Abstract
Antiprothrombin and anti–β2-glycoprotein I (β2-GPI) antibodies belong to the family of antiphospholipid (APL) antibodies and represent the phospholipid-dependent inhibitors of coagulation. They may be distinguished by analyzing the coagulation profiles generated by the comparison of the ratios of two coagulation tests, the Kaolin Clotting Time (KCT) and the dilute Russell's Viper Venom Time (dRVVT), commonly adopted for their diagnosis. The KCT profile is caused by antiprothrombin antibodies, whereas anti–β2-GPI antibodies are responsible for the dRVVT coagulation profile. The presence of aPL antibodies is frequently associated with acquired resistance to activated Protein C (APC-R), but limited information is available regarding the role of the different antibodies in its development. We studied the time-course of activated Factor V (FVa) generation and inactivation in the plasma of 42 patients with well-defined phospholipid-dependent inhibitors of coagulation: 24 displayed the dRVVT coagulation profile, whereas the other 18 cases showed the KCT profile. In normal pooled plasma, the peak values of FVa (mean ± standard deviation, [SD]: 16.307 ± 4.372 U/mL) were reached in 4 to 5 minutes and an almost complete inactivation (0.088 ± 0.123 U/mL) was obtained within 20 minutes. At this time point, values of residual FVa exceeding 2 SD the mean of controls (0.344 U/mL) were considered abnormal. Patients belonging to the KCT coagulation profile group reached the maximal amount of FVa in plasma (22.740 ± 7.693 U/mL, P = not significant v controls) within 4 to 5 minutes; at 20 minutes, the residual amount of FVa in plasma ranged from 0 to 1.09 U/mL (0.293 ± 0.298; P = .027), but it was found abnormal in only six of the 18 cases. The time-course of FVa in plasma of patients belonging to the dRVVT coagulation profile group differed from that of normal controls in that the peak values (10.955 ± 5.092 U/mL) were reached at 10 minutes and the amount of residual FVa at 20 minutes ranged from 0.320 to 14.450 U/ml (2.544 ± 3.580 U/mL;P = .0191 v normal controls and P = .0114v KCT group patients). Twenty of the 24 patients belonging to the dRVVT profile group had an abnormal inactivation of FVa (χ2 = 0.001 v KCT group patients). History of venous thrombosis was experienced by 15 patients: an abnormal rate of FVa inactivation was found in 11 of them (73%) versus 15 of the 27 cases without thrombosis (56%) (x2= 0.2556). The effect of affinity-purified IgG phospholipid-dependent inhibitors of coagulation on the time-course of FVa generation and inactivation in normal plasma was also investigated. Anti–β2-GPI, but not antiprothrombin antibodies, hampered the inactivation of FVa by endogenous APC, thus reproducing the behavior of the original plasmas. This effect was strictly β2-GPI–dependent. In conclusion, our findings confirm that anti–β2-GPI antibodies identify patients with phospholipid-dependent inhibitors of coagulation at increased risk of thrombosis and suggest acquired APC-R as a possible explanation of the pathogenesis of the thromboembolic events.
ANTIPHOSPHOLIPID (APL) antibodies represent a large group of autoantibodies that comprise, among the others, two phospholipid-dependent inhibitors of coagulation: anti–β2-glycoprotein I (β2-GPI)1-4 and antiprothrombin antibodies.5 Their nature may be identified on the basis of the distinctive coagulation profiles generated by the comparison of the ratios of two in-house assays, the Kaolin Clotting Time (KCT) and the diluted Russell's Viper Venom Time (dRVVT), thus avoiding the purification and characterization of the antibodies.6
The clinical relevance of APL antibodies resides in their association with arterial and venous thrombosis, recurrent miscarriages, and thrombocytopenia, which defines the so-called Antiphospholipid Syndrome (APS).7 Venous thrombosis is particularly frequent, as it occurs in about 20% of the patients.7 APL antibodies have been suggested to be involved in the development of the thromboembolic events and several hypotheses have been put forward. Among them, the interference of APL antibodies with the anticoagulant activity of the Protein C pathway has been studied by several investigators, who showed that APL antibodies inhibit the inactivation of activated Factor V (FVa) by activated Protein C (APC) on a phospholipid surface.8-12 The term “acquired” resistance to APC (APC-R) has been recently coined to identify this condition,13 which could explain, at least in part, the increased risk of venous thromboembolism of patients with APL antibodies. At present, it is unclear whether the “acquired” APC-R is a common property of all APL antibodies or pertains to a few antibody subsets. Therefore, in the present study, we have investigated the time-course of FVa generation and subsequent inactivation by endogenous APC in the plasma of a large group of patients with well-characterized phospholipid-dependent inhibitors of coagulation. The effect of affinity-purified anti–β2-GPI and antiprothrombin antibodies on the time-course of FVa generation and inactivation in normal plasma was also studied.
