Abstract
It is widely held that the plasminogen (Plg) system plays a role in inflammation through plasmin-mediated directional cell migration. However, substantial evidence for its involvement in the inflammatory response has been obtained from indirect studies and lacks firm biological confirmation. To directly characterize plasminogen's involvement in the inflammatory response, we used thioglycollate to induce a peritoneal inflammatory reaction in Plg(+/+),Plg(+/−), and Plg(−/−) mice. At 6 hours poststimulation, neutrophil recruitment into the peritoneum was maximal and similar between Plg(+/+), Plg(+/−), andPlg(−/−) mice. In contrast, monocyte recruitment was significantly diminished after 24 hours poststimulation inPlg(−/−) mice relative to Plg(+/+) mice. Lymphocyte recruitment also was blunted. Blood monocyte levels in these mice indicated that diminished recruitment into the peritoneum was not the result of a diminished source of cells in the circulation. Macrophage phagocytic function was similar between Plg(+/+) and Plg(−/−) mice. This study establishes a direct involvement of plasminogen in monocyte recruitment during a representative inflammatory response.
THE PLASMINOGEN SYSTEM, because of its ability to assemble on the surface of a number of cell types, has been implicated in directional cell migration associated with physiological responses, such as embryogenesis, development, and tissue remodeling1-3 and a number of pathophysiological events such as inflammation4 and tumor cell invasion.5This role in cell migration depends on the capacity of plasmin to either directly degrade a number of extracellular matrix proteins or to activate other proteases with matrix degrading capabilities. In vitro studies have identified urokinase (u-PA) and its receptor at the leading migratory edges of monocytes and smooth muscle cells.6,7 This complex can generate plasmin from cell-bound plasminogen thereby inducing a front of proteolysis, which can facilitate migration by degrading protein barriers or provisional matrices composed of fibrin. In vitro stimulated murine peritoneal macrophages produce and secrete a plasminogen activator, which has been identified as u-PA.8 More recently, an in vivo study on rat peritoneal macrophages indicated that procoagulant activity is expressed very early after intraperitoneal stimulation with the inflammatory agent, thioglycollate, followed by enhanced expression of fibrinolytic activity.4 Additionally, components of the plasminogen system may regulate the expression and/or activity of cytokines involved in inflammatory processes. For example, u-PA amplifies tumor necrosis factor-α secretion by THP monocytoid cells independent of plasmin.9 In addition, plasmin has been shown to release macrophage derived interleukin-1 (IL-1)10 and to activate transforming growth factor-β.11
Despite these relationships, controversy still exists over the involvement and importance of the plasminogen system in inflammation. For example, in a study assessing wound healing inPlg(−/−) mice, keratinocyte migration was impaired, but no effect on the inflammatory response at the wound site was noted.12 However, it has been shown that u-PA is required for a pulmonary inflammatory response to Cryptococcus neoformans.13 u-PA deficiency resulted in inadequate inflammatory cell recruitment, uncontrolled infection, and death. Recently, it has been shown that fewer CD45-immunoreactive leukocytes are present in the media and neointima of Plg(−/−) arteries relative to Plg(+/+) arteries during the early stages of a response to vascular injury.14 To directly address the role of plasminogen in the inflammatory response, this investigation used a classic acute peritoneal inflammation model in Plg(+/+),Plg(+/−), and Plg(−/−) mice. Inflammatory cell recruitment was differentially affected in plasminogen-deficient mice.
MATERIALS AND METHODS
Mice.
Plg(−/−) mice were developed and characterized as previously described.15 Mice were housed in microisolation cages on a 12-hour day/night cycle and fed a regular chow diet. Experimental mice were 10 to 12 weeks of age, of mixed gender, and appeared healthy during the course of the experiments. All animal experiments were performed in accordance with protocols approved by the Institutional Animal Care and Research Advisory Committee.
Peritoneal inflammation model.
