Abstract
The ligand for flt-3 (FLT3L) exhibits striking structural homology with stem cell factor (SCF ) and monocyte colony-stimulating factor (M-CSF ) and also acts in synergy with a range of other hematopoietic growth factors (HGF ). In this study, we show that FLT3L responsive hematopoietic progenitor cells (HPC) are CD34+CD38−, rhodamine 123dull, and hydroperoxycyclophosphamide (4-HC) resistant. To investigate the basis for the capacity of FLT3L to augment the de novo generation of myeloid progenitors from CD34+CD38− cells, single bone marrow CD34+CD38− cells were sorted into Terasaki wells containing serum-free medium supplemented with interleukin-3 (IL-3), IL-6, granulocyte colony-stimulating factor (G-CSF ), SCF (4 HGF ) ± FLT3L. Under these conditions, FLT3L recruited approximately twofold more CD34+CD38− cells into division than 4 HGF alone. The enhanced proliferative response to FLT3L was evident by day 3 and was maintained at all subsequent time points examined. In accord with these findings, we also show that transduction of CD34+CD38− cells with the LAPSN retrovirus is enhanced by FLT3L. The results of these experiments therefore indicate that increased recruitment of primitive HPC into cell cycle underlies the ex vivo expansion potential of FLT3L and also its ability to improve retroviral transduction of HPC.
A KEY FEATURE OF THE hematopoietic system in mammals is its hierarchical organization consisting of sequential cell populations, which are classified as such on the basis of differences in their proliferative potential and capacity for differentiation.1 Much of the exquisite sensitivity and flexibility of function of the hematopoietic system to signalled needs can be explained by the existence of multiple regulatory mechanisms acting at all levels within the hierarchy. A major influence on hematopoiesis is effected by hematopoietic growth factors (HGF ), many of which are produced by stromal cells of the bone marrow microenvironment and exert either stimulatory or suppressive effects on hematopoietic cell growth.2-7
Of considerable interest are those HGF that influence the proliferation and development of primitive hematopoietic stem cells and their immediate progeny. Such factors are likely to be of importance in cellular therapies, which involve ex vivo manipulation of the hematopoietic system, such as gene therapy or the expansion of either transplantable cells or their mature myeloid progeny.8-13 These HGF include interleukin-1 (IL-1), IL-6, IL-11, IL-12, leukemia inhibitory factor (LIF ), and stem cell factor (SCF ). All are regarded as synergistic HGF, which act to enhance the stimulatory activity of other HGF such as IL-3, the colony-stimulating factors or erythropoietin (EPO) and exhibit little or no effect alone on proliferation in vitro.14-17 Importantly, the most primitive hematopoietic progenitors (HPC) exhibit an almost obligatory requirement for combined stimulation by multiple synergistic HGF to elicit proliferation.18-20 This is in contrast to committed progenitor cell populations, which although demonstrating synergistic responses to combinations of HGF, are generally stimulated to proliferate by single HGF.21
A particularly potent synergistic HGF is SCF whose actions have been documented on both very primitive HPC and their immediate lineage-restricted clonogenic progeny.17,18,22-24 The actions of SCF are mediated by the product of the c-kit proto-oncogene, a receptor tyrosine kinase (RTK), which is a member of the platelet-derived growth factor (PDGF ) receptor superfamily that includes c-fms.25,26 Recently, an additional member of this receptor superfamily has been identified, flt3/flk2.27 The highly restricted expression of the flt3/flk2 RTK in both murine and human HPC demonstrated by initial studies28-30 suggested that the ligand for this receptor would play an important role in regulating the growth and development of early hematopoietic cells. The ligand (FLT3L) has since been shown to exhibit striking structural homology with SCF and monocyte colony-stimulating factor (M-CSF ) and in keeping with this, show synergy with a range of other HGF including IL-3, IL-6, IL-7, IL-11, IL-12, granulocyte colony-stimulating factor (G-CSF ), and granulocyte-macrophage CSF (GM-CSF ).31 32
Despite studies by a number of investigators, the phenotypic and functional characteristics of HPC that respond to this ligand remain to be completely defined. To date, the majority of these studies have examined the synergy between FLT3L and other HGF on total CD34+ cells,33-35 although Shah et al36 have reported that bone marrow CD34+38− cells, but not CD34+38+ cells, respond to FLT3L and two recent publications by Petzer37 38 describe a response of CD34+38− cells to FLT3L alone and in combination with other HGF. Herein we report a series of studies that show that FLT3L responsive cells are hierarchically more primitive HPC, CD34+CD38−, rhodamine 123dull, and resistant to 4 hydroperoxycyclophosphamide (4-HC). Furthermore, by culture of single CD34+CD38− cells, we show that increased recruitment of these cells into cell cycle underlies the ex vivo expansion potential of FLT3L. Finally, we propose that FLT3 is expressed at low to undetectable levels on the most primitive HPC, but is present at higher levels on HPC that have proliferative potential intermediate between primitive HPC and committed progenitors.
MATERIALS AND METHODS
Hematopoietic growth factors.Recombinant human FLT3L was generously provided by Dr Doug Williams (Immunex Corp, Seattle, WA) and was used at 100 ng/mL in all experiments. IL-3, IL-6, G-CSF, and SCF were all generously supplied by Amgen Inc (Thousand Oaks, CA), and EPO was purchased from Janssen Cilag (Auckland, New Zealand). Unless otherwise stated, all HGF were used at predetermined optimal concentrations; IL-3, 10 ng/mL; IL-6, 20 ng/mL; G-CSF, 100 ng/mL; SCF, 100 ng/mL; and EPO, 4 U/mL.39
Cell separation.Human bone marrow (BM) cells were obtained by aspiration from the iliac crest or sternum of normal adult volunteers with informed consent and the approval of the Ethics Committee of the Royal Adelaide Hospital. Mononuclear cells (MNC) were isolated by Ficoll-Hypaque gradient centrifugation40 (Lymphoprep, Nycomed, Oslo, Norway), then enriched for immature hematopoietic progenitor cells by differential agglutination using the lectin SBA (Vector Laboratories, Burlingame, CA) as previously described.41 42 The SBA− cells were washed in phosphate-buffered saline supplemented with 200 mmol/L D-galactose (Sigma, St Louis, MO) to remove residual lectin, then with Hanks' Balanced Salt Solution (HBSS) supplemented with 20 mmol/L HEPES buffer (pH 7.3) (GIBCO BRL, Victoria, Australia) and 5% heat inactivated fetal calf serum (FCS; P.A. Biologicals, Sydney, Australia) (HHF ) before immunofluorescent labeling.
Peripheral blood MNC were obtained from cyropreserved apheresis collections from patients undergoing therapeutic blood cell mobilization induced by high-dose cyclophosphamide. The methods for isolation of MNC were identical to those described previously9 and cells were finally resuspended in HBSS-FCS.
Immunolabeling and isolation of cells.Immunostaining of SBA- BM and blood MNC was performed at 4°C with HPCA-2–fluorescein isothiocyanate (FITC) (anti-CD34) with or without and Leu 17 (anti-CD38), both purchased from Becton Dickinson (Mountain View, CA) and appropriate isotype control antibodies conjugated to FITC or phycoerythrin (PE). Unbound antibody was removed by two washes of HHF and cells were resuspended to approximately 107/mL before fluorescent-activated cell sorting (FACS) on a FACStarPlus flow cytometer (Becton Dickinson) equipped with an argon laser emitting 488 nm light at 250 mW. Bulk CD34+, CD34+CD38+, or CD34+CD38− cells within the lymphocyte/blast region43 were sorted into Iscove's modified Dulbecco's media (IMDM) supplemented with 5% heat inactivated FCS. For experiments involving transduction of CD34+ cells with LAPSN retrovirus, a population of CD34+ cells expressing intermediate levels of CD38 (referred to as CD34+CD38+/−) were also sorted. Single cells with these phenotypes were sorted directly into wells of Terasaki plates containing 10 μL of serum-free precolony-forming unit (CFU) media and HGF. Two-color labeling of SBA− BM cells for CD34 and the vital fluorescent dye rhodamine 123 (Rh123) was performed by first incubating the cells for 45 minutes at 37°C in HBSS containing Rh123 (Molecular Probes, Eugene, OR) at 0.1 μg/mL. Excess internalized Rh123 was removed by incubating the cells with HBSS-FCS alone for 15 minutes at 37°C and then washing cells. Incubation of the Rh123-labeled cells with HPCA-2–PE was then performed as described above. CD34+ cells were sorted according to their level of Rh123 retention as described previously.42 Rh123 dull (low retention of Rh123) represented approximately 10% of the CD34+ population and the Rh123bright represented the 30% of CD34+ cells exhibiting the highest Rh123 retention.