MATERIALS AND METHODS
Patients.
Forty-two patients with APL antibodies were included in the present study. They were 8 men and 34 women, aged 19 to 76 years (median, 41 years). Only patients with persistently positive antibodies were enrolled. Patients with APL antibodies developed during or after an infectious disease were excluded. APL antibodies were associated with an underlying disease in 22 cases: systemic lupus erythematosus (SLE)/SLE-like diseases (n = 9), other autoimmune diseases (n = 6; vasculitis, autoimmune hemolytic anemia, rheumatoid arthritis, primary adrenocortical deficiency and two cases of Basedow's disease), other diseases (n = 7; thrombotic thrombocytopenic purpura, two cases of non-Hodgkin's lymphoma, two cases of chronic liver disease, and two cases of epilepsy). At the time of the study, seven patients were thrombocytopenic (platelet count <150 × 109/L). Eleven patients (32%) experienced at least two spontaneous, sequential miscarriages. APL antibodies were idiopathic in the other 20 patients. Clinical history was positive for thrombotic events in 21 patients (50%): 15 of them experienced at least one deep vein thrombosis and/or pulmonary embolism; peripheral arterial thrombosis, ischemic stroke and/or transient ischemic attacks were diagnosed in six cases. Diagnostic methods used for detecting thrombosis were ultrasonography or venography for deep vein thrombosis, radionuclide lung scanning or angiography for pulmonary embolism, arteriography for peripheral arterial occlusions, computerized tomography scan, magnetic resonance imaging, or angiography for cerebral thrombosis. The diagnosis of cerebral transient ischemic attack required a focal neurologic deficit resolved within 24 hours. Thirteen patients were on oral anticoagulation at the time of the study. Primary APS was diagnosed in 17 patients. Twenty normal, apparently healthy, subjects represented the control group.
Diagnosis of the phospholipid-dependent inhibitors of coagulation.
Venous blood was collected in plastic tubes containing one tenth volume of 3.8% sodium citrate and centrifuged at 2,500g for 20 minutes to obtain platelet-poor plasma. Plasma was divided in small aliquots and stored at −70°C until use. The revised criteria proposed by the Scientific Standardization Committee Subcommittee for Standardization of Lupus Anticoagulants were used for the diagnosis of the phospholipid-dependent inhibitors of coagulation.14 At the time of the study, all patients satisfied these criteria. Each test was performed in duplicate on a manual coagulometer and the mean values of the two determinations were used.15 Ratios were calculated by dividing the coagulation time of patients' plasma by that of normal pooled plasma.
Coagulation profiles were generated for each patient's plasma6: when the ratio of the KCT exceeded that of the dRVVT, the patient was allocated to the KCT coagulation profile group. In the opposite case, the patient was allocated to the dRVVT coagulation profile. We recently showed that the former profile is associated with the presence of antiprothrombin antibodies, whereas the latter one is caused by the presence of anti–β2-GPI antibodies.6 Both the KCT and the dRVVT were performed by “in-house” techniques.
Measurement of anti–β2-GPI antibodies. IgG and IgM anti–β2-GPI antibodies were measured essentially according to the enzyme-linked immunosorbent assay (ELISA) procedure described by Loizou et al16 for anticardiolipin (aCL) antibodies. IgG and IgM were expressed as G-antiphospholipid (GPL) and M-antiphospholipid (MPL) units according to Harris et al,17 1 U being equivalent to 1 μg of affinity-purified aCL antibodies/mL of sample. Values exceeding 15 GPL or MPL U were considered abnormal.
IgG and IgM anti–β2-GPI antibodies were also evaluated for their binging to β2-GPI in solid phase.18 Results were expressed as absorbance at 405 nm. Values exceeding 2 standard deviation (SD) the mean of controls (ie, 283 and 121 m-optical density [mOD] for IgG and IgM anti–β2-GPI antibodies, respectively) were considered abnormal.
Human β2-GPI, purified according to Polz,19 was a kind gift of Dr E.M. Bevers (Dept. of Biochemistry, Cardiovascular Research Institute, Maastricht University, The Netherlands).
Detection of antiprothrombin antibodies.
The binding of IgG and IgM antibodies to human prothrombin in solid phase and complexed to phosphatidylserine via calcium ions was evaluated by ELISA, as previously described.20
Purification of IgGs.