Plg(+/+), Plg(+/−), andPlg(−/−) mice (n = 8 to 13 per group) were injected intraperitoneally with 0.5 mL of a 4% Brewer thioglycollate medium solution (Difco Laboratories, Detroit, MI). At various timepoints after stimulation the mice were sacrificed by isoflurane inhalation (Abbott Laboratories, North Chicago, IL). The peritoneal cavity was then exposed and the exudate collected by washing the cavity with sterile saline using an 18-gauge catheter.
Cell counts and differentials.
Cell counts performed in triplicate on each peritoneal exudate sample were quantitated using a hemacytometer. A total of 105cells/exudate were subject to cytospin onto a glass slide and stained with Wright stain (EM Science, Gibsstown, NJ). Cell differentials were performed in duplicate, and values were expressed as mean ± standard error of the mean (SEM).
For blood leukocyte counts, samples were collected into EDTA microtainer tubes (Becton Dickinson, Rutherford, NJ) via cardiac puncture. After thorough mixing, 20 μL of blood was added to the reservoir of a Unopette microcollection system for leukocyte determination (Becton Dickinson). After allowing time for lysis of red blood cells (10 minutes) the leukocytes were counted in duplicate using a hemacytometer. Blood differentials were performed by spreading 5 μL of whole blood onto a glass slide and stained with Wright stain. Differentials were performed in duplicate on 100 leukocytes per slide.
Histology of peritoneum.
Plg(+/+) and Plg(−/−) mice were anesthetized 6, 24, 48, and 72 hours after thioglycollate stimulation and then perfused with Histochoice (Amersco, Solon, OH). A 5-cm2 section of peritoneal cavity wall was removed, examined for the presence of adhesions using a dissecting microscope, and then paraffin embedded. Five-micrometer sections of cross sectional areas of the cavity wall were stained with hematoxylin/eosin. Four fibrin identification sections were stained with phosphotungstic acid/hematoxylin (Rowley Biochemical Institute, Danvers, MA).
Electron microscopy of peritoneal macrophages.
Plg(+/+) and Plg(−/−) mice were anesthetized 72 hours after thioglycollate stimulation and then perfused with a fixative containing 1% paraformaldehyde, 1.25% glutaraldehyde (Electron Microscopy Sciences, Fort Washington, PA) in 0.1 mol/L cacodylate buffer, pH 7.4. After perfusion, macrophages were collected from peritoneal exudate and postfixed in cacodylate buffered OsO4 followed by staining with 1% uranyl acetate. Samples were then embedded in Spurr (Ernest F. Fullam Inc, Latham, NY) and sections were obtained using an MT 6000-xl ultramicrotome (Research and Manufacturing Company Inc, Tucson, AZ) and viewed with a JEOL 1200 EX II transmission electron microscope (JEOL USA Inc, Peabody, MA) at 3,000 to 25,000 magnification.
Phagocytosis of zymosan particles.
Zymosan (Sigma, St Louis, MO) was sterilized by autoclaving for 45 minutes at 120°C in phosphate-buffered saline (PBS) containing 1 mmol/L calcium and magnesium (PBS+). After autoclaving, the suspension was washed three times in PBS+ and stored at 4°C. Before use, the zymosan suspension was sonicated (water bath apparatus) to disrupt residual clumps, washed three times with PBS+, and resuspended (108 particles/mL) in PBS+.
Peritoneal macrophages, obtained 72 hours after thioglycollate stimulation, were adjusted to 2.5 × 105 cells/mL in Hank's balanced saline solution (HBSS) containing 25 mg/mL bovine serum albumin (BSA). A 0.5 mL aliquot of cell suspension was added to Lab-Tek 4 chamber culture slides (Nunc Inc, Naperville, IL) that were previously coated with gelatin. The cells were allowed to adhere for 30 minutes at 37°C. Nonadherent cells were removed by gentle washing with HBSS containing 25 mg/mL BSA. A suspension of 12.5 × 106 zymosan particles in HBSS/BSA were added to the adherent cells and incubated for 30 minutes at 37°C. Unbound particles were removed with gentle washing with PBS. Slides were stained with crystal violet (Sigma, St Louis, MO) for zymosan particle visualization and Wright stain for cell identification.