Expression of FLT3 receptor tyrosine kinase.The expression of FLT3 receptor tyrosine kinase on BM CD34+ cells was investigated by three-color immunolabeling using the phycoerythrin (PE)-conjugated antibody BV10.44 This anti-CD135 monoclonal antibody was raised by immunization of 4 to 8 week-old female Balb/c mice with the pro B-cell line, BV-173, obtained from the German Collection of Microorganisms and Cell Cultures, Branschwieg, Germany. The mice were injected five times intraperitoneally with 107 cells in 2-week intervals and 10 days after the last injection, an intrasplenic boost of 2 × 105 cells was applied. The spleens were removed 4 days later for fusion with the SP2/0 myeloma cell line. The resulting hybridomas were grown in RPMI 1640 (GIBCO) containing 10% FCS and hypoxanthine-aminopterin–thymidine (HAT; Sigma Chemicals, Munchen, Germany). Culture supernatants were screened on Ba/F3 cells transfected with the complete coding sequence of the human FLT3 cDNA,29 and hybridoma cells secreting antibodies selectively recognizing the transfectant cell line, but not the parental Ba/F3 cells, were cloned by limiting dilution. The clone was cultured on large scale in serum-free medium supplemented with 1% Nutridoma (Boehringer Mannheim, Mannheim, Germany) and antibody was purified from supernatants using T-Gel affinity columns (Bender & Hobein, Munich, Germany) as described by the manufacturer. The IgG1 isotype of the resulting monoclonal antibody, BV10, was determined by enzyme-linked immunosorbent assay (ELISA) (Boehringer Mannheim, Mannheim, Germany). Immunoprecipitation of Ba/F3-huFLT3 lysates resulted in the appearance of two bands of 130 and 155 kD, which correspond to the estimated molecular weights of differentially glycosylated FLT3 molecules.45
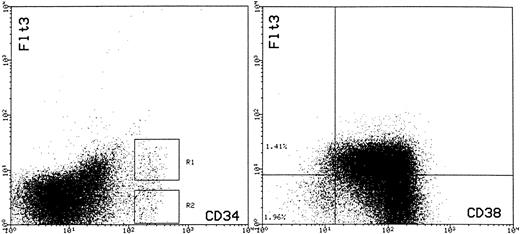
Cells were incubated at 4°C for 30 minutes with the IgG3 monoclonal anti-CD34, 43A1,46 then after washing, incubated sequentially with biotinylated anti-IgG3 followed by streptavidin tricolor (StrepTC; Caltag, San Francisco CA). Optimally diluted BV10-PE and CD38FITC (clone MCA971F, Serotec, Oxford, UK) was added during the third step of immunolabeling. To establish compensation settings and to assess the relative expression of FLT3, cells were also labeled with isotype control monoclonals directly conjugated to PE and FITC, only 43A1-StrepTC or CD38FITC and also two-color combinations of 43A1-StrepTC + IgG-PE and 43A1-StrepTC + CD38FITC. FACS of CD34+FLT3+ and CD34+FLT− cells was performed according to the sort regions shown in Fig 7 (left-hand panel). In one experiment, BM CD34+ cells were enriched to 98.6% purity using 561-Dynal beads (Dynal, Oslo, Norway) and then immunolabeled with CD38FITC (Serotec, UK) and BV10-PE or CD38FITC and IgG-PE. This enabled us to collect a large list mode data file and to more accurately assess FLT3 expression with respect to CD38 expression. The results of this experiment are shown in the right-hand panel of Fig 7.
Growth of myeloid (CFU-GM) and erythroid (BFU-E) progenitor cells from BM CD34+, CD34+CD38−, or CD34+CD38+ cells. Triplicate, 1 mL assays were established with 500 cells in either 6 HGF (10 ng/mL of IL-1, IL-3, IL-6, G-CSF, GM-CSF, and SCF ) or 6 HGF + FLT3L (shown by the solid bar) and scored at day 14. CFU-GM and BFU-E numbers refer to the number of colonies grown from 1,000 cells with the respective phenotypes. The results of three separate experiments are shown (mean + standard error of mean [SEM]).
Growth of myeloid (CFU-GM) and erythroid (BFU-E) progenitor cells from BM CD34+, CD34+CD38−, or CD34+CD38+ cells. Triplicate, 1 mL assays were established with 500 cells in either 6 HGF (10 ng/mL of IL-1, IL-3, IL-6, G-CSF, GM-CSF, and SCF ) or 6 HGF + FLT3L (shown by the solid bar) and scored at day 14. CFU-GM and BFU-E numbers refer to the number of colonies grown from 1,000 cells with the respective phenotypes. The results of three separate experiments are shown (mean + standard error of mean [SEM]).
Expression of FLT3 on CD34+ cells (left-hand panel) and coexpression of CD38 and FLT3 on CD34+ BM cells (right-hand panel). For analysis of FLT3 expression, BM MNC cells were stained simultaneously with HPCA-2–FITC and BV10-PE. Cells within region 1 (R1) were considered as CD34+FLT3+ and those in region 2 (R2) were considered as CD34+FLT3−. The right-hand panel shows expression of CD38 (FITC) and FLT3 (PE) on CD34+ cells enriched by 561-Dynal bead enrichment. The cell suspension contained 98.6% CD34+ cells. The quadstat is positioned to indicate the level of PE staining observed with the IgG-PE control.
Expression of FLT3 on CD34+ cells (left-hand panel) and coexpression of CD38 and FLT3 on CD34+ BM cells (right-hand panel). For analysis of FLT3 expression, BM MNC cells were stained simultaneously with HPCA-2–FITC and BV10-PE. Cells within region 1 (R1) were considered as CD34+FLT3+ and those in region 2 (R2) were considered as CD34+FLT3−. The right-hand panel shows expression of CD38 (FITC) and FLT3 (PE) on CD34+ cells enriched by 561-Dynal bead enrichment. The cell suspension contained 98.6% CD34+ cells. The quadstat is positioned to indicate the level of PE staining observed with the IgG-PE control.
Treatment of cells with 4-HC.Experiments involving exposure of cells to the cyclophosphamide derivative 4-HC were performed according to the method of Ottmann.47 In brief, BM MNC were resuspended at 1 × 107 cells/mL in IMDM supplemented with 10% FCS, then incubated for 30 minutes at 37°C with 120 μg/mL of 4-HC. A control tube was incubated under similar conditions with only media. After 30 minutes, the volume in each tube was doubled using IMDM-10%FCS then underlaid with a FCS “cushion”. Cells were then washed three times by centrifugation in IMDM-20%FCS to ensure residual 4-HC was removed. The resultant MNC suspension was then stained with HPCA-2-PE according to the method described above. CD34+ cells were sorted from both the treated and control tubes then placed into culture.
Clonogenic assays.Day 14 myeloid colony forming (CFU-GM) and burst forming unit-erythroid (BFU-E) assays were performed as described previously.48 Briefly, triplicate 1-mL cultures of 500 CD34+ cells were established in 35 mm plates in 0.9% methylcellulose in IMDM supplemented with 30% FCS and 3 mmol/L L-glutamine. Cultures were stimulated with 10 ng/mL of each of IL-1, IL-3, IL-6, G-CSF, GM-CSF, SCF, and 4 U EPO. After 14 days of incubation at 37°C in 5% CO2 , CFU-GM and BFU-E were scored using a dissecting microscope and standard methods for their identification.