Total IgG fractions were affinity-isolated from patients' and normal pooled plasmas over Protein A-sepharose CL-4B (Pharmacia Fine, Uppsala, Sweden). Affinity-purified IgG fractions containing high levels of anti–β2-GPI antibodies were prepared as previously described.2 The protein content of the preparations was assayed according to Sedmak and Grossberg.21
Evaluation of anticoagulant activity of IgG immunoglobulins.
The anticoagulant activity of IgG was evaluated by dRVVT both in normal human plasma and in β2-GPI–depleted plasma (prepared according to Galli et al20) as previously described.20 IgG were evaluated also by diluted activated partial thromboplastin time (aPTT) as follows: 50 μL of plasma were mixed with 25 μL IgG and 25 μL Thrombofax (Ortho, Raritan, NJ; 1:10 in Tris-buffered saline [TBS]). After a 3-minute incubation at 37°C, 50 μL 25 mmol/L CaCl2 was added to the mixture and the time to form the clot recorded. Each test was performed in duplicate and the results expressed as the mean of the two values. In the coagulation tests, total IgG preparations were used at the concentration of 5 mg/mL and anti–β2-GPI antibodies at the concentration of 0.5 mg/mL.
Assessment of FVa generation and inactivation in plasma.
The generation and inactivation of FVa was analyzed in defibrinogenated plasma essentially according to Marciniak and Romond.9 The effect of total IgG and aCL antibodies was evaluated in a similar way, on mixing 50 μL of normal pooled plasma with 25 μL of IgG instead of TBS. Total IgG preparations were used at the concentration of 5 mg/mL and anti–β2-GPI antibodies at the concentration of 0.5 mg/mL. Some experiments were similarly performed using β2-GPI–depleted human plasma.
Assessment of the R506Q (Factor V Leiden) mutation.
Genomic DNA was prepared from citrated blood by standard procedures. Mutant Factor V was detected by amplification of the Factor V gene by polymerase chain reaction (PCR) and digestion of the fragment with Mnl1.22 None of the patients with APL antibodies enrolled in the study were found to be carriers of the mutation. The plasmas of six carriers (heterozygous, n = 5; homozygous, n = 1) were selected for studying the time-course of FVa generation and inactivation.
Measurement of Protein C and Protein S.
Antigenic levels of Protein C and Protein S were measured by Asseraplate (Stago, Asnieres, France), according to the manufacturer's instructions. Their function was assessed by Protein C Reagent (Istituto Behring, Scoppito, Italy) and by Protein S Clotting-Test (Stago), according to the manufacturer's instructions. They were found normal in all patients who were not receiving oral anticoagulant treatment at the time of the study.
Statistical analysis.
The χ2 test and the Student's t-test for unpaired data were used: a P value < .05 was considered statistically significant.
RESULTS
Characterization of the coagulation profiles of patients with phospholipid-dependent inhibitors of coagulation.
Forty-two patients with phospholipid-dependent inhibitors of coagulation were enrolled in the study. The comparison of their coagulation profiles6 identified 24 patients to belong to the dRVVT group and the other 18 to the KCT group. The comparison was performed on the 1:1 mixture of patients' with normal pooled plasma in the 13 cases under oral anticoagulation.
The main clinical and laboratory data of the patients are reported in Table 1. The two groups differ with respect to both the prevalence and the titers of anti–β2-GPI antibodies (measured as aCL antibodies), which were significantly higher in the dRVVT profile group. When β2-GPI was used as the solid phase antigen, a clear trend towards a higher prevalence of anti–β2-GPI antibodies was observed in the dRVVT group, although statistical significance was not reached. The retrospective analysis of the clinical history of the patients showed that thromboembolic complications were statistically associated with the dRVVT profile (P = .0018).