Statistical analysis.
Results are expressed as the mean ± SEM. Significance of difference was determined by a one-way ANOVA or an unpaired Student'st-test.16
RESULTS
Leukocyte recruitment after peritoneal stimulation with thioglycollate.
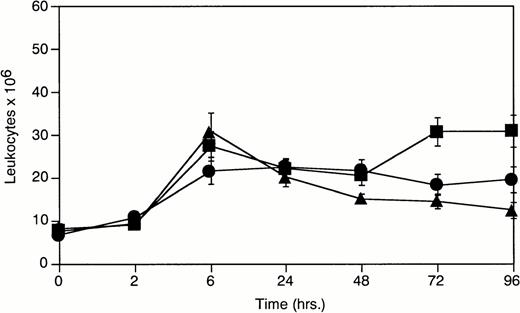
An analysis of total leukocyte levels in the peritoneal exudate ofPlg(+/+), Plg(+/−), andPlg(−/−) mice was made at various timepoints after thioglycollate induced inflammation (Fig1). Peak leukocyte recruitment occurred at 6 hours in all three genotypes. Peritoneal leukocyte levels diminished dramatically inPlg(−/−) mice after 6 hours relative toPlg(+/+) mice. The response in Plg(+/−) mice was intermediate to that observed in Plg(+/+) andPlg(−/−) mice and would appear to indicate a plasminogen dose dependency on sustained leukocyte recruitment. To further identify the inflammatory cells contributing to this response, cell differentials were performed.
Total peritoneal leukocytes 0 to 96 hours after peritoneal stimulation with thioglycollate. (-▪-)Plg(+/+), (-•-) Plg(+/−), and (-▴-)Plg(−/−). Unstimulated peritoneum is represented as t = 0. Values represent mean ± SEM. For Plg(+/+),Plg(+/−), and Plg(−/−) mice P = .003 at 72 hours poststimulation determined by one-way ANOVA
Total peritoneal leukocytes 0 to 96 hours after peritoneal stimulation with thioglycollate. (-▪-)Plg(+/+), (-•-) Plg(+/−), and (-▴-)Plg(−/−). Unstimulated peritoneum is represented as t = 0. Values represent mean ± SEM. For Plg(+/+),Plg(+/−), and Plg(−/−) mice P = .003 at 72 hours poststimulation determined by one-way ANOVA
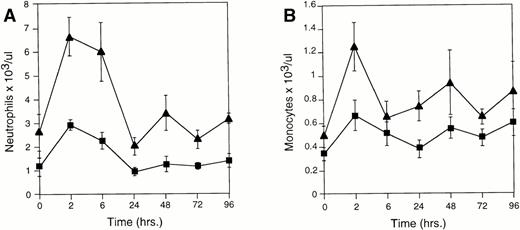
As documented by other investigators,17 18 neutrophil recruitment occurs early during the inflammatory response (Fig 2A). At 6 hours poststimulation, neutrophil levels were maximal and similar between Plg(+/+),Plg(+/−), and Plg(−/−) mice [24.07 ± 3.82 × 106 (n = 11) inPlg(−/−) mice, 15.15 ± 2.33 × 106 (n = 12) in Plg(+/−) mice, and 19.09 ± 2.66 × 106 (n = 13) inPlg(+/+) mice, P = not significant (NS)]. These levels diminished dramatically after 6 hours in all three genotypes.
Cell differentials of peritoneal leukocytes 0 to 96 hours after peritoneal stimulation with thioglycollate as determined by Wright stain. (A) Peritoneal neutrophils; (B) peritoneal macrophages; (-▪-) Plg(+/+), (-•-) Plg(+/−), and (-▴-) Plg(−/−). Unstimulated peritoneum is represented as t = 0. Values represent mean ± SEM. Using one-way ANOVA, P = NS for neutrophils at 6 hours poststimulation andP < .001 for macrophages at 72 hours poststimulation forPlg(+/+), Plg(+/−), and Plg(−/−) mice.