Pre-CFU cultures.The method for growing CD34+ cells under stroma-free HGF-dependent liquid culture conditions has been previously reported.9 In brief, 1,000 CD34+, CD34+CD38+ or C34+CD38− cells were cultured in 1 mL of serum-deprived pre-CFU medium (IMDM supplemented with 20 μg/mL of low-density lipoprotein (Sigma, Cat. #L-2139), 5 × 10−5 mol/L beta mercaptoethanol (BDH, Melbourne, Australia), 10 μg/mL of recombinant human insulin (Novo Nordisk, Denmark), 200 μg/mL of iron saturated human transferrin (Sigma, Cat. #T2158), 1% deionized bovine serum albumin (BSA) (Sigma batch #2153) and 4 mmol/L L-glutamine) together with the appropriate HGF in 24-well tissue culture plates. Triplicate 1-mL cultures were prepared for each cell population and each HGF combination under assessment. The cultures were fed with HGF at day 7 and harvested at day 14. A cell count was performed and a proportion of cells from each replicate was recultured for another 7 days for harvest at day 21. The cultures were split and refed again at weekly intervals. At each time point, the cell suspension was assayed for presence of CFU-GM.
Pre-CFU culture of single cells.Single CD34+, CD34+CD38+ and CD34+CD38− cells were sorted directly into Terasaki wells using the automated cell deposition unit of the FACStarPlus. Wells were examined within 24 hours using a 200× inverted phase microscope to determine the proportion receiving only a single cell. These wells were subsequently examined at 3, 7, and 14 days of culture to determine the number of cells generated from a single cell. At day 14, the contents of each Terasaki well were transferred into 90 μL of IMDM/10%FCS and the cell concentration determined after counting on a hemocytometer under phase contrast illumination.
Statistical analysis of data was performed using Statview 4.02 software (Abacus Concepts Inc, Berkerley, CA).
Retroviral transduction of hematopoietic progenitor cells.The (LAPSN) retrovirus, which contains the alkaline phosphatase and neomycin resistance genes was generously provided by Dr Dusty Miller (Fred Hutchinson Cancer Center, Seattle, WA). The LAPSN virus was packaged in PA317 and viral containing supernatant was filtered (0.45 μmol/L) before addition to target cells. Immunophenotypically defined subpopulations of bone marrow CD34+ cells were obtained by FACS, prestimulated for 2 days in 1 mL of IMDM supplemented with 30% FCS, 1% BSA and various combinations of HGF and to enhance expression of the amphotropic receptor, they were incubated for 16 hours in phosphate-free DMEM before addition of the first aliquot of virus containing supernatant. Cells were then transferred into wells of a 24-well plate coated with 25 μg/mL fibronectin and viral supernatant added together with HGF. The target cells were exposed to fresh viral containing PA317 supernatant every 12 hours for 3 days, then cultured for a further 5 days before the contents of wells were harvested and the proportion of infected cells determined by staining for alkaline phosphatase (Sigma). Levamisole was included in all samples stained to eliminate background endogenous alkaline phosphatase.
RESULTS
Effect of FLT3 ligand on growth of myeloid and erythroid progenitors.Initial studies were performed to investigate the potential of FLT3L to stimulate the growth of committed progenitors in standard semisolid clonogenic assays. Previous studies from this laboratory have identified the combination of IL-1, IL-3, IL-6, G-CSF, GM-CSF, SCF (6 HGF ), and EPO as a potent stimulus for myeloid and erythroid colony growth. Clonogenic assays were performed on sorted CD34+ cells obtained from BM and mobilized peripheral blood. As is evident from Fig 1, the addition of FLT3L to 6 HGF + EPO increased the number of myeloid colonies (CFU-GM) significantly (P = .07), but did not alter the number of erythroid bursts (BFU-E) grown from BM CD34+ cells. Analogous results were obtained with CD34+ cells derived from mobilized peripheral blood. There was no difference in cloning of BFU-E, but addition of FLT3L to the 6 HGF + EPO combination resulted in an average increase of 33.6% more CFU-GM, which was significantly more than that grown with only 6 HGF + EPO (P = .09, n = 3). These data confirm previous studies showing the lack of effect of FLT3L on erythroid progenitor cells, but showed the marked potentiation by FLT3L on the growth of myeloid progenitor cells.35 49-52 Additional experiments were therefore performed to further characterize the FLT3L responsive clonogenic cells within the CD34+ population.
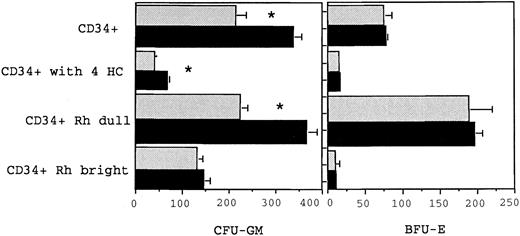
Growth of myeloid (CFU-GM) and erythroid (BFU-E) progenitor cells from BM CD34+, CD34+Rhodamine 123 dull, CD34+ Rhodamine 123 bright, and CD34+ cells following 4-HC treatment. The different cell populations were cultured in either 6 HGF or 6 HGF + FLT3L (shown in the solid bars) and colonies scored at day 14. The mean and standard error from triplicate plates from a single representative experiment are shown. CFU-GM and BFU-E numbers refer to the number of colonies grown from 1,000 cells with the respective phenotypes. The number of CFU-GM in the total CD34+ fraction, the CD34+ Rhodamine dull and the 4-HC–treated CD34+ cells was significantly greater (as indicated by the *) when cultured with 6 HGF + FLT3L (P = .05, .06, and .05, respectively) than with 6 HGF.
Growth of myeloid (CFU-GM) and erythroid (BFU-E) progenitor cells from BM CD34+, CD34+Rhodamine 123 dull, CD34+ Rhodamine 123 bright, and CD34+ cells following 4-HC treatment. The different cell populations were cultured in either 6 HGF or 6 HGF + FLT3L (shown in the solid bars) and colonies scored at day 14. The mean and standard error from triplicate plates from a single representative experiment are shown. CFU-GM and BFU-E numbers refer to the number of colonies grown from 1,000 cells with the respective phenotypes. The number of CFU-GM in the total CD34+ fraction, the CD34+ Rhodamine dull and the 4-HC–treated CD34+ cells was significantly greater (as indicated by the *) when cultured with 6 HGF + FLT3L (P = .05, .06, and .05, respectively) than with 6 HGF.
FLT3 ligand responsive clonogenic cells are CD38−, Rhodamine 123 dull, and resistant to 4-HC.Clonogenic assays were performed on CD34+CD38+ and CD34+CD38− cells, CD34+ cells sorted on the basis of their ability to retain the supravital dye rhodamine123 (Rh123), and also on CD34+ cells exposed to 4-HC. A significant increase in CFU-GM (P = .02, n = 4) was observed in cultures of CD34+CD38− cells when grown in 6 HGF + EPO + FLT3L and compared with growth stimulated by 6 HGF + EPO (Fig 1). In contrast, the cloning efficiency of CFU-GM and BFU-E derived from CD34+CD38+ cells was not altered by the addition of FLT3L (P = .17). However, in all experiments, when cells were cultured with FLT3L, there was a notable increase in the size of CFU-GM colonies. Approximately 30% of CFU-GM from the CD34+CD38− cells were multicentric and macroscopic, with diameters ranging from 0.5 to 1.5 mm; much larger than those present when 6 HGF was used as a stimulus.
Both CD34+Rh123dull, and CD34+Rh123bright cells, obtained according to previously defined sort criteria53 were cultured with either 6 HGF + EPO or 6 HGF + EPO + FLT3L. As shown in Fig 2, there was again no effect of FLT3L on cloning of BFU-E from either of the sorted populations, in accord with the data from previous clonogenic assays performed on unfractionated CD34+ cells. Similarly, growth of CFU-GM from the CD34+Rh123bright cells was not significantly increased by addition of FLT3L to cultures. In contrast, there was an increase in the number of CFU-GM from both CD34+ and CD34+Rh123dull cells when FLT3L was added to 6 HGF + EPO (P = .05; Fig 2). The CD34+Rh123dull cells were the most responsive subset with 64.3% more CFU-GM produced when FLT3L was added to cultures.