Clinical and Laboratory Characteristics of 42 Patients With Phospholipid-Dependent Inhibitors of Coagulation Classified According to Their Coagulation Profile
| . | Coagulation Profile . | P . | |
|---|---|---|---|
| dRVVT (n = 24) (%) . | KCT (n = 18) (%) . | ||
| Age (yrs) | |||
| Mean ± SD | 41.5 ± 12.8 | 47.5 ± 16.8 | NS |
| Median; range | 39; 19-70 | 43; 20-76 | |
| Sex | |||
| M/F | 8/16 | 0/18 | .0065 |
| Anti–β2-GPI antibodies | |||
| Cardiolipin in solid-phase | 24 (100) | 13 (72) | .0059 |
| Mean ± SD (GPL and/or MPL units) | 122 ± 61 | 46 ± 52 | .0001 |
| β2-GPI in solid-phase | 16 (67) | 7 (39) | NS |
| Associated diseases | 12 (50) | 10 (56) | NS |
| SLE/SLE-like | 5 | 4 | |
| Other autoimmune disorders | 4 | 2 | |
| Other diseases | 3 | 4 | |
| Thrombosis | 17 (71) | 4 (22) | .0018 |
| Venous | 13 | 2 | .004 |
| Arterial | 4 | 2 | |
| Recurrent abortions (≥2) | 6 (25) | 5 (28) | NS |
| Thrombocytopenia | 2 (8) | 5 (28) | NS |
| . | Coagulation Profile . | P . | |
|---|---|---|---|
| dRVVT (n = 24) (%) . | KCT (n = 18) (%) . | ||
| Age (yrs) | |||
| Mean ± SD | 41.5 ± 12.8 | 47.5 ± 16.8 | NS |
| Median; range | 39; 19-70 | 43; 20-76 | |
| Sex | |||
| M/F | 8/16 | 0/18 | .0065 |
| Anti–β2-GPI antibodies | |||
| Cardiolipin in solid-phase | 24 (100) | 13 (72) | .0059 |
| Mean ± SD (GPL and/or MPL units) | 122 ± 61 | 46 ± 52 | .0001 |
| β2-GPI in solid-phase | 16 (67) | 7 (39) | NS |
| Associated diseases | 12 (50) | 10 (56) | NS |
| SLE/SLE-like | 5 | 4 | |
| Other autoimmune disorders | 4 | 2 | |
| Other diseases | 3 | 4 | |
| Thrombosis | 17 (71) | 4 (22) | .0018 |
| Venous | 13 | 2 | .004 |
| Arterial | 4 | 2 | |
| Recurrent abortions (≥2) | 6 (25) | 5 (28) | NS |
| Thrombocytopenia | 2 (8) | 5 (28) | NS |
Abbreviation: NS, not significant.
Evaluation of acquired APC-R: time-course of FVa generation and inactivation in plasma.
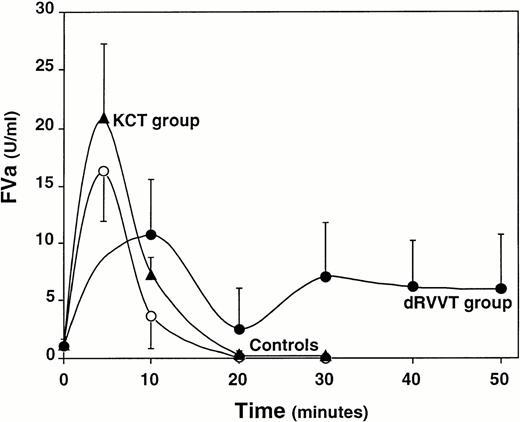
First, the time-course of FVa generation and inactivation was evaluated in normal pooled plasma and in plasma of nine normal controls. The peak values of FVa were reached within 4 to 5 minutes from calcium addition (mean ± SD, 16.307 ± 4.372 U/mL) and an almost complete inactivation by endogenous APC was achieved at 20 minutes (mean ± SD, 0.088 ± 0.123 U/mL) (Fig 1). Levels of residual FVa in plasma at 20 minutes exceeding 2 SD the mean of controls (ie, 0.334 U/mL) were considered abnormal. In six patients with the R506Q mutation (Factor V Leiden), the maximal amount of FVa generated in plasma (mean ± SD, 32.537 ± 29.624 U/mL) was approximately twice that of normal subjects (P = .3173). In all cases, the residual amount of FVa at 20 minutes was higher than that of the control group (range, 0.370 to 14.120 U/mL; mean ± SD, 5.352 ± 5.965 U/mL; P = .004). Therefore, the assay could easily distinguish carriers of the R506Q mutation, responsible for the APC-R, from normal individuals.
Time-course of FVa generation and inactivation in the plasma of normal controls (○), 18 patients belonging to the KCT coagulation profile group (▴), and 24 patients belonging to the dRVVT coagulation profile group (•). Results are presented as the mean values of the three groups for each time point; bars represent 1 SD.
Time-course of FVa generation and inactivation in the plasma of normal controls (○), 18 patients belonging to the KCT coagulation profile group (▴), and 24 patients belonging to the dRVVT coagulation profile group (•). Results are presented as the mean values of the three groups for each time point; bars represent 1 SD.