Cell differentials of peritoneal leukocytes 0 to 96 hours after peritoneal stimulation with thioglycollate as determined by Wright stain. (A) Peritoneal neutrophils; (B) peritoneal macrophages; (-▪-) Plg(+/+), (-•-) Plg(+/−), and (-▴-) Plg(−/−). Unstimulated peritoneum is represented as t = 0. Values represent mean ± SEM. Using one-way ANOVA, P = NS for neutrophils at 6 hours poststimulation andP < .001 for macrophages at 72 hours poststimulation forPlg(+/+), Plg(+/−), and Plg(−/−) mice.
Monocyte recruitment, which occurs later during the inflammatory response,19,20 peaked at 72 hours poststimulation inPlg(+/+) mice. This response was severely compromised inPlg(−/−) mice [11.50 ± 1.48 × 106 (n = 11) in Plg(−/−) mice versus 25.94 ± 2.84 × 106 (n = 13) in Plg(+/+) mice at 72 hours poststimulation, P < .001] (Fig 2B). Moreover, plasminogen deficiency dramatically diminished sustained monocyte recruitment: The level of macrophages appeared to plateau at 24 hours poststimulation in the plasminogen deficient mice whereas recruitment continued to increase in the wild type mice. Interestingly, levels in the Plg(+/−) mice were intermediate (14.70 ± 2.05 × 106 at 72 hours poststimulation) indicative of a gene dosage dependency consistent with intermediate antigen levels present in plasma [35 ± 2 μg/mL (n = 3) forPlg (+/−) mice versus 84 ± 8 μg/mL (n = 4) forPlg(+/+) mice].15 Additional studies using fluorescent-labeled peritoneal macrophages injected intraperitoneally into thioglycollate stimulated Plg(+/+) andPlg(−/−) mice indicated that the observed difference in monocyte recruitment was not attributable to differences in recovery of macrophages in Plg(+/+) andPlg(−/−) mice (data not shown).
However, a minor component of the inflammatory response in this model sustained recruitment of lymphocytes also was compromised in plasminogen-deficient mice: 1.06 ± 0.15 × 106 (n = 13) for Plg(+/+) mice versus 0.66 ± 0.11 × 106 (n = 11) for Plg(−/−) mice at 72 hours poststimulation P < .05.
Blood leukocyte levels after peritoneal thioglycollate stimulation.
To determine if diminished levels of peritoneal macrophages are caused by a diminished source of monocytes in circulation, cell differentials were performed on blood collected from Plg(+/+),Plg(+/−), and Plg(−/−) mice during the inflammatory response. The early increase in circulating neutrophils and monocytes is reflective of a response to the inflammatory agent. This effect is seen most dramatically in the neutrophil response (Fig 3A). The slightly increased neutrophil levels in the peritoneum ofPlg(−/−) relative to Plg(+/+) mice, at 6 hours poststimulation, may be the result of increased levels of these cells in circulation. However, the diminished response in monocyte recruitment into the peritoneum cannot be explained by a diminished source in circulation (Fig 3B). In addition, the enhanced levels of monocytes in blood coincident with inflammation inPlg(−/−) mice would suggest that accumulation of monocytes in peripheral circulation is unaffected by plasminogen deficiency.
Blood cell differentials 0 to 96 hours after peritoneal thioglycollate stimulation. (A) Blood neutrophils and (B) blood monocytes from Plg(+/+) and Plg(−/−) mice. (-▪-) Plg(+/+) and (-▴-)Plg(−/−). Blood cell levels from unstimulated mice are represented as t = 0. Values represent mean ± SEM. Monocyte P < .05 at 2 and 24 hours poststimulation using Student's t-test for Plg(+/+) andPlg(−/−) mice. Neutrophils P < .05 at all timepoints poststimulation using Student's t-test forPlg(+/+) and Plg(−/−) mice .
Blood cell differentials 0 to 96 hours after peritoneal thioglycollate stimulation. (A) Blood neutrophils and (B) blood monocytes from Plg(+/+) and Plg(−/−) mice. (-▪-) Plg(+/+) and (-▴-)Plg(−/−). Blood cell levels from unstimulated mice are represented as t = 0. Values represent mean ± SEM. Monocyte P < .05 at 2 and 24 hours poststimulation using Student's t-test for Plg(+/+) andPlg(−/−) mice. Neutrophils P < .05 at all timepoints poststimulation using Student's t-test forPlg(+/+) and Plg(−/−) mice .
Microscopy of peritoneum and macrophages.
During the inflammatory process in the peritoneum, the mesothelial lining of the cavity transforms from a nonadhesive to an adhesive surface.21,22 Eventually fibrinous adhesions are found to accumulate on the mesothelial surface and are eventually resolved by mesothelium-derived proteases.23 A lack of resolution of these adhesions could potentially affect migration of inflammatory cells. Analysis of peritoneal cavity lining of Plg(+/+) andPlg(−/−) mice at the light microscopic level indicated development of fibrinous adhesions associated with the mesothelium as early as 6 hours poststimulation. These adhesions appeared patchy and loosely associated with the cavity wall. Electron microscopic analysis of recruited macrophages did not show fibrin on the cell surface.
Macrophage phagocytic function.
Because macrophages were the most prominent cell type affected by plasminogen deficiency during an inflammatory response, an analysis of their function in Plg(+/+) and Plg(−/−) mice was made. For this purpose, phagocytic potential was assessed using zymosan particles. The percent of macrophages that ingested zymosan [83.00 ± 1.25 (n = 3) for Plg(+/+) mice versus 82.67 ± 1.79 (n = 3) for Plg(−/−) mice] as well as the number of particles per phagocytic cell [9.89 ± 0.21 (n = 3) for Plg(+/+) mice versus 10.65 ± 0.31 (n = 3) forPlg(−/−) mice] were similar between the two genotypes. This would indicate that a macrophage function, in this case phagocytic potential, is not affected by plasminogen deficiency.
DISCUSSION
Proteases play a major role in the inflammatory response by contributing to tissue injury and remodeling as well as regulating the activation and function of inflammatory cells. The plasminogen system has been implicated in playing a role in the inflammatory response by virtue of the capacity of inflammatory cells to synthesize and assemble components of the system on their surface.4,7,8,24 In addition, the main components of the fibrinolytic system, plasminogen, plasminogen activators, and inhibitors have been identified in exudates and extracts of inflamed tissues.25 26
To directly analyze plasminogen's involvement in the inflammatory response this study used a classic acute inflammatory model.Plg(+/+), Plg(+/−), andPlg(−/−) mice were exposed to the inflammatory agent, thioglycollate, through intraperitoneal injection, and the cellular response quantitated. Total leukocyte recruitment indicated that the initial response was equivalent between the genotypes but a sustained response was compromised in the absence of plasminogen.
An analysis of the specific cellular response indicated that the kinetics of recruitment as well as the quantitative response of neutrophils was equivalent between the different genotypes and occurred early during inflammation.27 Prior studies have shown that neutrophils are able to degrade subendothelial matrix via an elastase-dependent process, and therefore, plasminogen may play a less significant role in mechanisms involved in neutrophil recruitment during an inflammatory response.27 The early but brief neutrophil response during inflammation is consistent with other studies.4 It is known that the lifespan of neutrophils in tissues is short the result of emigration via the lymphatics or apoptosis leading to phagocytosis by macrophages.28 29 At later timepoints after stimulation, the predominant recruited cell types are monocytes that continue to migrate to the site and eventually differentiate into long-lived macrophages.
An analysis of the monocyte response indicated that monocyte recruitment is severely compromised in Plg(−/−) mice and intermediate in Plg(+/−) mice suggesting a gene dosage dependency of the response. Macrophages are capable of generating procoagulant and fibrinolytic activity and the coordinate expression of these two activities may contribute to inflammatory and healing processes by regulating fibrin turnover.4,30 In addition, both plasmin and plasmin-derived fibrin(ogen) degradation products have been identified as leukocyte chemoattractants, and therefore, diminished levels of these chemoattractants may partly explain impaired monocyte recruitment.31-33
During peritoneal inflammation the mesothelial cells that line the interior cavity of the peritoneum become activated and fibrinolytic activity is temporarily diminished resulting in deposition of fibrin and collagen fibrils on the peritoneal surface. Eventually, the plasminogen activating activity of the peritoneal mesothelium leads to fibrinolysis and degradation of extracellular matrix protein. In vitro studies assessing macrophage mediated extracellular matrix degradation in u-PA (−/−) and t-PA (−/−) mice indicated a dependence on Plg and, thus, would implicate Plg in cell migratory events associated with extracellular matrix degradation.34In addition, the observed presence of patchy fibrin-like adhesions along the peritoneal cavity could potentially effect transmesothelial migration into the cavity.
Levels of macrophage colony forming cells after thioglycollate stimulation are dependent on the presence of promonocyte bone marrow cells and circulating monocytes.35 During an acute inflammatory response in the peritoneal cavity both peritoneal macrophages and monocytes in peripheral blood increase in number.36 Therefore, an analysis of blood monocyte levels was performed to determine whether diminished recruitment was reflective of a diminished source in Plg(−/−) mice. Circulating levels of monocytes were comparable betweenPlg(+/+) and Plg(−/−) mice and even slightly elevated in Plg(−/−) mice during the timecourse of the study. In addition, the comparable kinetics of an increase in circulating monocytes between Plg(+/+) andPlg(−/−) mice after thioglycollate stimulation would appear to suggest that emigration of monocytes from bone marrow is not affected by plasminogen deficiency.
Plasminogen deficiency may result in diminished recruitment of subpopulations of monocytes or lymphocytes. It has been shown that the recruitment of CD4+ population of T lymphocytes is specifically affected in u-PA–deficient mice during pulmonarycryptococcus infection13; and, although lymphocytes are not a prominent cell type in this peritoneum inflammatory model migration of lymphocytes appear to be compromised inPlg(−/−). An assessment of macrophage phagocytic function, a property developed early during differentiation, was made between Plg(+/+) and Plg(−/−) mice and was found to be comparable.
While the mechanisms of inflammatory cell recruitment may vary depending on the specific tissue target site, as observed in the P-Selectin/ICAM-1 double mutant mice,37 this study directly shows a role for plasminogen in an inflammatory response specifically in processes that regulate sustained leukocyte recruitment. Additional studies assessing the response to other peritoneal stimulants as well as the inflammatory response to other sites are currently ongoing.
ACKNOWLEDGMENT
We thank Dr Meredith Bond, Dr Kandice Marchant, John Gabrovsek, and Frankie Whaller for assistance in microscopy studies and Suzanne Turner and Gene Lazuta for assistance in preparing this manuscript.
Supported by National Institutes of Health Grant No. HL17964 and Human Frontiers of Science Grant No. RG 363/95M.
Address reprint requests to Victoria A. Ploplis, PhD, Joseph J. Jacobs Center for Thrombosis and Vascular Biology, Cleveland Clinic Foundation, 9500 Euclid Ave, Cleveland, OH 44195.
The publication costs of this article were defrayed in part by page charge payment. This article must therefore be hereby marked “advertisement” in accordance with 18 U.S.C. section 1734 solely to indicate this fact.


This feature is available to Subscribers Only
Sign In or Create an Account Close Modal