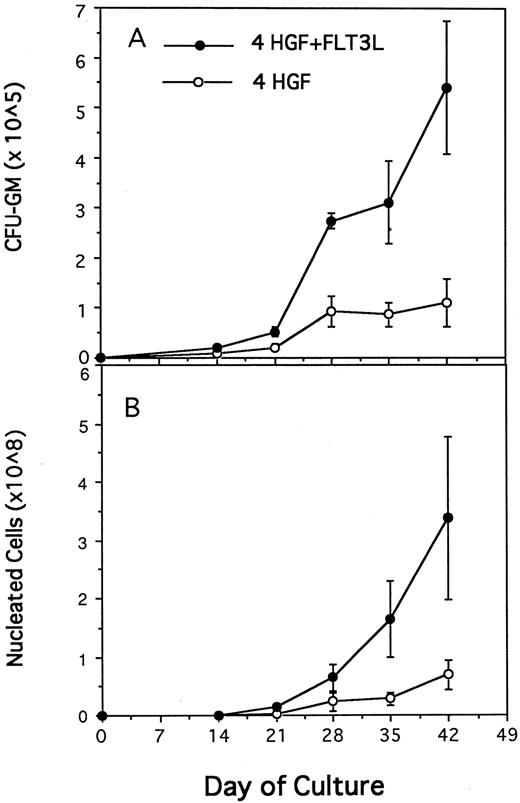
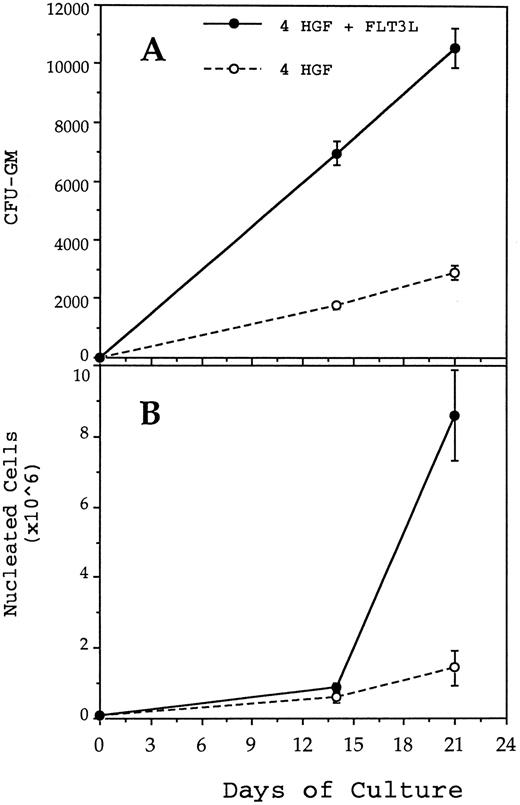
Generation of CFU-GM (A) and nucleated cells (B) from 1,000 CD34+CD38− BM cells in pre-CFU culture stimulated with either 4 HGF (IL-3, IL-6, G-CSF, and SCF ) or 4 HGF + FLT3L. At each time point, the mean and standard error from three separate experiments with different sources of BM are presented. CD34+CD38− cells were cultured in wells of a 24-well plate. At day 14 and at weekly intervals thereafter, the cultures were subjected to 1 in 5 splits and refed with fresh media and HGF.
Generation of CFU-GM (A) and nucleated cells (B) from 1,000 CD34+CD38− BM cells in pre-CFU culture stimulated with either 4 HGF (IL-3, IL-6, G-CSF, and SCF ) or 4 HGF + FLT3L. At each time point, the mean and standard error from three separate experiments with different sources of BM are presented. CD34+CD38− cells were cultured in wells of a 24-well plate. At day 14 and at weekly intervals thereafter, the cultures were subjected to 1 in 5 splits and refed with fresh media and HGF.
We next investigated whether quiescent CD34+ cells, spared from the cytotoxic effects of 4-HC were capable of responding to FLT3L and generated CFU-GM or BFU-E colonies. Incubation of CD34+ cells with 4-HC resulted in a 5.5-fold reduction in the number of 6 HGF responsive CFU-GM cloned (Fig 2). Notably, cultures stimulated with FLT3L contained significantly more CFU-GM than those assayed in the presence of 6 HGF + EPO alone (P = .05, n = 3). Collectively, the results of these clonogenic assays suggest that FLT3L preferentially stimulates the proliferation of a minor subset of primitive myeloid progenitor cells within the CD34+ population, which are characterized by their resistance to 4-HC, low retention of Rh123, and lack of expression of CD38. These are all characteristics of hierarchically more primitive progenitors as demonstrated by the capacity of such cells to generate nascent lineage restricted clonogenic progenitors in long-term culture stimulated either by coculture with marrow stromal cells or cytokines.53-55 Subsequent experiments were therefore performed to examine the effect of FLT3L on growth of CD34+CD38− cells in stromal-free cytokine-dependent pre-CFU culture.
FLT3 ligand increases production of nucleated cells and nascent CFU-GM from CD34+CD38− cells.Previous studies from this laboratory have shown that the combination of IL-3, IL-6, G-CSF, and SCF, when used at 10 ng/mL, 10 ng/mL, 100 ng/mL, and 100 ng/mL, respectively (4 HGF ), is superior to 6 HGF for generation of nascent CFU-GM from CD34+ cells.39 Therefore, pre-CFU cultures were established by plating CD34+CD38− cells in either 4 HGF or 4 HGF + FLT3L. Production of nucleated myeloid cells and CFU-GM were monitored weekly for 6 weeks. The results shown in Fig 3 represent the data from five separate experiments performed with different normal adult BM cells. The generation of CFU-GM at weekly time points is shown in Fig 3A. Culture of CD34+CD38− cells with 4 HGF + FLT3L resulted in significantly more CFU-GM generated at days 14, 21, 28, 35, and 42 (P = .0026, .0094, .005, .06, and .07, respectively) than with cultures stimulated with 4 HGF. Of note was the absolute number of CFU-GM present at day 42. An average of 541,199 (range, 251,736 to 893,000) CFU-GM were generated at day 42 from 1,000 CD34+CD38− cells when cultured with 4 HGF + FLT3L. In comparison, the same number of CD34+CD38− cells generated an average of 109,246 CFU-GM (range, 0 to 220,000) at day 42 after culture in only 4 HGF.
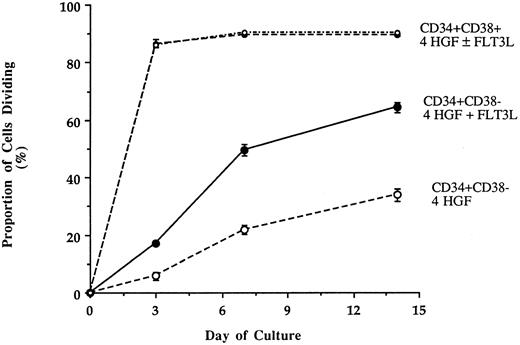
Recruitment of single CD34+CD38+ and CD34+CD38− cells into cell division when cultured with either IL-3 + IL-6 + G-CSF + SCF (4 HGF ) or 4 HGF + FLT3L. Cells were deposited into wells of a Terasaki plate each containing 10 μL of serum-free pre-CFU media. Wells containing a single cell were examined at 3, 7, and 14 days to determine the proportion of single cells undergoing division. The results are the means and standard error of three experiments performed with different sources of adult human BM.
Recruitment of single CD34+CD38+ and CD34+CD38− cells into cell division when cultured with either IL-3 + IL-6 + G-CSF + SCF (4 HGF ) or 4 HGF + FLT3L. Cells were deposited into wells of a Terasaki plate each containing 10 μL of serum-free pre-CFU media. Wells containing a single cell were examined at 3, 7, and 14 days to determine the proportion of single cells undergoing division. The results are the means and standard error of three experiments performed with different sources of adult human BM.
The corresponding production of nucleated cells in the same cultures is shown in Fig 3B. At each time point, there were significantly more nucleated cells present in 4 HGF + FLT3L stimulated cultures (P values ranging from .06 to .0058, paired t test, 95% confidence limits) than in the 4 HGF cultures. At day 42, an average of 3.3 × 108 cells were generated from 1,000 CD34+CD38− cells when cultured in 4 HGF + FLT3L compared with 6.9 × 107 with 4 HGF alone. Thus, FLT3L was found to markedly potentiate the de novo generation of mature myeloid cells from CD34+CD38− cells.
FLT3L increases recruitment of single CD34+CD38- cells into cell cycle.To investigate the basis for the capacity of FLT3L to augment ex vivo expansion of myeloid progenitors from CD34+CD38− cells, single BM CD34+CD38+ or CD34+CD38− cells were sorted into Terasaki wells containing serum-deprived media supplemented with 4 HGF with or without FLT3L. Wells were scored on days 3, 7, and 14 to determine the proportion of cells of each phenotype, which divide in response to the two different HGF combinations. The responses of single CD34+CD38+ and CD34+CD38− were very different (Fig 4). Single CD34+CD38+ cells responded rapidly to either 4 HGF or 4 HGF + FLT3L as evidenced by the high proportion of cells dividing within 3 days (89.1% and 88.7%, respectively). During the remainder of culture, only a few more single CD34+CD38+ cells were recruited into cell cycle. At day 14, 90.3% and 89.7% of cells cultured in 4 HGF or 4 HGF + FLT3L, respectively, had divided.
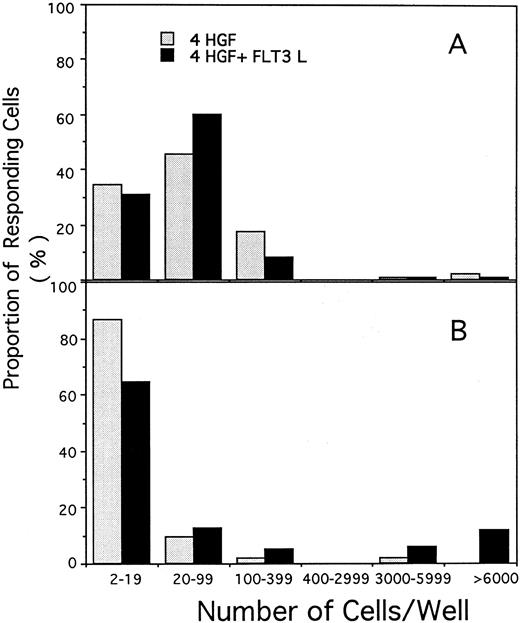
Proliferative response of single CD34+CD38+ (A) or CD34+CD38− cells (B) cultured in serum-free pre-CFU media stimulated with either 4 HGF or 4 HGF + FLT3L (shown as the solid columns). The number of viable cells present after 14 days of culture was determined for each single cell that divided. The results are from one of the experiments performed to generate data shown in Fig 4.
Proliferative response of single CD34+CD38+ (A) or CD34+CD38− cells (B) cultured in serum-free pre-CFU media stimulated with either 4 HGF or 4 HGF + FLT3L (shown as the solid columns). The number of viable cells present after 14 days of culture was determined for each single cell that divided. The results are from one of the experiments performed to generate data shown in Fig 4.
In contrast, a significantly greater proportion of CD34+CD38− cells divided when cultured in 4 HGF + FLT3L as compared with those cultured in 4 HGF (P = .015, .0003, and .001 for days 3, 7, and 14, respectively). The enhanced proliferative response in the presence of FLT3L was evident by day 3 and was maintained at all subsequent times examined. Thus, at day 14, 62.5% of single CD34+CD38− cells had divided when stimulated with 4 HGF + FLT3L as compared with 34.7% with 4 HGF. At day 14, in the first two experiments, we observed a trend for more cells in wells cultured in 4 HGF + FLT3L as compared with those stimulated with 4 HGF. Therefore, in a further experiment, we counted the number of cells generated at day 14 from single CD34+CD38− or CD34+CD38+ cells cultured in either 4 HGF or 4 HGF + FLT3L. The results of this analysis are shown in Fig 5. Proliferation of single CD34+CD38+ cells was not enhanced by the addition of FLT3L to the 4 HGF combination. However, the number of cells generated from single CD34+CD38− cells was increased by the addition of FLT3L to the cultures. Culture with 4 HGF resulted in an average of 23.4 cells/well (range, 2 to 120, n = 48 wells). In contrast, stimulation with 4 HGF + FLT3L resulted in an average of 875 cells/well (range, 2 to 9,000, n = 96 wells), representing 35-fold more cells/well. This increase in the average number of cells/well is primarily explained by a small proportion (11.9% of responding cells) of single CD34+CD38− cells generating greater than 6,000 cells when stimulated with 4 HGF + FLT3L. The difference in cell production between the two groups was highly significant (P = .001, Paired t test).
Ex vivo culture of PB CD34+ cells in serum-free pre-CFU media stimulated with 4 HGF or 4 HGF + FLT3L. The graph shows the number of CFU-GM (A) and nucleated myeloid cells (B) generated from 1,000 PB CD34+ cells after 14 and 21 days of culture. The results are the mean and standard errors from three cultures established with CD34+ cells obtained from cryopreserved apheresis collections following high-dose cyclophosphamide administration.
Ex vivo culture of PB CD34+ cells in serum-free pre-CFU media stimulated with 4 HGF or 4 HGF + FLT3L. The graph shows the number of CFU-GM (A) and nucleated myeloid cells (B) generated from 1,000 PB CD34+ cells after 14 and 21 days of culture. The results are the mean and standard errors from three cultures established with CD34+ cells obtained from cryopreserved apheresis collections following high-dose cyclophosphamide administration.
The results of these experiments indicate that increased recruitment of primitive HPC cells into cell cycle and thereafter increased proliferation of recruited cells underlies the ex vivo expansion potential of FLT3L. Given these findings, we proceeded to determine whether the addition of FLT3L to 4 HGF could improve the incidence of retroviral transduction into CD34+CD38− BM cells.
FLT3L increases retroviral transduction of CD34+CD38− cells.In all experiments, between 5,000 and 10,000 CD34+CD38+, CD34+CD38+/− or CD34+CD38− cells were cultured together with LAPSN containing PA317 supernatant. The retroviral titer of the supernatant was determined to be at least 1 × 106 virions/mL. In the first set of experiments, we compared the ability to transduce CD34+CD38+, CD34+CD38+/− or CD34+CD38− cells when stimulated with either 4 HGF or 4 HGF + FLT3L. As indicated in Table 1, transduction of CD34+CD38+ and CD34+CD38+/− cells was independent of FLT3L in the culture system and resulted in approximately 20% of cells expressing alkaline phosphatase. In contrast, the transduction of CD34+CD38− cells, was increased by the addition of FLT3L to the 4 HGF combination. A series of subsequent experiments were then performed to compare transduction of only CD34+CD38− cells when grown with different HGF combinations. In the first, the combination of IL-3 + IL-6 + SCF, which has been widely used for retroviral gene therapy protocols, was compared with 4 HGF and 4HGF + FLT3L. In accord with the previous experiments, we showed that a significantly higher proportion (P = .0047) of CD34+CD38− cells were transduced when FLT3L was added to the 4 HGF combination (Table 2). Of note was the very low proportion of alkaline phosphatase positive cells present after culture in only IL-3 + IL-6 + SCF. In two of the three experiments, there were very few viable cells remaining at the end of culture and the proportion of transduced cells could not be determined.
Retroviral Transduction of CD34+ Cell Subsets: Effect of FLT3L
| Cells . | 4 HGF . | 4 HGF + FLT3L . |
|---|---|---|
| CD34+CD38+ | 20 (39/192) | 19 (33/173) |
| CD34+CD38+/− | 15 (34/226) | 22 (43/196) |
| CD34+CD38− | 7.5 (12/159) | 15 (28/182) |
| Cells . | 4 HGF . | 4 HGF + FLT3L . |
|---|---|---|
| CD34+CD38+ | 20 (39/192) | 19 (33/173) |
| CD34+CD38+/− | 15 (34/226) | 22 (43/196) |
| CD34+CD38− | 7.5 (12/159) | 15 (28/182) |
CD34+CD38+, CD34+CD38+/−, and CD34+CD38− cells were cultured with LAPSN retrovirus with either 4 HGF or 4 HGF + FLT3L. Cells were harvested after 2.5 days in the presence of retrovirus and total of 10 days culture in HGF. Cytocentrifuge preparations were stained for akaline phosphatase and the number of positive cells were expressed as a proportion of total cells. The figures in the table represent the percentage of alkaline phosphatase positive cells (number of positive/total cells examined).