Subsequently, the time-course of FVa generation and inactivation was measured in the plasma of 42 patients with phospholipid-dependent inhibitors of coagulation. In the 13 cases under warfarin treatment at the time of the study, this measurement was performed in the 1:1 mixture of patients' with normal pooled plasma. This was based on the results of experiments performed in plasmas of seven patients under stable oral anticoagulation for valvular heart prosthesis without APL antibodies or the R506Q mutation: the increased levels of residual FVa at 20 minutes measured in the undiluted plasmas (range, 6.500 to 8.140 U/mL; mean ± SD, 7.413 ± 0.605 U/mL, P = .0001v controls) came down to 0.130 to 0.610 U/mL in the 1:1 mixture with normal pooled plasma. Therefore, levels of FVa exceeding the upper value of this range (ie, 0.610 U/mL) were considered abnormal for APL patients under oral anticoagulation.
In the 42 patients with APL antibodies, the peak values of FVa generated in plasma (14.173 ± 13.523 U/mL) were similar to those of normal plasmas. Conversely, abnormal rates of FVa inactivation by endogenous APC were observed in 26 cases (62%), indicative for the acquired APC-R. Eleven of them (42%) experienced venous thromboembolic events versus only four of the 16 patients (25%) with a normal rate of FVa inactivation. There was a trend towards a correlation between the acquired APC-R and deep vein thrombosis, which did not reach statistical significance (P = .2556).
The time-course of FVa generation and inactivation was analyzed according to the coagulation profiles of the 42 patients (Table 1 and Fig 1). The prevalence of the acquired APC-R was significantly higher in patients belonging to the dRVVT profile group (83%) compared with those of the KCT profile group (33%) (χ2 = 0.001). The level of FVa residual at 20 minutes measured in the plasma of patients belonging to the dRVVT group (range, 0.320 to 14.450 U/mL; mean ± SD, 2.544 ± 3.580 U/mL) was signficantly higher than that of both the control group (P = .0191) and that of patients belonging to the KCT coagulation profile group (range, 0.0 to 1.090 U/mL; mean ± SD, 0.293 ± 0.298 U/mL) (P = .0114). When compared with controls, the KCT group patients also had an abnormal inactivation of FVa (P = .027), even though the levels of residual FVa at 20 minutes of the six pathologic cases ranged from 0.43 to only 1.090 U/mL. No correlation between anti–β2-GPI antibody titers and the levels of residual FVa in plasma was found.
Effect of anti–β2-GPI and antiprothrombin antibodies on the time-course of FVa generation and inactivation in normal and in β2-GPI–depleted plasma.
IgG preparations were isolated from the plasma of eight patients with phospholipid-dependent inhibitors of coagulation. They were three IgG affinity-purified by means of cardiolipin-containing liposomes (Table 2, numbers 1-3) and five total IgG fractions (Table 2, numbers 4-8). Their behavior in ELISA assays for anti–β2-GPI and antiprothrombin antibodies, as well as their effect on the dRVVT and diluted aPTT performed in normal pooled plasma and in β2-GPI–deficient plasma is presented in Table 2. The first four IgG preparations had high titers of anti–β2-GPI and antiprothrombin antibodies measured by ELISA and were able to inhibit the dRVVT and the dilute aPTT performed in normal plasma, but not in β2-GPI–deficient plasma. Therefore, they were classified as anti–β2-GPI antibody preparations (formerly defined aCL-type A antibodies).6 IgG preparations number 5-8 had low titers of anti–β2-GPI antibodies, high content of antiprothrombin antibodies, and displayed anticoagulant activity in both a dRVVT and dilute aPTT system, irrespective of the presence or absence of β2-GPI in plasma. Therefore, they were classified as antiprothrombin antibodies.6
