposure to virus. Moreover, this was accompanied by a several-fold increase in viral receptor (pit-1) messenger RNA transcripts in the target cells. Using this prestimulation protocol, repeated daily exposure to new virus (3×) did not alter the proportion of transduced cells over that obtained with a single exposure. Adult human BM cells able to engraft immunodeficient (NOD/SCID-β2M−/−) mice were also efficiently transduced (10%-20% GFP+ human lymphoid and myeloid cells present 6-8 weeks after transplant) using a 6-day prestimulation and infection protocol. A clinically useful efficiency of retrovirus-mediated gene transfer to transplantable adult human BM stem cells can thus be obtained with a protocol that allows their semisynchronous activation into cycle and concomitant increased expression of virus receptor transcripts before virus exposure.
Introduction
Retroviral vectors are able to introduce new genes stably and at high efficiency into the genome of many types of mammalian cells, including primitive hematopoietic cells.1Transplantable hematopoietic stem cells are particularly attractive targets for the gene therapy of various inherited and acquired disorders because of their ability to completely and permanently replace the hematopoietic system of engrafted hosts. However, despite unequivocal evidence of the retroviral transduction of transplantable hematopoietic stem cells from adult murine bone marrow with these properties,2-5 results with their adult human counterparts have been generally disappointing. This may be due, at least in part, to the recent recognition that cells detectable in vitro as long-term culture-initiating cells (LTC-IC) 6,7 or in vivo as repopulating cells8,9 require prolonged cytokine stimulation to be maximally activated.10-15 In addition, the many differences between these cells and progenitors detectable as in vitro colony-forming cells (CFC)7,12,16 have meant that conditions for achieving high levels of gene transfer to CFC have not proven to be applicable for transplantable stem cells, whereas when transplantable stem cells are assessed at nonlimiting numbers (per recipient), gene transfer efficiencies are similar to those measured for LTC-IC.13 17
Nevertheless, from clinical studies, it is clear that transplantable human hematopoietic cells can be transduced with retroviral vectors, albeit at a low efficiency to date.18-22 This has highlighted the need both for systematic investigations of variables that may independently (or in concert) limit stem cell transduction and for careful validation of any improvements suggested using surrogate in vivo assays of transplantable human hematopoietic stem cell activity. Such an approach was first reported using bg/nu/xid (bnx) mice as recipients of human cells and, in this case, a transduction efficiency of about 1% to human repopulating cells was detected.23,24 The particular advantage of this xenotransplant model is that assessment of engraftment for year-long periods is possible.24 More recently, the use of SCID25,26 and then nonobese diabetic/SCID (NOD/SCID)27 and NOD/SCID-β2microglobulin−/−(NOD/SCID-β2M−/−)28 mice as recipients of transplantable human hematopoietic cells has become popular because of the high levels of engraftment that may be obtained in these mice.29,30 Several studies using NOD/SCID recipients have now reported efficient levels of transduction of transplantable human hematopoietic stem cells (20%-50%) but, in most cases, these have been limited to cells obtained from cord blood (CB).13,17,31-35 Although up to 20% gene transfer efficiency to transplantable stem cells obtained from the marrow of adult baboons prestimulated with cytokines for 5 to 7 days in vivo was reported,36 less impressive results have been obtained in the one analogous study undertaken with adult human bone marrow (BM) cells.32
The purpose of the present study was to analyze a number of variables known to affect retroviral transduction efficiency as they apply to transplantable stem cells present in adult human BM. The specific variables evaluated were the cytokine combination used to stimulate the cells, their rate of entry into division, the duration of their stimulation prior to virus exposure, alterations in their expression of virus receptor messenger RNA (mRNA), and the duration of the final period of exposure to virus. Because the transplantable stem cell population in adult BM is several-fold less frequent than that in CB, we used FACS-purified CD34+CD38− BM cells to anticipate the mitogenic and viral receptor mRNA responses of functionally defined stem cells. This also made it possible to keep the multiplicity of infection high without requiring the use of very large volumes of virus-containing medium (VCM) in experiments where functional end points were used. In addition, we took advantage of our previous observation that tissue culture dishes can be used effectively to colocalize the virus and the target cells even in the absence of fibronectin or the CH-296 fragment.37 The present studies have allowed the definition of a protocol that reproducibly results in 10% to 20% transduced transplantable stem cells from freshly isolated normal adult BM.
Materials and methods
BM cells
Human BM cells were either from aspirate samples obtained from healthy individuals donating marrow for clinical allogeneic transplantation or were from cadaveric BM samples obtained from the Northwest Tissue Center (Seattle, WA). For both, approved institutional procedures including informed consent were followed. Low-density (<1.077 g/cm3) cells were first isolated by centrifugation on Ficoll-Hypaque (Pharmacia, Uppsala, Sweden) and then cryopreserved in fetal calf serum (FCS) with 10% DMSO (Sigma Chemicals, St Louis, MO) at −135°C until required. Aliquots of cells were then thawed and cells expressing lineage (lin) antigens found on mature erythroid, granulopoietic, megakaryopoietic, and lymphoid cells removed immunomagnetically as described38 (using a StemSep column from StemCell Technologies Inc, Vancouver, BC).