Retroviral Transduction of CD34+CD38− Cells Is Enhanced by FLT3L
| HGF . | Experiment 1 . | Experiment 2 . | Experiment 3 . |
|---|---|---|---|
| Control | 0 | 0 | 0 |
| IL-3, IL-6, SCF | ND | 2 (5/250) | ND |
| 4 HGF | 8 (11/143) | 5 (13/250) | 11 (16/150) |
| 4 HGF + FLT3L | 17 (27/173) | 14 (36/260) | 22 (41/168) |
| HGF . | Experiment 1 . | Experiment 2 . | Experiment 3 . |
|---|---|---|---|
| Control | 0 | 0 | 0 |
| IL-3, IL-6, SCF | ND | 2 (5/250) | ND |
| 4 HGF | 8 (11/143) | 5 (13/250) | 11 (16/150) |
| 4 HGF + FLT3L | 17 (27/173) | 14 (36/260) | 22 (41/168) |
Abbreviation: ND, not determined.
Three different sources of BM CD34+CD38− cells were cultured with LAPSN retrovirus without HGF (control), with IL-3 + IL-6 + SCF, 4 HGF and 4 HGF + FLT3L. Cells were examined for presence of alkaline phosphatase and the percentage of positive (positive cells/total cells examined) expressed as a proportion of total cells. A significantly (P = .0047) greater proportion of the CD34+CD38− cells were transduced when cultured with 4 HGF + FLT3L.
Ex vivo expansion of peripheral blood CD34+ cells is increased by FLT3L.Pre-CFU cultures were performed with CD34+ cells isolated from the apheresis collections of three patients undergoing therapeutic blood progenitor cell mobilization. In accord with the results of pre-CFU culture of BM CD34+ cells, the addition of FLT3L to 4 HGF stimulated culture of PB CD34+ cells resulted in a significant increase in the number of nascent CFU-GM generated after 14 and 21 days (P = .05 and .005, respectively) (Fig 6). At day 14, there was no significant difference between the number of nucleated cells generated by cultures stimulated with 4 HGF or 4 HGF + FLT3L, although an average of 44% more nucleated cells were present in those cultures stimulated by 4HGF + FLT3L. However, at day 21, significantly more nucleated cells (P = .05) were generated in cultures stimulated with 4 HGF + FLT3L than in cultures stimulated with only 4 HGF.
FLT3 expression by CD34+ hematopoietic progenitor cells.Collectively, the above data indicates that the capacity to enhance the ex vivo expansion of CD34+ HPC is in part due to the recruitment of primitive HPC with greater proliferative potential than those recruited by the 4 HGF combination. To further investigate the basis of this effect of FLT3L, we chose to examine the expression of the FLT3 receptor tyrosine kinase on the BM CD34+ population using the recently described antihuman FLT3 monoclonal antibody BV10.44 Three-color flow cytometric analysis using BV-10 in combination with antibodies to CD34 and CD38 was performed on BM MNC from four normal donors. As shown in Fig 7, FLT3 was expressed at readily detectable levels on approximately 55% ± 5.1% (n = 4) of CD34+ cells.
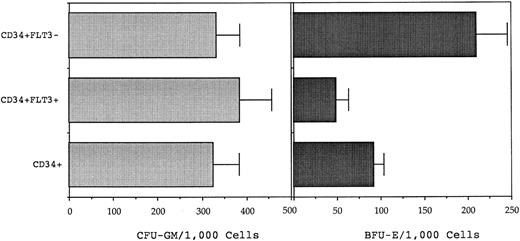
Subsequently, FACS was performed to separate BM CD34+ cells into ostensibly FLT3+ and FLT3− subpopulations according to the sort regions (R1 and R2) illustrated in Fig 7. Clonogenic assays performed on the sorted populations isolated from four normal donor BM samples showed that CFU-GM were present at approximately equivalent incidence in the CD34+FLT3+ and CD34+FLT3− fractions (mean of 385 and 333 CFU-GM/103, respectively; Fig 8). Conversely, BFU-E numbers were consistently higher in the CD34+FLT3− subpopulation compared with the corresponding CD34+FLT3+ fraction (P = .0014) or to the total CD34+ population (P = .009).
Clonogenic assay of CD34+FLT3+, CD34+FLT3−, and CD34+ cells performed in IL-1, IL-3, IL-6, G-CSF, GM-CSF, SCF (each added at 10 ng/mL) and 4 IU of erythropoietin. The number of myeloid (CFU-GM) and erythroid (BFU-E) clonogenic cells was determined at day 14 after culture of 500 cells of each phenotpye. The graph shows the mean (± SEM) CFU-GM and BFU-E/1,000 input cells from culture of four normal BM samples. BFU-E were enriched in the CD34+FLT3− fraction as compared with the CD34+FLT3+ cells (P = .0014) or total CD34+ cells (P = .009). The incidence of CFU-GM was equivalent in each fraction.
Clonogenic assay of CD34+FLT3+, CD34+FLT3−, and CD34+ cells performed in IL-1, IL-3, IL-6, G-CSF, GM-CSF, SCF (each added at 10 ng/mL) and 4 IU of erythropoietin. The number of myeloid (CFU-GM) and erythroid (BFU-E) clonogenic cells was determined at day 14 after culture of 500 cells of each phenotpye. The graph shows the mean (± SEM) CFU-GM and BFU-E/1,000 input cells from culture of four normal BM samples. BFU-E were enriched in the CD34+FLT3− fraction as compared with the CD34+FLT3+ cells (P = .0014) or total CD34+ cells (P = .009). The incidence of CFU-GM was equivalent in each fraction.
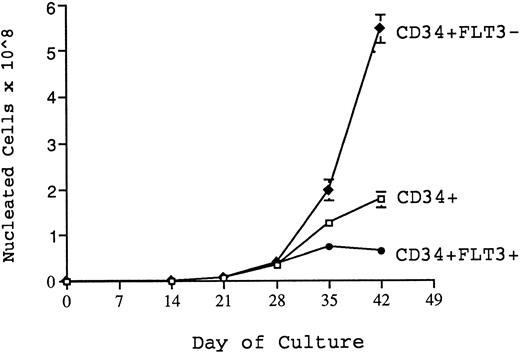
Production of nucleated cells over 6 weeks of culture from 1,000 CD34+, CD34+FLT3+, and CD34+FLT3− cells cultured in 4 HGF + 100 ng/mL FLT3-L. The graph shows results (mean + SEM from triplicate wells) from one representative experiment. At 4 weeks, CD34+FLT3− cells produced significantly (P = .05) more cells than either the unfractionated CD34+ cells or the CD34+FLT3+ cells. At both 5 and 6 weeks of culture, significantly greater nucleated cell generation was achieved from CD34+FLT3− cells than that achieved by either CD34+ or CD34+FLT3+ cells (P values of .08, .0016, and .03, .005 for weeks 5 and 6, respectively). In contrast, the CD34+FLT3+ cells were unable to sustain production beyond 4 weeks.
Production of nucleated cells over 6 weeks of culture from 1,000 CD34+, CD34+FLT3+, and CD34+FLT3− cells cultured in 4 HGF + 100 ng/mL FLT3-L. The graph shows results (mean + SEM from triplicate wells) from one representative experiment. At 4 weeks, CD34+FLT3− cells produced significantly (P = .05) more cells than either the unfractionated CD34+ cells or the CD34+FLT3+ cells. At both 5 and 6 weeks of culture, significantly greater nucleated cell generation was achieved from CD34+FLT3− cells than that achieved by either CD34+ or CD34+FLT3+ cells (P values of .08, .0016, and .03, .005 for weeks 5 and 6, respectively). In contrast, the CD34+FLT3+ cells were unable to sustain production beyond 4 weeks.