Characterization of the Immunologic and Anticoagulant Properties of Purified Anti–β2-GPI and Antiprothrombin IgG Antibodies
| APL Antibodies . | aβ2-GPI (GPL U) . | aHPT (mOD) . | aHPT/PS (mOD) . | Anticoagulant Activity (s) . | ||
|---|---|---|---|---|---|---|
| daPTT . | dRVVT . | dRVVT . | ||||
| (normal pooled plasma) . | (β2-GPI–deficient plasma) . | |||||
| Anti–β2-GPI | ||||||
| 1. | 170 | 1,923 | 1,817 | 94.8 | 47.6 | 51.3 |
| 2. | 96 | 1,881 | 2,012 | 67.6 | 35.7 | 49.9 |
| 3. | 100 | 802 | 1,071 | 67.9 | 41.7 | 50.7 |
| 4. | 25 | 1,418 | 544 | 61.5 | 35.8 | 51.0 |
| Antiprothrombin | ||||||
| 5. | 1 | 444 | 378 | 89.1 | 40.5 | 59.4 |
| 6. | 1 | 976 | 1,007 | 84.9 | 39.2 | 73.4 |
| 7. | 2 | 1,074 | 424 | 70.7 | 32.8 | 67.2 |
| 8. | 2 | 1,098 | 501 | 89.1 | 40.8 | 76.2 |
| Normal IgG | 1 | 238 | 256 | 57.5 | 28.5 | 49.9 |
| APL Antibodies . | aβ2-GPI (GPL U) . | aHPT (mOD) . | aHPT/PS (mOD) . | Anticoagulant Activity (s) . | ||
|---|---|---|---|---|---|---|
| daPTT . | dRVVT . | dRVVT . | ||||
| (normal pooled plasma) . | (β2-GPI–deficient plasma) . | |||||
| Anti–β2-GPI | ||||||
| 1. | 170 | 1,923 | 1,817 | 94.8 | 47.6 | 51.3 |
| 2. | 96 | 1,881 | 2,012 | 67.6 | 35.7 | 49.9 |
| 3. | 100 | 802 | 1,071 | 67.9 | 41.7 | 50.7 |
| 4. | 25 | 1,418 | 544 | 61.5 | 35.8 | 51.0 |
| Antiprothrombin | ||||||
| 5. | 1 | 444 | 378 | 89.1 | 40.5 | 59.4 |
| 6. | 1 | 976 | 1,007 | 84.9 | 39.2 | 73.4 |
| 7. | 2 | 1,074 | 424 | 70.7 | 32.8 | 67.2 |
| 8. | 2 | 1,098 | 501 | 89.1 | 40.8 | 76.2 |
| Normal IgG | 1 | 238 | 256 | 57.5 | 28.5 | 49.9 |
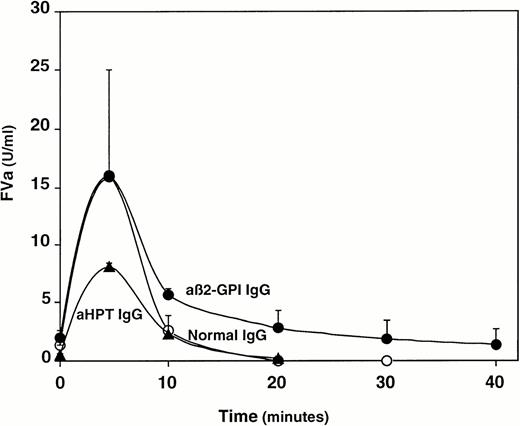
The effect of the eight IgG fractions on the time-course of FVa generation and inactivation was studied in normal pooled plasma (Fig 2): anti–β2-GPI antibodies (Table 3, preparations number 1-4), but not antiprothrombin antibodies (Table 3, preparations 5-8), were able to hamper the inactivation of FVa by endogenous APC. Thus, the different phospholipid-dependent inhibitors of coagulation reproduced the pattern displayed by the original plasmas. The acquired APC-R induced by anti–β2-GPI antibodies was clearly dependent on the IgG concentration (data not shown). It was also strictly β2-GPI–dependent, as the four IgG preparations containing anti–β2-GPI antibodies failed to cause acquired APC-R in β2-GPI–depleted plasma (Table 3).
Effect of isolated anti–β2-GPI IgG antibodies (•) (n = 4) and antiprothrombin antibodies (▴) (n = 4) on the time course of FVa generation and inactivation in normal pooled plasma. IgG from normal pooled plasma served as control IgG (○). Results are presented as the mean values for each time point; bars represent 1 SD.
Effect of isolated anti–β2-GPI IgG antibodies (•) (n = 4) and antiprothrombin antibodies (▴) (n = 4) on the time course of FVa generation and inactivation in normal pooled plasma. IgG from normal pooled plasma served as control IgG (○). Results are presented as the mean values for each time point; bars represent 1 SD.
Effect of Purified APL Antibodies on the Inactivation of FVa in Normal and β2-GPI–Deficient Plasma
| APL Antibodies . | Residual FVa at 20 Minutes (U/mL) . | |
|---|---|---|
| Normal . | β2-GPI–Deficient Plasma . | |
| Anti–β2-GPI | ||
| 1. | 3.02 | 0.53 |
| 2. | 1.17 | 0.77 |
| 3. | 4.78 | 0.63 |
| 4. | 2.45 | 0.74 |
| Antiprothrombin | ||
| 5. | 0.50 | ND |
| 6. | 0.12 | ND |
| 7. | 0.25 | ND |
| 8. | 0.06 | ND |
| Normal IgG | 0.01 | 0.93 |
| APL Antibodies . | Residual FVa at 20 Minutes (U/mL) . | |
|---|---|---|
| Normal . | β2-GPI–Deficient Plasma . | |
| Anti–β2-GPI | ||
| 1. | 3.02 | 0.53 |
| 2. | 1.17 | 0.77 |
| 3. | 4.78 | 0.63 |
| 4. | 2.45 | 0.74 |
| Antiprothrombin | ||
| 5. | 0.50 | ND |
| 6. | 0.12 | ND |
| 7. | 0.25 | ND |
| 8. | 0.06 | ND |
| Normal IgG | 0.01 | 0.93 |
Abbreviation: ND, not determined.