Flow cytometry and cell sorting
The CD34+ and CD34+CD38−cells were isolated by FACS from the lin− BM cells after being stained with antihuman CD34-Cy5(8G12) and CD38-phycoerythrin (PE) (Leu17; Becton Dickinson, San Jose, CA) antibodies. They were then washed twice with phosphate-buffered saline (PBS; StemCell), the last washing step containing propidium iodine (PI; Sigma Chemicals) to allow exclusion of PI+ (nonviable) cells.39For some experiments, single cells were deposited using the single-cell deposition unit of the FACS (FACStar Plus, Becton Dickinson) into 96-well plates (Life Technologies, Burlington, ON) preloaded with serum-free medium and cytokines (see below). To determine the efficiency of gene transfer to cultured CD34+ cells, aliquots were stained with antihuman CD34-Cy5 and CD38-PE and then washed twice with PBS, the last washing step again containing PI, as described above. Cells expressing green fluorescence protein (GFP+ cells) were detected by their increased fluorescence intensity within the fluorescence one channel. Gene transfer efficiencies to CD34+ cells were calculated by dividing the number of GFP+ CD34+ cells by the total number of CD34+ cells present. Cells harvested from the marrow of NOD/SCID-β2M−/− mice were stained with antihuman CD45-PE (Hle1, Becton Dickinson) and antihuman CD71-PE (OKT9, prepared in our laboratory) or antihuman CD15-PE (80H5, Coulter, Burlington, ON), or antihuman CD19-PE (Leu12, Becton Dickinson), antihuman CD20-PE (Leu16, Becton Dickinson), and antihuman CD34-Cy5 antibodies. Cy5-, fluorescein isothiocyanate (FITC)- and PE-conjugated mouse Ig-stained cells were used as negative controls for each population analyzed, and gates were set to include more than 99.9% of all events obtained with these controls.
Recombinant human cytokines
Recombinant interleukin (IL)-3 and granulocyte-macrophage colony-stimulating factor (GM-CSF) were gifts from Novartis (Basel, Switzerland), flt3-ligand (FL) was a gift from Immunex Corporation (Seattle, WA), thrombopoietin (TPO) was a gift from Genentech (San Francisco, CA), and erythropoietin was a gift from StemCell. Steel factor (SF) was purified from supernatants of COS cells that had been transfected with the corresponding human complementary DNA (cDNA). Hyper-IL-6 (H-IL-6) was purified from plasmid-transfected yeast supernatants by anion exchange chromatography and gel filtration as described earlier.40
Single-cell division studies
Single CD34+CD38−cells were cultured in Iscove medium containing BIT (StemCell Technologies), 10−4mol/L 2-mercaptoethanol (Sigma), 40 μg/mL low-density lipoproteins (Sigma), and either FL (100 ng/mL) + SF (100 ng/mL) + IL-3 (20 ng/mL), or FL + SF + IL-3 and H-IL-6 (40 ng/mL), or FL + SF + IL-3 + H-IL-6 + TPO (50 ng/mL). Each well was assessed visually each day. The day of each first cell division (appearance of 2 refractile cells in a well) was noted and the total number of clones present, that is, number of wells with more than one cell also recorded.
Retroviral vector and transduction protocol
The KA125 retrovirus (kindly provided by Dr P. Leboulch, MIT-Harvard, Boston, MA), which contains the gene of the humanized redshifted GFP molecule (EGFP; Clontech, Palo Alto, CA) under the control of a long terminal repeat (LTR) derived from mouse stem cell virus (MSCV)41 and the Neorgene under the control of a phosphoglycerate kinase promoter was packaged in PG13 cells and VCM harvested as described previously.13 FACS-purified CD34+ or CD34+CD38− BM cells were suspended at 2 × 105 cells/mL and incubated as indicated in serum-free medium supplemented with FL + SF + IL-3, FL + SF + IL-3 + H-IL-6, or FL + SF + IL-3 + H-IL-6 + TPO, as described above. The cells were then washed once with Iscove medium containing 2% FCS, resuspended in the same volume of VCM supplemented with 5 μg/mL protamine sulfate (Sigma, Oakville, ON), and fresh cytokines and were then transferred in this medium into tissue culture dishes (Corning, Cambridge, MA) that had been previously loaded with VCM for 2 hours at room temperature.37 When repeated exposure to new virus was undertaken, the cells were harvested after 24 hours, centrifuged, and resuspended in the same volume of new VCM plus protamine sulfate and cytokines and transferred into new VCM-pretreated tissue culture dishes as described above. Two days after the last exposure to new VCM, cells were harvested for FACS analyses and in vitro assays. For the in vivo experiments, the cells were prestimulated as before for 3 (or 4) days, as indicated, prior to exposure to virus. For the 3-day prestimulated cells, an equal volume of new cytokine and protamine sulfate-supplemented VCM was added on the 2nd and 3rd day of transduction and then on the 4th day, the cells were harvested and injected directly into mice. For the 4-day prestimulated cells, incubation with VCM was for 2 days and then cells were harvested and injected into mice (ie, after the same overall period in culture of 6 days).
In vitro progenitor cell assays
The CFC assays were performed in methylcellulose cultures as previously described.7 The proportion of G418-resistant CFC was determined by comparing the number of CFC detectable in cultures with and without 1.7 mg/mL G418 (dry weight, active drug concentration = 1.26 mg/mL, GibcoBRL, Burlington, ON). Less than 1% of CFC from nontransduced cells gave detectable colonies when assayed in the presence of 1.7 mg/mL G418 in every experiment. LTC-IC were assayed by measuring the total number of CFC present after culturing the test cells for 6 weeks at 37°C on murine fibroblast feeders previously engineered to produce human IL-3, SF, and G-CSF.7 The proportion of G418-resistant LTC-IC was then inferred from the proportion of G418-resistant CFC measured in the 6-week-old LTC-IC.
NOD/SCID-β2M−/− mouse reconstitution assays
Breeding, maintenance, and total body irradiation of NOD/SCID-β2M−/− mice (original breeding pairs kindly provided by Dr L. Schulz, Jackson Laboratory, Bar Harbor, ME)28 were carried out as described for NOD/SCID mice.17 Briefly, transduced and mock-transduced human BM cells plus 106 irradiated (1500 cGy) human BM carrier cells in less than 0.5-mL volumes were injected intravenously into mice previously given 350 cGy of 137Cs γ rays. Six to 8 weeks later, the mice were killed and the cells obtained by flushing both femurs and tibias with PBS were then stained for FACS analysis.13
Retrovirus receptor expression
For each sample, 5 × 103 cells of each population to be analyzed were lysed in 50 μL of GIT buffer (5 mol/L guanidine isothiocyanate, 20 mmol/L 1,4-dithiothreitol [DTT], 25 mmol/L sodium citrate [pH = 7.0], 0.5% sarcosyl) and the nucleic acids precipitated in ethanol using 4 mg glycogen as a carrier. Reverse transcription and global amplification of the cDNA was performed using the procedure of Brady and colleagues,42 as modified by Sauvageau and coworkers16 and Jiang and associates.43 The amplified cDNA was electrophoresed in 1% agarose gels and transferred to nylon membrane (Hybond-N, Amersham, Pharmacia, Piscataway, NJ) for hybridization, first with a cDNA probe for pit-1 isolated from the POJ75 plasmid44 (provided by Dr M. van Zeijl, Wyeth-Ayerst, Pearl River, NY) and then with GAPDH (isolated from a plasmid obtained from Dr G. Krystal, Terry Fox Laboratory, Vancouver, BC).
The pit-1 transcript levels were measured by phosphoimage analysis using a Storm 860 phosphoimager and APPS software (Molecular Dynamics, Sunnyvale, CA). Each measurement was corrected for the signal level obtained in the RT− control, which was hybridized to the same filter under the same conditions. Filters were then stripped and rehybridized to the GAPDH probe. For comparisons of transcript levels between fresh or cultured cells from the same tissue sample, each normalized transcript signal was expressed as a ratio relative to the normalized transcript signal measured in the corresponding initial CD34+CD38− cells.
Results
Time course analysis of single-cell cultures of CD34+CD38− BM cells stimulated to divide by different cytokine combinations
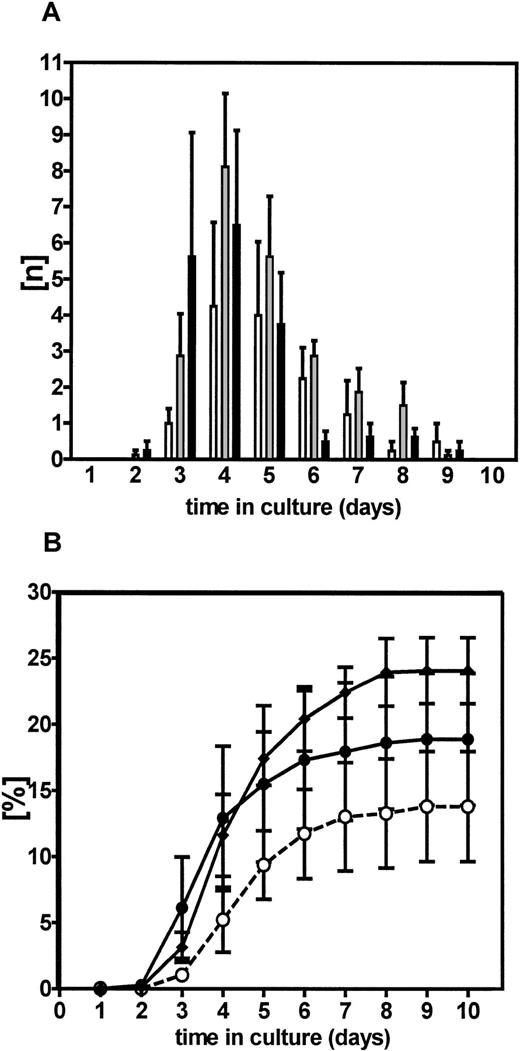
Because the majority of cells in human CB that are able to engraft NOD/SCID mice are CD34+CD38−8,17,45 and the CD34+CD38− population in adult BM is known to represent a highly enriched source of a closely related progenitor type, that is, LTC-IC,16,17 we anticipated that this population would also constitute a highly enriched suspension of transplantable human stem cells. Thus, the responses of adult BM CD34+CD38− cells to cytokine stimulation might provide a reasonable approximation of the kinetics of activation of transplantable stem cells. Figure 1 shows the combined results of 4 experiments in which the proliferative response of adult marrow CD34+CD38− cells to 3 different cytokine combinations was examined. The combination of FL + SF + IL-3 was tested because it had been previously found to stimulate large amplifications of LTC-IC in similar cultures of CD34+CD38− adult BM cells.46,47 The additions of H-IL-6 ± TPO to this combination were studied because of recent evidence that activation of gp13014,48,49 and c-mpl50-53 may enhance the amplification of repopulating cells. H-IL-6 has the advantage that it can activate gp130 independent of expression of any α receptor (eg, for IL-6) and has been found to mimic effects obtained on murine long-term repopulating cells with IL-11.14 Although the proportion of PI− CD34+CD38−cells that subsequently proliferated was low in all 4 BM samples used for these particular experiments, a consistent pattern was seen with the first cell divisions starting only 48 hours after initiation of the cultures, regardless of the cytokine combination used (Figure 1). Thereafter, the number of wells containing 2 cells or more increased rapidly to reach a plateau by 7 to 8 days of culture, at which time the frequencies of responsive cells were found to be highest in cultures containing FL + SF + IL-3 + H-IL-6 (± TPO, 19% ± 5% and 24% ± 2%, respectively, in these experiments) and slightly lower in cultures containing FL + SF + IL-3 only (14% ± 4%).
Kinetics of activation of CD34+CD38− BM stem cells.
Kinetics of initiation of clone (doublet) formation by single CD34+CD38− BM cells cultured individually in 96-well plates in serum-free medium supplemented with either FL + SF + IL-3 (■, ○), FL + SF + IL-3 + HIL-6 (░, ♦), or FL + SF + IL-3 + HIL-6 + TPO (▪, ●). Panel A shows the number of wells containing a first doublet as a function of time. Panel B shows the same data as a cumulative plot. Values shown are the mean ± SEM of data pooled from 4 independent experiments.
Kinetics of activation of CD34+CD38− BM stem cells.
Kinetics of initiation of clone (doublet) formation by single CD34+CD38− BM cells cultured individually in 96-well plates in serum-free medium supplemented with either FL + SF + IL-3 (■, ○), FL + SF + IL-3 + HIL-6 (░, ♦), or FL + SF + IL-3 + HIL-6 + TPO (▪, ●). Panel A shows the number of wells containing a first doublet as a function of time. Panel B shows the same data as a cumulative plot. Values shown are the mean ± SEM of data pooled from 4 independent experiments.
Changes in pit-1 mRNA levels in cytokine-activated CD34+CD38−BM cells
Viral infection also depends on the level of expression of the specific receptor that allows the initial binding of the virus to the surface of the target cell. The receptor responsible for binding viruses carrying the GALV envelope produced by PG-13 cells has been identified as pit-1.54 We therefore also undertook a time-course study of pit-1 mRNA expression in cultured CD34+CD38− cells using the FL + SF + IL-3 + H-IL-6 cytokine combination. Figure2 shows the results obtained with 3 different marrow samples. A strong induction of pit-1 expression occurred between the 1st and 3rd day in all 3 experiments (2- to 11-fold increase), which was then variably sustained, whereas only a slight increase in GAPDH transcript levels (1.1- to 2.6-fold) was seen in the same analyses. A 1- to 3-fold increase in pit-1 transcripts was also seen between day 0 and day 1, but this was equally true for GAPDH and may be due to nonspecific increases in transcription that occur during the first 24 hours after cells have been previously cooled.55
Time-course study of changes in pit-1 expression in cultures of CD34+CD38− BM cells stimulated with FL + SF + IL-3 + H-IL-6.
RNA obtained from cells immediately after their purification and then 1, 3, and 5 days later was reverse transcribed and amplified by polymerase chain reaction as described in Materials and methods, and the resultant cDNA electrophoresed, blotted, and hybridized first with a pit-1 probe and then a GAPDH probe. The results of 3 independent experiments are shown in the top 2, middle 2, and bottom 2 rows of autoradiographs, respectively.
Time-course study of changes in pit-1 expression in cultures of CD34+CD38− BM cells stimulated with FL + SF + IL-3 + H-IL-6.
RNA obtained from cells immediately after their purification and then 1, 3, and 5 days later was reverse transcribed and amplified by polymerase chain reaction as described in Materials and methods, and the resultant cDNA electrophoresed, blotted, and hybridized first with a pit-1 probe and then a GAPDH probe. The results of 3 independent experiments are shown in the top 2, middle 2, and bottom 2 rows of autoradiographs, respectively.
Effect of prestimulation on the efficiency of transducing CD34+ BM cells
In a next series of experiments, we examined the effect of varying the duration of initial exposure to each of the same 3 cytokine combinations on the efficiency of transduction following subsequent exposure to virus. Accordingly, CD34+ cells were first prestimulated for 1 to 5 days and then incubated with a GFP/neor VCM for a standard 48-hour period at the end of which the frequency of transduced cells (or progenitors) present was determined. In this case, the transduction protocol involved exposing the target cells once to cytokine-supplemented VCM on tissue culture dishes that had been preloaded with virus in the absence of fibronectin, as previously described.37 As shown in Figure3, under these conditions, even without any prestimulation, approximately 30% of the CD34+ cells present 2 days after infection had become GFP+ and approximately 50% of the CFC in the same cultures were G418 resistant. These impressive efficiencies of gene transfer to intermediate types of progenitors extend to human BM cells, results we have previously obtained with human CB cells transduced on preloaded tissue culture dishes.13 The frequencies of transduced CD34+CD38− cells and LTC-IC in the same cultures were, however, much lower (<10%). As also shown in Figure 3, an expected increase in transduction efficiency of all cell types was obtained when the cells had been prestimulated with cytokines for at least 24 hours before virus exposure, with maximum frequencies of transduced cells obtained after 1 to 3 days of prestimulation, depending on the cytokines present and the cell type assessed.13,56 Only in the case of cells defined by a CD34+CD38− phenotype did an even more prolonged period of prestimulation (3-5 days) result in higher apparent transduction levels. However, it should be noted that at least part of this latter effect may be due to the culture-induced acquisition of a CD34+CD38− phenotype by originally CD34+CD38+ cells.35
Analysis of gene transfer efficiencies.
Gene transfer efficiencies to CD34+ cells, CD34+CD38− cells, CFC, and LTC-IC-derived CFC were obtained after prestimulation of CD34+CD38− BM cells with FL + SF + IL-3 (open bars), FL + SF + IL-3 + HIL-6 (hatched bars), or FL + SF + IL-3 + HIL-6 + TPO (black bars) for various intervals followed by a single exposure to VCM. Gene transfer rates were assessed by FACS 48 hours after the first exposure of the cells to virus to determine the proportion of GFP+CD34+ or GFP+CD34+CD38− cells, or by plating the cells directly, or after 6 weeks in LTC, in methylcellulose to measure the proportion of G418-resistant CFC and LTC-IC–derived CFC, respectively. Values shown are the mean ± SEM of data pooled from 3 independent experiments.
Analysis of gene transfer efficiencies.
Gene transfer efficiencies to CD34+ cells, CD34+CD38− cells, CFC, and LTC-IC-derived CFC were obtained after prestimulation of CD34+CD38− BM cells with FL + SF + IL-3 (open bars), FL + SF + IL-3 + HIL-6 (hatched bars), or FL + SF + IL-3 + HIL-6 + TPO (black bars) for various intervals followed by a single exposure to VCM. Gene transfer rates were assessed by FACS 48 hours after the first exposure of the cells to virus to determine the proportion of GFP+CD34+ or GFP+CD34+CD38− cells, or by plating the cells directly, or after 6 weeks in LTC, in methylcellulose to measure the proportion of G418-resistant CFC and LTC-IC–derived CFC, respectively. Values shown are the mean ± SEM of data pooled from 3 independent experiments.
Effect of varying the period of virus exposure after a fixed (3-day) period of prestimulation on the efficiency of transducing CD34+ BM cells
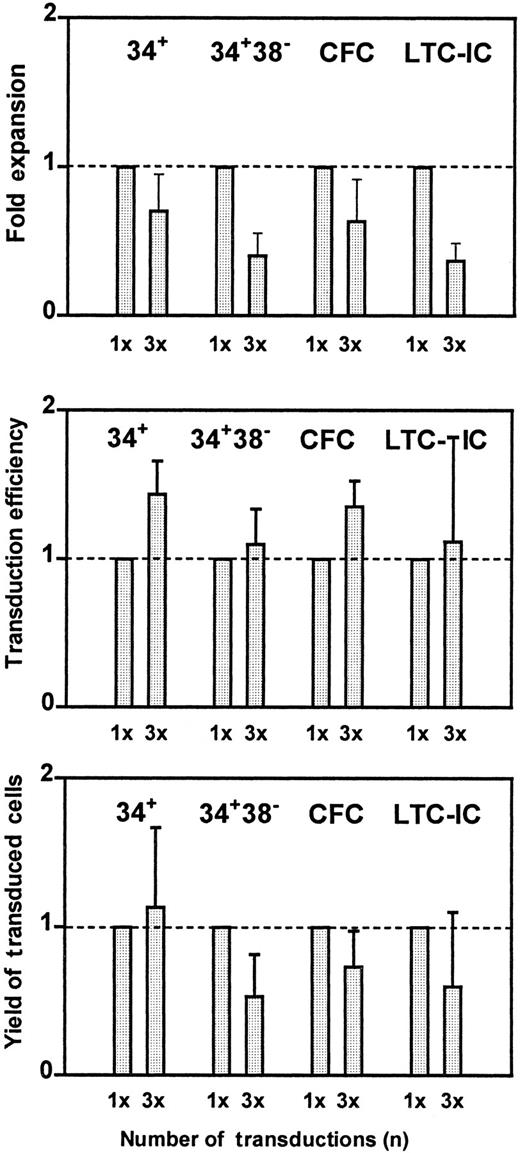
Because the highest efficiency of gene transfer to LTC-IC was obtained when BM cells were stimulated with FL + SF + IL-3 + H-IL-6 (Figure 3), we focused on this cytokine combination to determine whether any further improvement in transduction would be obtained if the cells were exposed to the virus for a more prolonged period. Figure 4 shows the combined results of 3 independent experiments in which CD34+ BM cells were first prestimulated for 3 days and then exposed to new VCM either once or on each of 3 successive days, with assessment of gene transfer efficiencies 2 days after the last exposure to new VCM (ie, a total of 2 days and 4 days of virus exposure, respectively). The results show that the proportion of transduced cells (all types) obtained was the same with both infection protocols (Figure 4, middle panel). However, the yields of cells after 3 cycles of infection were slightly (although not significantly, P > .05) reduced (Figure 4, upper panel). Accordingly, the yields of transduced cells were also slightly less (Figure 4, lower panel). This suggested that any gain anticipated from the expected further expansion of primitive cells in such cultures after additional time was counterbalanced by losses due to the daily cell manipulations and possibly due to the serum present in the VCM in which the cells were incubated.
Cell expansion, transduction efficiency, and yield of transduced cells.
Cell expansion (top), transduction efficiency (middle), and yield of transduced cells (bottom) were measured after stimulation of CD34+ BM cells for 3 days in serum-free medium supplemented with FL + SF + IL-3 + HIL-6 and then exposure to VCM once for a total of 48 hours or 3 times over a total period of 96 hours. To reduce the variation of the data due to differences in absolute transduction efficiencies between different experiments, the results for the second protocol have been expressed as a fraction of the corresponding result obtained with the first protocol in the same experiment. These values have then been pooled and the mean ± SEM shown (n = 3).
Cell expansion, transduction efficiency, and yield of transduced cells.
Cell expansion (top), transduction efficiency (middle), and yield of transduced cells (bottom) were measured after stimulation of CD34+ BM cells for 3 days in serum-free medium supplemented with FL + SF + IL-3 + HIL-6 and then exposure to VCM once for a total of 48 hours or 3 times over a total period of 96 hours. To reduce the variation of the data due to differences in absolute transduction efficiencies between different experiments, the results for the second protocol have been expressed as a fraction of the corresponding result obtained with the first protocol in the same experiment. These values have then been pooled and the mean ± SEM shown (n = 3).
Transduction efficiency of human BM repopulating cells
In a final series of experiments, we used the cytokine prestimulation conditions found to optimize transduction of adult BM LTC-IC to assess gene transfer efficiencies to repopulating cells. In view of preliminary data indicating that higher levels of human cell engraftment could be obtained in NOD/SCID-β2M−/− mice than in NOD/SCID mice,15,30 and the known low frequency of repopulating cells in adult human BM,9 57 we chose to use NOD/SCID-β2M−/− mice as recipients. Because there was no need to wait for GFP expression in vitro in these experiments, the cells were injected immediately into mice on finishing the period of exposure to VCM. In the first 3 experiments, FACS-purified CD34+CD38− BM cells were thus first prestimulated for 3 days in serum-free medium containing FL + SF + IL-3 + H-IL-6 (days 1, 2, and 3). On day 4, the cells were suspended in VCM supplemented with the same cytokines and protamine sulfate and transferred to virus-preloaded tissue culture dishes (in the absence of fibronectin). On each of the next 2 days (days 5 and 6), the culture volume was successively doubled by the addition of new cytokine and protamine sulfate-supplemented VCM. One day after the last exposure to new VCM (day 7), all cells were harvested from the dishes and the progeny of 26 000 or 33 000 initial CD34+CD38− cells injected intravenously into each of 10 sublethally irradiated NOD/SCID-β2M−/− mice. Two of these mice died and, of the remaining 8, 4 were found to be engrafted with more than 0.5% human lymphoid (CD19/20+) and more than 0.5% human myeloid (CD15+) cells when the marrow of the mice was assessed 6 to 8 weeks later (20 000 events analyzed). A 5th mouse showed 81 CD19/20+ events of 34 000 analyzed and 13 CD15+ events of 48 000 analyzed. The other 3 did not contain detectable levels of human cells. The average level of engraftment with human (CD45/71+) cells in the 5 positive mice was 4% ± 3%, of which 23% ± 6% were GFP+(Table 1). In 2 subsequent experiments the number of cells injected per mouse was increased approximately 2- to 3-fold (ie, each mouse was injected with the harvested progeny of 53 000 or 80 000 initial CD34+CD38− cells). In addition, in these experiments, half of the cells were subjected to a slightly modified transduction protocol in which the initial period of prestimulation was extended by another day and the period of exposure to VCM was shortened by 1 day with no manipulation of the cells after the first transfer to VCM. Only 1 of the 16 mice transplanted in these latter 2 experiments died, and all of the remaining 15 were engrafted with both human lymphoid and myeloid cells 6 to 8 weeks after transplant (> 30 CD15+ and > 60 CD19/20+ events per 20 000 analyzed in 13 mice, with 7 and 19, and 12 and 30, respectively, seen in the other 2). As summarized in Table 2, despite somewhat lower overall levels of engraftment, all mice contained detectable numbers of GFP+ (lymphoid and myeloid) human cells (overall range = 2%-37%). Interestingly, there was no difference in either the level of human cell engraftment or the proportion of GFP+ human cells between recipients of cells that had been transduced with the 2 different protocols.
Transduction of adult human BM cells capable of repopulating NOD/SCID-β2M−/− mice
| No. of cells per mouse* . | % of human cells† . | % GFP+ human cells‡ . | ||||
|---|---|---|---|---|---|---|
| CD45/71+ . | CD19/20+/34− . | CD15+ . | CD45/71+ . | CD19/20+/34− . | CD15+ . | |
| 26 000 | 0.6 | 0.2 | 0.04 | 25 | 31 | 31 |
| 33 000 | 5.0 | 0.5 | 1.5 | 16 | 28 | 22 |
| 33 000 | 3.8 | 1.0 | 0.8 | 24 | 17 | 31 |
| 33 000 | 2.5 | 0.4 | 0.7 | 32 | 14 | 37 |
| 33 000 | 7.4 | 0.7 | 2.1 | 18 | 13 | 21 |
| No. of cells per mouse* . | % of human cells† . | % GFP+ human cells‡ . | ||||
|---|---|---|---|---|---|---|
| CD45/71+ . | CD19/20+/34− . | CD15+ . | CD45/71+ . | CD19/20+/34− . | CD15+ . | |
| 26 000 | 0.6 | 0.2 | 0.04 | 25 | 31 | 31 |
| 33 000 | 5.0 | 0.5 | 1.5 | 16 | 28 | 22 |
| 33 000 | 3.8 | 1.0 | 0.8 | 24 | 17 | 31 |
| 33 000 | 2.5 | 0.4 | 0.7 | 32 | 14 | 37 |
| 33 000 | 7.4 | 0.7 | 2.1 | 18 | 13 | 21 |
CD34+CD38− cells were stimulated for 3 days with FL + SF + IL-3 + H-IL-6 and then exposed to new virus daily for 3 days. Twenty-four hours after the last addition of fresh virus, the cells were harvested and injected into sublethally irradiated NOD/SCIDβ2M−/− mice. These were sacrificed 6 weeks later and their marrow cells then analyzed for the presence of human cells expressing GFP as described in Materials and methods. Results are shown only for the 5 of 8 mice from 3 experiments in which engraftment of the mice with human cells was seen, where engraftment was defined as the presence of ≥ 5 lymphoid (CD34−CD19/20+) and ≥ 5 myeloid (CD15+) human cells per 20 000 viable cells analyzed.
This refers to the number of CD34+CD38−cells at the start of the transduction protocol, the progeny of which were injected into each NOD/SCID-β2M−/−mouse.
Values shown are the proportion of all viable (PI−) cells retrieved from the BM of the mice that showed reactivity with antibodies to the human cell surface antigens shown.
Proportion of cells in each subpopulation of human cells indicated that were GFP+.
Comparison of the engraftment level and the efficiency of gene transfer to adult human BM cells capable of repopulating NOD/SCID-β2M−/− mice using 2 slightly different 6-day transduction protocols
| No. of cells per mouse* . | Protocol A . | Protocol B . | ||
|---|---|---|---|---|
| % human CD45/71+ cells . | % GFP+ human CD45/71+ cells . | % human CD45/71+ cells . | % GFP+ human CD45/71+ cells . | |
| 53 000 | 1.0 | 3 | 2.1 | 2 |
| 53 000 | 1.2 | 19 | 0.3 | 9 |
| 53 000 | 0.3 | 2 | 1.1 | 13 |
| 53 000 | 1.0 | 4 | ||
| 80 000 | 1.9 | 23 | 2.9 | 13 |
| 80 000 | 11.0 | 16 | 4.3 | 20 |
| 80 000 | 1.0 | 37 | 2.5 | 21 |
| 80 000 | 1.5 | 33 | 2.1 | 12 |
| Mean ± SEM | 2.4 ± 1.2 | 17 ± 5 | 2.2 ± 0.5 | 13 ± 2 |
| No. of cells per mouse* . | Protocol A . | Protocol B . | ||
|---|---|---|---|---|
| % human CD45/71+ cells . | % GFP+ human CD45/71+ cells . | % human CD45/71+ cells . | % GFP+ human CD45/71+ cells . | |
| 53 000 | 1.0 | 3 | 2.1 | 2 |
| 53 000 | 1.2 | 19 | 0.3 | 9 |
| 53 000 | 0.3 | 2 | 1.1 | 13 |
| 53 000 | 1.0 | 4 | ||
| 80 000 | 1.9 | 23 | 2.9 | 13 |
| 80 000 | 11.0 | 16 | 4.3 | 20 |
| 80 000 | 1.0 | 37 | 2.5 | 21 |
| 80 000 | 1.5 | 33 | 2.1 | 12 |
| Mean ± SEM | 2.4 ± 1.2 | 17 ± 5 | 2.2 ± 0.5 | 13 ± 2 |
CD34+CD38− were stimulated for 3 days (protocol A) or 4 days (protocol B) with FL + SF + IL-3 + HIL-6 and then exposed to VCM for 72 hours (3×) or 48 hours (1×), respectively. At the end of this period, the cells were harvested and injected into sublethally irradiated NOD/SCID-β2M−/− mice. The mice were then analyzed 6 to 8 weeks later as described in the footnote to Table 1.
This refers to the number of CD34+CD38−cells at the start of the transduction protocol, the progeny of which were injected into each NOD/SCID-β2M−/−mouse.
Discussion
The potential of retroviral vectors for transducing new genes into primitive human hematopoietic cells was first demonstrated more than a decade ago58 and the first successful clinical application of this technology in an autotransplant setting was reported in 1993.18 Transduction protocols for achieving efficient gene transfer to transplantable human stem cells from CB preparations are now well established.13,17,31-33 However, comparable results for the hematopoietic stem cells present in adult human BM have not been achieved. This is likely due, at least in part, to the practical advantages of working with CB where the frequency of transplantable stem cells appears to be much higher, even within the CD34+ population, as determined from quantitative measurements using NOD/SCID recipients.8,9,57 Moreover, because primitive hematopoietic cells in CB and adult BM respond differently to many cytokines59,60 and with different kinetics,10-13 15 transduction conditions optimized for transplantable CB stem cells would not necessarily be ideal for their adult BM counterparts.
In the present study, we confirmed the prolonged period (7-8 days) required for all CD34+CD38− adult BM cells to complete a first division, even when stimulated by potent cytokine combinations (Figure 1). These included FL + SF + IL-3 + H-IL-6 ± TPO, which ultimately recruits a higher proportion of CD34+CD38− adult BM cells to divide than FL + SF + IL-3 only, although the latter is already sufficient to stimulate a large expansion of LTC-IC.12Because of the rapid recruitment of CD34+CD38−adult BM cells into division between the 4th and 7th days, this period was anticipated to be optimal for transducing a useful fraction of those with stem cell activity, as confirmed by subsequent experiments. It is also interesting to note that the initial 2-day delay in cell cycle entry of CD34+CD38− adult BM cells is shared by CD34+CD38− CB cells although, in the latter case, this is followed by a more rapid recruitment phase (4 days for CD34+CD38− CB cells13 versus 6-7 days for CD34+CD38− BM cells to show maximal recruitment into division) allowing for a shorter overall CB stem cell transduction protocol.13,17 The slower rate of entry into cycle of cytokine-activated CD34+CD38− adult BM cells suggests that, in vivo, more achieve a deeper level of quiescence than those present in CB, as borne out by a recent comparative analysis of their patterns of gene expression before and after cytokine activation.61
An additional factor potentially limiting stem cell transduction is the expression of receptors for the particular virus used.62Here we show that cytokine activation of CD34+CD38− adult BM cells consistently up-regulated their expression of the pit-1 gene, which encodes the receptor for the GALV pseudotyped viruses produced by PG13 cells.63 Interestingly, there was no change in pit-1 transcripts until after the first day of cytokine exposure, after which these increased rapidly to reach a maximum by day 3, which was then sustained for at least 2 more days (Figure 2). These findings provide an additional explanation for the need for an initial period of prestimulation demonstrated empirically (Figure 3) and extend topit-1 the cytokine response pattern previously documented for other viral receptor genes.62,64 65
To increase the multiplicity of infection, we took advantage of our recent observation that virus can be concentrated on tissue culture dishes, even in the absence of fibronectin or fibronectin fragments.37 Interestingly, under these conditions we found no increase in transduction efficiency or yield of recovered LTC-IC (Figure 4) or in vivo repopulating cells (Table 2) by repeated exposure to new virus every day (3 ×) as compared to a single exposure. This finding probably reflects a balance between the loss in all cells incurred by the extra manipulations associated with the repeated transduction protocol and any gain achieved by a more prolonged exposure to higher titer VCM.
Detection of transduced repopulating adult BM cells was facilitated by the use of NOD/SCID-β2M−/−recipients. Thus transplantation of the cultured progeny of 2.6 to 8 × 104 CD34+CD38− cells resulted in 20 of 23 evaluable mice (3 died) becoming engrafted with readily detectable levels of human cells (0.3%-11% of all cells present in the marrow of these mice at 6-8 weeks after transplant), which included both lymphoid (CD34−CD19/20+) and myeloid (CD15+) progeny. This establishes that nonlimiting numbers of (> 1) pluripotent repopulating human stem cells had been injected into each mouse in these experiments.8 This is a prerequisite condition to infer gene transfer efficiencies from the levels of GFP+ cells obtained within the in vivo regenerated human populations that would otherwise be subject to all-or-none effects. GFP+ cells were, in fact, present in all 20 engrafted mice, and in 12 of these, comprised more than 15% of the human cells, with a maximum value of 37%. Furthermore, the GFP+ cells were represented in both lymphoid and myeloid progeny populations and at approximately equivalent levels (R2 = 0.6, P < .05) consistent with their origin from a transduced cell with multilineage repopulating potential. Our more recent investigation of human cells that engraft NOD/SCID-β2M−/− mice indicate that these mice may be transiently repopulated for up to 8 weeks by a subpopulation of human stem cells with more restricted functions in addition to those capable of more prolonged hematopoiesis, which exclusively repopulate NOD/SCID mice (Glimm H, Eisterer W, Lee K, et al, manuscript in preparation). It will, therefore, be important to extend the present studies in the future to other transplant models that would provide more definitive evidence of gene transfer to adult human stem cells with longer term reconstituting activity. The experiments described here suggest that this should be possible, moreover, under conditions where exposure to fibronectin may not be necessary.
Acknowledgments
The authors thank Jessyca Maltman and Karen Lambie for expert technical assistance; members of the Stem Cell Assay Service laboratory for initial processing of human BM cells; the staff of the FACS facility for assistance in cell sorting; Yvonne Yang for typing the manuscript; Dr Krystal, Dr Leboulch, Dr van Zeijl, Novartis, Cangene, Genentech, and StemCell for generous gifts of reagents; and Dr Schultz for mice.
Supported by a grant from the National Heart, Lung, and Blood Institute (P01 55435) (C.J.E. and R.K.H.); a grant from the Dr Mildred Scheel Stiftung für Krebsforschung, Bonn, Germany (B.H.); and a grant from the Deutsche Forschungsgemeinschaft, Bonn, Germany, and the Stiftung Innovation Rheinland Pfalz, Mainz, Germany (S.R.-J.). C. Eaves was a Terry Fox Cancer Research Scientist of the National Cancer Institute of Canada.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
References
Author notes
Connie J. Eaves, Terry Fox Laboratory, 601 W 10th Ave, Vancouver, BC, V5Z 1L3; e-mail:connie@terryfox.ubc.ca.



This feature is available to Subscribers Only
Sign In or Create an Account Close Modal