In addition, the same sorted subpopulations were assayed in pre-CFU culture to determine which subpopulation had the greatest capacity over time for denovo generation of hematopoietic cells under these culture conditions. Shown in Fig 9 are data from a representative experiment (one of four) demonstrating production of nucleated cells from CD34+, CD34+FLT3+ and CD34+FLT3− cells over 6 weeks when stimulated by 4 HGF + FLT3 L in pre-CFU culture. There was no significant difference in the number of nucleated cells generated from each cell type over the first 3 weeks of culture. However, at 4 weeks, the CD34+FLT3− cells produced significantly (P = .05) more cells than either the unfractionated CD34+ cells or the CD34+FLT3+ cells. The increased capacity for CD34+FLT3− cells to generate myeloid cells was also evident at both 5 and 6 weeks of culture and remained significantly greater than that achieved by either CD34+ or CD34+FLT3+ cells (P values of .08, .0016, and .03, .005 for weeks 5 and 6, respectively). Notably, 1,000 CD34+FLT3− cells were able to support a 50,000-fold expansion of nucleated cells over 6 weeks of culture. In contrast, the CD34+FLT3+ cells were unable to sustain increasing cell production beyond 4 weeks. These results may, in part, be explained by the relative proportion of CD38− cells in the FLT3+ and FLT3− fractions. Figure 7 shows the typical pattern of expression of both FLT3 and CD38 on CD34+ cells. Approximately 60% to 70% of CD34+CD38+ cells express readily detectable levels of FLT3. In contrast, a lower percentage (25% to 31%) of CD34+CD38− cells express equivalent levels of FLT3 to that expressed by CD34+CD38+ cells. It is notable that the majority of CD34+CD38− cells express low levels of FLT3 (in comparison to isotype controls and the CD34+CD38+ cells) and that only the very occasional CD34+CD38− cell could be considered as FLT3−. Therefore, it is more appropriate to refer to the the FLT3− population as being FLT3low/−.
DISCUSSION
Although a number of recent reports have examined the effect of the recently identified FLT3L on the growth of human hematopoietic progenitor cell populations in vitro,31,33-35,51,52 there is little known concerning the identity and phenotypic characteristics of the primitive progenitor cell populations that respond to this cytokine. In this report, we show that FLT3L acts preferentially on a minor subpopulation of clonogenic CD34+ cells characterized by their low retention of rhodamine 123, resistance to 4-HC, and low/absent expression of the CD38 antigen. These are all characteristics of hierarchically primitive hematopoietic progenitors as demonstrated by the capacity of such cells to initiate and sustain hematopoietic cell development in various in vitro and in vivo assay systems.53-55 These data prompted us to examine the effect of FLT3L on the growth of CD34+CD38− cells in cytokine driven, stromal cell-free suspension cultures. Previous studies from this and other laboratories have shown that the combination of IL-3, IL-6, G-CSF, and SCF (4 HGF ) provides a potent stimulus for growth of primitive human hematopoietic progenitors (pre-CFU) under these conditions.39 56 In accord with these data, 4 HGF in the present study consistently supported mean 500-fold and 80,000-fold increases in the numbers of CFU-GM and total cells, respectively, at 7 weeks following initiation of cultures with 1,000 CD34+CD38− cells. Despite this considerable expansion of hematopoietic cells, the further addition of FLT3L resulted in a significant enhancement of cell generation over that obtained in cultures stimulated with 4 HGF alone, corresponding to a 330,000-fold expansion of total cells numbers at this 7-week time point.
These data clearly show the remarkable capacity of FLT3L to potentiate the ex vivo expansion of human HPC and are in accord with, although considerably more impressive, than the previous observations of the effects of this cytokine on primitive human and murine HPC.35,49,57-59 Moreover, these studies provide a further example of the potent synergy demonstrated by FLT3L with other cytokines, an observation in agreement with a number of previous reports documenting the synergistic enhancement of colony formation effected by FLT3L when added in combination with cytokines such as IL-3, IL-6, G-CSF, and SCF.33,35 60
Single cell deposition studies performed under serum-deprived conditions to investigate the basis for the potent enhancement of hematopoiesis by FLT3L confirmed the preferential action of this cytokine on primitive (CD34+CD38−) HPC and in addition showed that the activity of FLT3L on these largely quiescent progenitors was direct and not dependent on either serum components or accessory cell populations. Most significantly, these single cell studies show that FLT3L recruited approximately twofold more CD34+CD38− cells than in cultures stimulated by the 4 HGF combination alone. Not only were more CD34+CD38− cells stimulated to divide, but importantly, for retroviral-mediated transduction of HPC, the rate of recruitment was enhanced by FLT3L. Furthermore, the primitive HPC recruited by FLT3L showed at a single cell level significantly greater proliferation on average than those stimulated to proliferate by 4 HGF. This was in large part due to the specific recruitment by FLT3L of a minor subpopulation compromising approximately 12% of CD34+CD38− cells which, over the 2-week time course of the assay, were identified by their capacity to generate greater than 6,000 cells per input HPC. Cells with this proliferative potential were only rarely detected in cultures stimulated with 4 HGF.
Collectively, these data suggest that the marked potentiation of HPC expansion promoted by FLT3L is at least partially due to the increased recruitment of primitive progenitor cells with enhanced proliferative potential. This explanation is supported by the recent studies of Shah et al36 who demonstrated similar results with single CD34+CD38− cells cultured on preformed irradiated BM stromal cells supplemented with HGF. Additional evidence for the action of FLT3L on primitive HPC within the CD34+CD38− compartment comes from a study reported by Petzer et al37 where FLT3L alone or in combination with other HGF including SCF, IL-3, IL-6, G-CSF, and β-nerve growth factor was found to expand long-term culture initiating cells (LTC-IC) within the CD34+CD38− cell population. In a further study, Petzer et al38 reported that in serum-deprived, stroma free cultures of 200 CD34+CD38− cells, aside from thrombopoietin, FLT3L was the only factor that, on its own was able to stimulate expansion of LTC-IC numbers to above input levels. Also relevant in this regard are recent studies showing that FLT3L accelerates the cycling of 5-flurouracil–resistant murine HPC by shortening the G1 phase of the cell cycle.61 To date, comparable studies have not been performed using human HPC, but if a similar acceleration of cycling is subsequently shown, then this additional activity of FLT3L must also be considered in assessing the overall contribution of FLT3L to the ex vivo growth of human HPC.
Of relevance to this issue is the pattern of expression of the FLT3 receptor tyrosine kinase by primitive human HPC. Small et al62 have previously shown that human flt3 mRNA is restricted to the CD34+ population in adult human BM. This pattern of expression is ostensibly analogous to that previously described for the distribution of the FLT3 receptor on mouse fetal liver and adult BM cells as determined either by flow cytometry using monoclonal antibody to murine flt3/flk-2 or by binding studies on sorted cell populations using radiolabeled ligand.35,63 These studies showed readily detectable levels of FLT3 protein on cell populations known to be enriched in stem cell activity as shown by their coexpression of antigens such as AA4, c-kit, and sca-1. In accord with these observations, flow cytometric analysis by McKenna et al35 using biotinylated human FLT3L identified FLT3 expression only on CD34+ cells in adult human BM, the great majority of which appeared to be FLT3+ albeit at a low level. A recent study by Rosnet et al64 using a monoclonal antibody SF1.340, to human FLT3 showed low levels of FLT3 expression on approximately 2% of adult BM MNC. Notably, FLT3 expression was detected on only a minor subpopulation (≈25%) of CD34+ cells. However, functional studies were not performed to compare the biological properties of FLT3+ and FLT3− subpopulations. A more recent study by Rappold et al65 performed with a different anti-FLT3 monoclonal antibody, 4G8, suggests that between 63% to 82% of BM CD34+ coexpress FLT3. In addition three-color phenotypic analysis indicated that the most primitive HPC defined as CD34+ CD38−, CD71low, HLA-DR-, CD117+, and Thy-1+ express low levels of FLT3.
In the current study, BV10 was found to subdivide the CD34+ population into numerically similar proportions to those found by Rosnet et al.64 Clonogenic assay of the two subpopulations showed equivalent numbers of CFU-GM in each fraction, but a significant enrichment for erythroid progenitors in the CD34+FLT3− subpopulation, in accord with the well-documented lack of response of these progenitors to FLT3L.49-52 Thus, the pattern of binding of BV10 to CD34+ cells appears to closely parallel the activity of FLT3L on clonogenic HPC in vitro. The CD34+FLT3+ and CD34+FLT3− subpopulations were also assayed for their capacity to sustain the generation of nascent CFU-GM and their myeloid progeny in long-term suspension cultures stimulated by 4 HGF + FLT3L. At early time points, up to day 14, the two populations showed approximately equivalent activities under these conditions, but at later periods, the FLT3− subpopulation consistently exhibited a significantly greater and more prolonged generation capacity, while there was a concomitant decline in that from the FLT3+ fraction.
Consistent with these data, three-color flow cytometric analysis showed heterogeneous expression of FLT3 on CD34+CD38− cells with readily detectable levels on approximately 45% to 50% of the population and low to undetectable levels on the remainder. These analyses are consistent with those performed by Rosnet64 and Rappold65 who showed heterogeneous expression of FLT3 on CD34+ cells from adult human BM. Notably, FLT3+ cells were found to be CD71lo and c-kit+ (characteristics of primitive HPC42,66) and to exhibit high levels of HLA-DR, a feature of more mature clonogenic progenitors. Based on the above data, it is therefore tempting to speculate that hierarchically primitive human HPC responsible for sustained hematopoiesis at later time points in culture are CD34+CD38− FLT3low/−. Moreover, that CD34+CD38−FLT3+ cells represent a transit population with proliferative potential intermediate between that of FLT3low/− HPC and committed progenitors. Functional studies of cells isolated by FACS based on this composite three-color phenotype will be required to investigate this hypothesis. However, in support of this proposal are data from a recent study by Zeigler et al30 who showed that on murine bone marrow and fetal liver-derived progenitors, FLT3 was only expressed by a subpopulation of actively cycling AA4 + sca-1 + c-kit + CD34+ cells with more limited repopulating activity in vivo. A higher proportion of HPC lacking FLT3 were noncycling and exhibited a significantly greater long-term repopulating activity than their FLT3+ counterparts. The results of phenotypic and functional studies reported herein support the thesis that the most primitive HPC express relatively low levels of FLT3.
In summary, these studies clearly illustrate the potent activity of FLT3L on the growth of primitive human HPC and show at least one means by which this cytokine is able to support considerable expansion of hematopoietic tissue ex vivo. These data have a number of important implications. First, based on its remarkable capacity to enhance ex vivo expansion of human HPC, FLT3L may be of clinical benefit in the transplantation setting, particularly in situations in which the size of the hematopoietic graft is limiting, where there are obvious potential benefits to be gained from expansion of HPC numbers by ex vivo culture. A clear example of this is umbilical cord blood which, because of limited numbers of cells available, is currently viewed as being safely applied for only pediatric transplants.67 However, it will be important to determine whether transplantable cells persist after such ex vivo culture manipulations. In this regard, it is interesting to note that although FLT3L in combination with 4 HGF almost doubles the number of CD34+ CD38−HPC recruited by 4 HGF alone, there still remains approximately 40% of cells with this phenotype, which do not respond (at least in terms of proliferation) to this five cytokine combination. Given previous studies showing the hierarchical ordering of primitive murine HPC according to their growth factor requirements,68 it is reasonable to speculate that hierarchically more primitive human HPC, including cells with marrow repopulating potential, may be found within this five cytokine nonresponsive subpopulation of CD34+CD38− cells. Accordingly, such cells would be predicted to exhibit a requirement for additional (or alternative) cytokines and/or different culture conditions to those described here to be recruited into cycle. Assuming that culture conditions are sufficient to maintain the viability of this progenitor population, it is thus conceivable that the use of 4 HGF + FLT3L as described herein may facilitate large fold expansion of late HPC without compromising the engraftment potential of the population as a whole. This possibility is currently under investigation.
A second important clinical implication of our findings regarding FLT3L is the benefit of this molecule in promoting retroviral gene transfer into primitive human HPC. In accord with the ability of FLT3L to increase the recruitment of CD34+CD38− cells, there was a twofold increase in retroviral transduction of these cells when cultured in the presence of FLT3L and 4 HGF. We speculate that the increased rate of transduction of CD34+CD38− cells is most likely related to the increased proportion of cells, which are induced to divide by FLT3L. Of note in our studies was the poor viability and low rate of retroviral transduction of CD34+CD38− cells when cultured with IL-3, IL-6, and SCF. This result is in contrast to other studies that report that this combination of HGF results in high levels of retroviral transduction of CD34+ cells.69,70 Studies performed in our laboratory show that less than 5% of CD34+CD38− cells are recruited into cycle within 7 days by IL-3, IL-6, and SCF, a result that correlates with the low degree of retroviral transduction of primitive HPC when cultured with the same HGF combination. It was also notable that for CD34+CD38+ cells, where greater than 90% of cells can be recruited into cell cycle by 4 HGF, that FLT3L did not enhance retroviral-mediated transduction of these cells. It was, however, unexpected that only 20% of CD34+CD38+ cells were transduced despite a considerably greater proportion of the cells dividing when cultured with 4 HGF or 4 HGF and FLT3L. This low rate of transduction may be related to expression of the amphotropic receptor71 on CD34+CD38+ cells, although this was not determined in the present study. Alternatively, the transduced CD34+CD38+ cells may have a reduced proliferative capacity in comparison to untransduced cells and therefore progeny of the transduced cells would represent a small proportion of the total cells at the time alkaline phosphatase staining was performed. In conclusion, while FLT3L may conceivably improve retroviral transduction of a proportion of CD34+ CD38− cells, it is still necessary to develop ex vivo culture conditions to induce cycling of transplantable stem cells.
ACKNOWLEDGMENT
We gratefully acknowledge Dr Bill Sheridan and Dr Ian McNiece of AMGEN for kindly providing SCF, G-CSF, IL-1, IL-3, IL-6, and GM-CSF and also Dr Doug Williams of IMMUNEX for kindly providing FLT3L. We also acknowledge and thank Dr L.B. To and the clinical hematologists from the Division of Hematology for assisting with procurement of bone marrow from adult volunteers. We thank Alan Bishop and Judy Haywood for excellent assistance with flow cytometry and also James Wyatt for assisting with studies on single cells.
Supported by grants in aid from the Anti-Cancer Foundation of the Universities of South Australia.
Address reprint requests to David Haylock, Leukaemia Research Unit, Institute of Medical and Veterinary Science, PO Box 14, Rundle Mall, Adelaide, Australia 5000.

![Fig. 1. Growth of myeloid (CFU-GM) and erythroid (BFU-E) progenitor cells from BM CD34+, CD34+CD38−, or CD34+CD38+ cells. Triplicate, 1 mL assays were established with 500 cells in either 6 HGF (10 ng/mL of IL-1, IL-3, IL-6, G-CSF, GM-CSF, and SCF ) or 6 HGF + FLT3L (shown by the solid bar) and scored at day 14. CFU-GM and BFU-E numbers refer to the number of colonies grown from 1,000 cells with the respective phenotypes. The results of three separate experiments are shown (mean + standard error of mean [SEM]).](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/90/6/10.1182_blood.v90.6.2260/3/m_bl_0002f1.jpeg?Expires=1769488494&Signature=X~haRSw01~gFRhY1Eg1qw0DvOmQzzQNsGInFi23plx8xNDRYOBAJqSgQPZknai~f1~3l76ZKZsqGBmY-pc4k8LCR1jb5mouKccLQzrctrmqE7nmVpYEVu7UMTRrdMT8VyvEicQEzh2LFO3vZak1HfwIdFLGkVGAJHqVcM7j8P1uB9gNhlynaZSHD69-tcFGmSu87xQ2PgFjmgbjxHHdpZHRAC5ur9oVpp6kV1cHa0j1y1SA0RONitrlgckefE6kLGg9eXuoqffdnO9eWoNNmYWYxcCAKOYBy-~irmTwZVYSHF1UJ9PiuSWfSTIh71nIs-JqyLFrdyhFJEyL77ZXOdQ__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)
This feature is available to Subscribers Only
Sign In or Create an Account Close Modal