DISCUSSION
Interference of APL antibodies with the anticoagulant activity of the PC system has been reported by several investigators.8-13Along with the resistance to APC displayed by plasmas from patients carrying the R506Q mutation (ie, the FV Leiden),22 the term acquired APC-R has been recently coined to define this property,13 which may, in part, explain the increased thrombotic risk of patients with APL antibodies. The prevalence of this acquired APC-R has not yet been clearly established because single cases or small series of patients have been investigated so far and also because the type of assay used to evaluate the effect of APL antibodies on the anticoagulant activity of protein C (PC) system greatly influences the results. Here, we have shown impairment of the inactivation of FVa by endogenous APC in approximately two thirds of a large group of patients with phospholipid-dependent inhibitors of coagulation. Acquired APC-R could not be due to the presence of FV Leiden because none of the APL-positive patients enrolled in this study was found hetero- or homozygous for the R506Q mutation. The reported prevalence could neither be an overestimation due to the warfarin treatment received by a substantial number of cases. In fact, being aware of the effect of oral anticoagulants on PC function, we evaluated the inactivation FVa by APC in the 1:1 mixture of warfarin-treated patients' plasma with normal pooled plasma.
The assay used here to assess the time-course of FVa generation and inactivation in plasma is a two-stage, PTT-based test, which could easily discriminate plasmas of carriers of the R506Q mutation from those of normal controls. The interference of phospholipid-dependent inhibitors of coagulation with this type of assays is a well known in vitro phenomenon, which makes the results of the majority of the coagulation tests currently used to evaluate APC-R in APL-positive patients unreliable. The experimental conditions we used to assess the anticoagulant activity of APC in plasma allowed us to kinetically follow the generation and subsequent inactivation of FVa beyond clot formation.9 The peak values of FVa measured in our patients' plasmas and in control plasmas were similar; this makes unlikely the possibility that the reduced rate of FVa inactivation observed in the majority of our samples was due to incomplete FV activation by thrombin. Conversely, as we did not measure the level and activity of PC inhibitor and α1-antitrypsin in the patients' plasmas, we cannot exclude a possible influence of these inhibitors on the anticoagulant activity of APC. However, under conditions very similar to ours, Marciniak and Romond9 showed that the full inactivation of FVa requires the presence in its active form of no more than 2% of the PC available in plasma. The same investigators reported impairment or even abolishment of FVa inactivation in all of the 15 plasmas with phospholipid-dependent inhibitors of coagulation analyzed, due to IgG fractions containing the phospholipid-dependent inhibitors of coagulation.9 This elegant work was performed before it became evident that APL antibodies and, in particular, phospholipid-dependent inhibitors of coagulation, are heterogenous and display different specificities. Therefore, it does not provide information on whether the interference with the anticoagulant activity of APC is a common property of these inhibitors or it is expressed by specific subgroups. We approached this problem by analyzing the time-course of FVa generation and inactivation in plasma according to the coagulation profiles of the patients: acquired APC resistance was found to correlate with the dRVVT rather than with the KCT coagulation profile. We have recently shown that anti–β2-GPI antibodies are responsible for the former coagulation profile, whereas the latter one is due to the presence of antiprothrombin antibodies.6Moreover, the retrospective analysis of the clinical history of the patients showed that the dRVVT profile is statistically associated with an increased risk of thromboembolic complications.6 Our present data confirm the relationship between historical thrombosis and the dRVVT profile and provide a possible pathogenetic explanation of such an association. In fact, isolated anti–β2-GPI antibodies, but not antiprothrombin antibodies, were able to induce acquired APC-R, thus reproducing the procoagulant effect observed in the original plasmas. This effect of anti–β2-GPI antibodies was strictly β2-GPI–dependent, as it was abolished in plasma depleted of this protein. Similar results were recently reported by Matsuda et al,23 who demonstrated the ability of a rabbit anti–β2-GPI antibody to induce APC-R only when β2-GPI was present in plasma. β2-GPI has been shown to inhibit the inactivation of FVa by APC at physiologic concentrations of Protein S and FVa.24 This effect was proportional to the amount of β2-GPI present and was more pronounced at low phospholipid concentrations. Based on these data, β2-GPI was suggested to act via competition with APC for the binding to phospholipids.24Although they do not clarify the fine details of the acquired APC-R induced by anti–β2-GPI antibodies, these findings and our present data suggest the possibility that anti–β2-GPI antibodies may cause this phenomenon by enhancing the binding of β2-GPI to the phospholipid surface. Therefore, acquired APC-R would be due to the same mechanism responsible for the in vitro anticoagulant effect of anti–β2-GPI antibodies. Further experiments will be necessary to elucidate this point.
In contrast with Smirnov et al11 findings, the prevalence of acquired APC-R reported here cannot be explained by the ability of antiphosphatidylethanolamine (anti-PE) antibodies to interfere with the anticoagulant activity of APC by competing with this protein for the binding to PE. In fact, a survey of IgG anti-PE antibodies performed in our laboratory showed their presence in only 10% of APL-positive patients (unpublished observation). Also the possibility that β2-GPI competes with APC for the binding to PE seems unlikely because we failed to show binding of purified β2-GPI, either alone or in combination with anti–β2-GPI antibodies, to this phospholipid in solid phase (data not shown).
Despite the interference of anti–β2-GPI antibodies with the anticoagulant activity of APC, we did not find a correlation between acquired APC-R and the titers anti–β2-GPI antibodies measured in plasma either as aCL antibodies or using β2-GPI as solid phase antigen. Our data are at variance with those recently reported by Martinuzzo et al,12 who found that acquired APC-R in APL-positive patients was statistically associated with the presence of anti–β2-GPI antibodies rather than with that of the phospholipid-dependent inhibitors of coagulation. Once more, these conflicting results highlight the heterogeneity of the methodology used for the detection of APL antibodies, whose standardization is far from being reached. This is underlined also by the low prevalence of anti–β2-GPI antibodies we found in patients belonging to the dRVVT profile group using the ELISA with β2-GPI in solid phase. Theoretically, all of them would be expected to have anti–β2-GPI antibodies measured by this system. However, one must keep in mind that the identification of the different phospholipid-dependent inhibitors of coagulation by comparing the ratios of the dRVVT and the KCT is somewhat artificial, and no complete overlap with the ELISA's results is to be expected. Moreover, the higher prevalence of plasmas reacting with β2-GPI bound to cardiolipin (88%) than with β2-GPI directly bound to high-activated polyvinylchloride (PVC) plates (55%) closely resembles that recently reported for antiprothrombin antibodies,20 suggesting that the former ELISA system is more efficient than the latter one in identifying anti–β2-GPI antibodies. Interestingly, the titers of anti–β2-GPI antibodies (measured with cardiolipin in solid phase) were significantly higher in patients belonging to the dRVVT than to the KCT coagulation profile. The most likely explanation is that the presence of anti–β2-GPI antibodies, which are responsable for the former coagulation profile,6 is a necessary condition only in plasmas displaying the dRVVT profile. Indirectly, this is also supported by the higher prevalence of anti–β2-GPI antibodies in the dRVVT than in the KCT profile (Table 1).
In conclusion, the present report confirms the association between thromboembolic events and the anti–β2-GPI antibodies and shows their relationship with the impairment of the anticoagulant activity of the PC system. The resulting acquired APC-R might represent one of the possible pathogenetic mechanisms responsible for the thrombotic risk of this subgroup of APL-positive patients. It is also tempting to speculate that coagulation tests might appear more efficient than ELISAs in identifying the anti–β2-GPI antibodies that represent a possible risk factor for thromboembolic events. However, more work is required to improve the specificity of both coagulation tests and immunoassays for the detection of APL antibodies with respect to the clinical events of the antiphospholipid syndrome.
ACKNOWLEDGMENT
We wish to thank Drs E.M. Bevers and J. Rosing (Department of Biochemistry, Cardiovascular Research Institute Maastricht, Maastricht University, The Netherlands) for critically reviewing this manuscript. Dr E.M. Bevers is also acknowledged for kindly giving us β2-GPI–depleted plasma.
Address reprint requests to Monica Galli, MD, PhD, Divisione di Ematologia, Ospedali Riuniti, L.go Barozzi, 1, 24100 Bergamo, Italy.
The publication costs of this article were defrayed in part by page charge payment. This article must therefore be hereby marked “advertisement” in accordance with 18 U.S.C. section 1734 solely to indicate this fact.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal