Abstract
Unexpected clonal variability was observed in the content of β-globin mRNA in erythropoietin receptor (EpoR)-transfected Ba/F3 cells before and after exposure to erythropoietin (Epo). Of 11 clones selected by virtue of G418 resistance and positive EpoR expression, 5 clones showed high levels of βmajor-globin mRNA before Epo exposure, with subsequent Epo treatment causing little or no increase in globin mRNA. Five clones had undetectable levels of globin mRNA before Epo stimulation, and they did not accumulate globin mRNA when exposed to Epo, exhibiting resistance to the differentiation inducing action of Epo. Only one clone exhibited the expected phenotype, a low level of globin mRNA before exposure to Epo, and a significant Epo-dependent accumulation of globin mRNA. Phosphorylation of tyrosyl residues of the EpoR, Stat5, and JAK2 occurred upon Epo stimulation in clones representing each category. Furthermore, electrophoretic mobility shift assays using a Stat5 consensus sequence showed a difference in the nuclear binding component among these clones. These findings indicate that (1) the attainment of EpoR+ Ba/F3 clones with the anticipated sensitivity to both the growth and differentiation inducing actions of Epo is a rare event and (2) STAT5 transcription factors were differently activated by Epo in clones that differed in sensitivity to Epo.
INTERACTION OF cytokines with their cognate receptors results in both the proliferation and differentiation of a specific lineage of hematopoietic progenitors.1 The molecular mechanism(s) by which cytokine receptors exert these paradoxical effects is unknown. Delineation of the decision mechanism that leads cells to undergo self-renewal or to commit to a differentiation pathway is central to an understanding of the regulation of hematopoiesis. The lineage-specific cytokine erythropoietin (Epo) appears to be particularly suitable for studies designed to define the events responsible for the induction of differentiation because of (1) its definitive physiologic functions in support of clonal proliferation and the induction of the terminal maturation of relatively late lineage erythroid precursors to produce mature erythrocytes2 and (2) the existence of cell lines which respond to Epo by the accumulation of hemoglobin, a specific marker of erythroid differentiation.
Erythroleukemia cell lines such as TSA83,4 and SKT6,5 established with various isolates of the Friend virus, Rauscher erythroleukemia cells,6 and J2E erythroid cells,7 are known to respond to Epo by undergoing differentiation. These cell lines express the Epo receptor (EpoR), but their growth is not primarily dependent on Epo. In contrast to these cell lines, interleukin-3 (IL-3)–dependent bone marrow-derived cell lines, which express negligible amounts of endogenous EpoR, are unique in that they acquire responsiveness to Epo after the introduction of the EpoR by transfection. Depending on the cell type, Epo can act as a growth-promoting factor (eg, FDC-P18 and DA-39 cell lines) or as a growth-promoting and differentiation-inducing factor (eg, Ba/F39-15 and B6SUtA16 cell lines). These cell systems have contributed to the identification of a number of key signaling molecules associated with Epo stimulation, including JAK2, STAT5, MAPK, PI 3-kinase, SH-PTP1, etc (reviewed by Watowich et al17 ).
A significant advantage intrinsic to IL-3–dependent cell lines is the flexibility of introducing modified constructs of the EpoR to define functional domains. However, the transfection of chimeric receptors in which the extracellular or intracellular domain of the EpoR is substituted by that of other types of cytokine receptors into Ba/F3 cells has created controversial interpretations as to which domain of the EpoR specifies erythroid maturation and as to whether the cytosolic portion of other cytokine receptors has the ability to elicit an erythroid-specific differentiation signal.11,12 15
Transfection of a receptor of interest into IL-3–dependent cells in all cases relies upon the selection of clones with a drug resistance marker that allows the subsequent exposure of the clones to a cytokine to test the response. In this report, we describe the clonal variation in β-globin mRNA content in Ba/F3 cells expressing murine wild-type EpoR selected by using G418 resistance. Some of these clones exhibited high levels of β-globin mRNA in the absence of Epo stimulation, whereas some clones failed to accumulate globin mRNA upon stimulation by Epo. Although variation in kinetics as well as in the extent of accumulation of β-globin mRNA in various clones of Ba/F3 cells expressing the EpoR has been noted previously,9 this phenomenon was not followed-up in detail and the significance was not discussed. The clonal variability in G418-selected clones contrasts to homogeneity in β-globin mRNA expression in Ba/F3 clones selected for growth in Epo-containing medium.18 The selection of clones in Epo-containing medium permitted the quantification of differentiation-inducing capacities of various mutated constructs of the EpoR.18 The clonal variability observed in the present report shows the difficulty in using G418 selection as a means of developing receptor-positive Ba/F3 cells in studies designed to define the functional domains of the receptor.
MATERIALS AND METHODS
Cells and reagents.Murine IL-3–dependent Ba/F3 cells19 (supplied by Dr H.F. Lodish, Whitehead Institute for Biomedical Research, Cambridge, MA) were maintained in RPMI 1640 medium supplemented with 10% fetal bovine serum (FBS) and 10% conditioned medium from WEHI-3B cells (WEHI-CM) as a source of IL-3. Before initiating experiments, Ba/F3 cells were cloned in medium containing 0.8% methylcellulose and the entire experiment was derived from a single clone. Human recombinant Epo in both bovine serum albumin-stabilized and carrier-free formulations was generously donated by the R.W. Johnson Pharmaceutical Research Institute (Raritan, NJ).
Transfection and selection.Construction of the EpoR expression plasmid p75/15-EpoR has been described previously.16 The plasmid contains the murine EpoR gene under the control of the human metallothionein IIA promoter and the bacterial neo gene, which confers resistance to the antibiotic G418. Ba/F3 cells (1 × 107) were subjected to electroporation with 10 μg of plasmid DNA as previously described.16 Cells were washed and incubated for 1 day to allow for expression. For selection, cells were plated at a density of 3 × 105 cells/dish (60 × 15 mm) in 5 mL of medium containing 0.8% methylcellulose and 0.8 mg/mL of G418. Colonies were selected on day 11 and propagated in the presence of G418 until consistent cellular growth was established in suspension. Thereafter, G418 was removed from the medium.
Stimulation of cells with Epo.For analysis of β-globin mRNA, cells maintained in IL-3–containing medium were collected by centrifugation and washed with 10 mL of RPMI 1640 medium containing 10% FBS three times and then exposed to 0.5 U/mL of Epo or 0.5 U/mL of Epo and 5 ng/mL of IL-3 (recombinant murine IL-3; R & D Systems, Minneapolis, MN). For immunoprecipitations and preparation of nuclear extracts, cells were washed as described for β-globin mRNA analysis and stimulated with Epo for 10 minutes as described in each section. A starvation period after removal of IL-3 was not included in these studies.
Radioiodination of Epo and 125I-Epo binding.One microgram (125 U) of carrier-free human recombinant Epo was radioiodinated using the IODO-GEN method as previously described.16 The specific activity of 125I-Epo was 2.5 × 105 cpm/ng. The biologic activity of 125I-Epo, measured by the ability to promote proliferation of a clone of Ba/F3 cells expressing the EpoR, was decreased by 90% as a result of the iodination. To measure the specific binding of 125I-Epo to cells, 3 × 106 cells in duplicate were incubated with 2.5 U/mL of 125I-Epo in the presence or absence of 250 U/mL of unlabeled Epo at 37°C for 30 minutes and radioactivity associated with cells was determined.16 Epo binding was maximum between 15 and 30 minutes at 37°C. The degree of Epo binding at maximum at 37°C was equivalent to that at equilibrium reached at about 17 hours at 4°C. The Friend erythroleukemia cell line 745-PC4, which was assumed to express a constant level of Epo binding capacity, was included as a positive control.
Northern hybridizations.Total cellular RNA was isolated using a Trizol reagent (Life Technologies, Inc, Gaithersburg, MD). RNA (10 μg) was separated by agarose/formaldehyde gel electrophoresis, stained with acridine orange, transferred to a nitrocellulose membrane, immobilized, and hybridized with 32P-labeled cDNA probes as described previously.16 Because the staining allowed visualization of 28s and 18s rRNAs, the membrane was cut just below the 18s rRNA mark, and upper and lower portions were subjected to hybridization with actin and β-globin probes, respectively. To obtain a murine βmajor-globin cDNA probe, a 458-bp fragment corresponding to the entire murine βmajor-globin cDNA20 was amplified by reverse transcription-polymerase chain reaction from dimethyl sulfoxide-treated Friend cell RNA and cloned into pRc/CMV (Invitrogen, San Diego, CA). The presence of βmajor-globin cDNA was verified by DNA sequencing. The actin probe, a 2-kb Pst I fragment of chicken β-actin from pA1,21 was used to monitor RNA loading. A 1.8-kb Kpn I fragment of mouse EpoR cDNA from pXM-EpoR22 was used to probe for endogenously as well as exogenously derived EpoR mRNAs.
Immunoprecipitations and Western blot analyses.Cells at a density of 107 cells/mL were either not stimulated or stimulated with 50 U/mL of Epo at 37°C for 10 minutes. At the end of the incubation, ice-cold Hank's Balanced Salt Solution (HBSS) containing 1 mmol/L Na3VO4 and 5 mmol/L EDTA was added and cells were collected by centrifugation in an Eppendorf microcentrifuge for 4 seconds. Cells (2 × 107 cells/mL) were solubilized in a lysis buffer consisting of 0.5% NP-40, 50 mmol/L N-2-hydroxyethylpiperazine-N′-2-ethanesulfonic acid (HEPES), pH 7.4, 100 mmol/L NaF, 10 mmol/L sodium pyrophosphate, 2 mmol/L Na3VO4 , 2 mmol/L EDTA, 50 μg/mL of aprotinin, 40 μg/mL of leupeptin, and 1 mmol/L phenylmethanesulfonyl fluoride (PMSF ) and cell lysates were cleared by centrifugation for 3 minutes in a microcentrifuge. The supernatant (190 μL) was mixed with 4 μg of antiphosphotyrosine monoclonal IgG 4G10 (UBI, Lake Placid, NY) overnight. The immune complex was absorbed onto 25 μL of protein A-agarose (Pierce Chemical Co, Rockford, IL), washed three times with the lysis buffer, and released in 30 μL of 2× Laemmli's sample buffer23 by heating at 100°C for 5 minutes. The immune complex was subjected to sodium dodecyl sulfate-7.5% polyacrylamide gel electrophoresis (SDS-PAGE)23 and electrophoretically transferred to a nitrocellulose membrane. The membrane was probed, successively, with 4G10, rabbit anti-STAT5b polyclonal IgG (Santa Cruz Biotechnology, Inc, Santa Cruz, CA), and rabbit antimouse JAK2 polyclonal antiserum (UBI), each at a concentration of 1 μg/mL. Detection was by the enhanced chemiluminescence (ECL) system (Amersham Corp, Arlington Heights, IL).
Electrophoretic mobility shift assay (EMSA).24To prepare nuclear extracts, 2 × 107 cells were either not stimulated or stimulated with 100 U/mL of Epo at 37°C for 10 minutes in a volume of 0.5 mL. Cells were collected by centrifugation and washed once with HBSS containing 1 mmol/L Na3VO4 and 5 mmol/L EDTA. Cells were solubilized in 100 μL of the lysis buffer by vigorous vortexing and the cytoplasmic extract was separated from the nuclear pellet by centrifugation at 1,300g for 4 minutes in the microcentrifuge. Nuclear pellets, washed once with the lysis buffer without NP-40, were extracted with 50 μL of nuclear extraction buffer consisting of 20 mmol/L HEPES, pH 7.4, 0.42 mol/L NaCl, 25% glycerol, 1.5 mmol/L MgCl2 , 0.2 mmol/L EDTA, 0.5 mmol/L dithiothreitol, and 0.5 mmol/L PMSF. Nuclear extracts were cleared by centrifugation at 16,000g for 2 minutes. Protein concentrations of cytoplasmic and nuclear extracts, as determined by Bradford assays,25 were 13.0 and 1.25 mg/mL, respectively. STAT5 gel shift oligonucleotide (sc-2566; Santa Cruz) was end-labeled with [γ-32P]ATP and polynucleotide kinase. The nuclear extract (10 μL) was incubated with 105 cpm of the probe and 2 μg of poly[d(I-C)] at room temperature for 15 minutes and analyzed by 5% PAGE in 0.25× TBE (Tris-borate/EDTA electrophoresis buffer).24
RESULTS
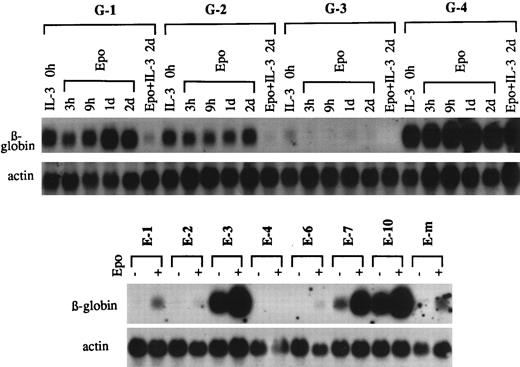
To assess the responsiveness of a factor-dependent hematopoietic cell line to a cytokine, conventional methodology uses the transfection of the cytokine receptor with a drug resistance selection marker followed by a determination of the response of drug-resistant clones to the cytokine. The EpoR expression plasmid, p75/15-EpoR, contained the functional EpoR gene as well as the neo gene that conferred resistance to G418.16 Ba/F3 cells were subjected to electroporation with p75/15-EpoR and colonies were developed in semisolid medium containing G-418. To determine whether Epo was capable of transmitting a differentiation signal in G418r clones, cells were washed and exposed to Epo, and the expression of the βmajor-globin gene before and after Epo stimulation was determined by Northern hybridization. The G-418 selection procedure produced a number of unexpected results (Fig 1, top panel). First, in three of four clones (G-1, G-2, and G-4), substantial amounts of β-globin mRNA were present before Epo stimulation (IL-3 0 hours). Subsequent Epo stimulation produced little increase in β-globin mRNA in these clones. The accumulation of β-globin mRNA in the presence of IL-3 appeared to be contradictory to the repeatedly reported inhibitory role of IL-3 in the induction of differentiation by lineage-specific cytokines.13,16 26-28 However, in agreement with these previously reported results, simultaneous exposure to Epo and IL-3 (Epo + IL-3 2 days) decreased the level of β-globin mRNA in clones G-1 and G-2, but not in clone G-4. In contrast to clones G-1, G-2, and G-4, clone G-3 exhibited a low level of β-globin before Epo exposure, and globin mRNA levels in this clone remained low after Epo stimulation.
Levels of β-globin mRNA before and after stimulation with Epo in G418r clones of Ba/F3 cells from two independent transfections. (Top panel) G418r clones (designated G-1 through G-4) maintained in IL-3–containing medium (IL-3 0 hours) were washed and exposed to either Epo alone for 3 hours, 9 hours, 1 day, and 2 days or to Epo and IL-3 for 2 days. Total cell RNA was hybridized with βmajor-globin and actin probes. (Bottom panel) G418r clones (designated E-1 through E-10) and a mixture of G418r cells propagated in suspension culture (designated E-m) derived from a separate transfection were washed and exposed to Epo for 2 days. The β-globin mRNA contents before and after Epo exposure were measured.
Levels of β-globin mRNA before and after stimulation with Epo in G418r clones of Ba/F3 cells from two independent transfections. (Top panel) G418r clones (designated G-1 through G-4) maintained in IL-3–containing medium (IL-3 0 hours) were washed and exposed to either Epo alone for 3 hours, 9 hours, 1 day, and 2 days or to Epo and IL-3 for 2 days. Total cell RNA was hybridized with βmajor-globin and actin probes. (Bottom panel) G418r clones (designated E-1 through E-10) and a mixture of G418r cells propagated in suspension culture (designated E-m) derived from a separate transfection were washed and exposed to Epo for 2 days. The β-globin mRNA contents before and after Epo exposure were measured.
These unexpected findings led us to obtain new G418r clones from a separate transfection to ensure that they were not erroneous results. Again, two of three clones exhibited high levels of globin mRNA before stimulation with Epo and one showed no globin mRNA accumulation before or after exposure to Epo (data not shown). A third independent transfection was performed to determine whether the expression of the EpoR itself was the factor that determined whether Ba/F3 cells committed to a differentiation pathway in the absence of Epo. To accomplish this, Ba/F3 cells were subjected to electroporation with either p75/15 (the plasmid lacking EpoR cDNA) or p75/15-EpoR and the levels of β-globin mRNA before Epo stimulation were determined in 10 G418r clones for each plasmid. None of the clones from the transfection of the vector alone was a positive expressor of globin mRNA (data not shown). The bottom panel in Fig 1 shows levels of globin mRNA in G418r clones transfected with p75/15-EpoR before and after exposure to Epo for 2 days. Two clones (E-3 and E-10) exhibited high levels of globin mRNA before Epo exposure. In contrast, the levels of globin mRNA in four clones (E-1, E-2, E-4, and E-6) were negligible in IL-3–containing medium and remained low after treatment with Epo. Only one clone (E-7) exhibited an expected phenotype, ie, a low level of globin mRNA in IL-3–containing medium and an Epo-dependent accumulation of globin mRNA.
Ba/F3 cells are capable of responding to some chemical inducers of differentiation.29 Because the pathways involved in inducing differentiation by chemical agents and by cytokines may overlap, the possibility exists that G418 present at a concentration of 0.8 mg/mL in the selection medium contributed to the induction of β-globin mRNA in some of the G418r clones. The finding that no induction of β-globin mRNA occurred in Ba/F3 cells treated with G418 at concentrations ranging from 0.6 to 1.2 mg/mL for 2 days (data not shown) indicates that G418 itself does not cause differentiation.
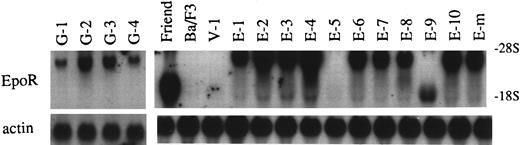
To examine the relationship between the differences in phenotype and the levels of the EpoR in G418r clones of Ba/F3 cells, the relative levels of β-globin mRNA before and after stimulation with Epo, the degree of Epo-induced cell growth, and the 125I-Epo binding capacity are summarized in Table 1. In addition, quantitative as well as qualitative aspects of EpoR mRNA in these clones were assessed by Northern hybridization (Fig 2), because exogenous EpoR mRNA was distinguishable from endogenous EpoR mRNA by size.16 Clones E-5, E-8, and E-9 lacked Epo-dependent growth, in agreement with a low degree of specific Epo binding. All of the other clones exhibited variable degrees of Epo-dependent growth, as well as of specific Epo binding. These two parameters showed a positive relationship. In contrast, the level of EpoR, shown by various means, did not correlate with the sensitivity or resistance of clones to the differentiation inducing action of Epo.
Summary of the β-Globin mRNA Content, the Epo-Dependent Cell Growth, and the Epo Binding Capacity of G418rClones of Ba/F3 Cells Transfected With an EpoR Expression Plasmid
| Clone . | β-Globin mRNA . | Epo-Dependent Growth . | ||||
|---|---|---|---|---|---|---|
| . | −Epo . | +Epo . | Cells/mL ×10−5 . | Fold Increase . | 125I-Epo Binding . | |
| . | . | . | d 0 . | d 2 . | . | (cpm/3 × 106 cells) . |
| G-1 | ++ | ++ | 1.2 | 6.83 | 5.7 | 2,740 |
| G-2 | + | + | 1.2 | 8.66 | 7.2 | 7,250 |
| G-3 | − | − | 1.2 | 11.6 | 9.7 | 5,740 |
| G-4 | +++ | +++ | 1.2 | 14.3 | 11.9 | 5,380 |
| E-1 | − | ± | 0.44 | 5.23 | 11.9 | 6,540 |
| E-2 | − | − | 0.41 | 9.88 | 23.9 | 8,540 |
| E-3 | ++ | +++ | 0.35 | 8.63 | 24.3 | 6,920 |
| E-4 | − | − | 0.46 | 9.55 | 21.0 | 15,600 |
| E-5 | − | NM | 0.44 | 0.50 | 1.1 | 830 |
| E-6 | − | − | 0.48 | 1.70 | 3.5 | 2,160 |
| E-7 | ± | +++ | 0.46 | 5.63 | 12.2 | 5,930 |
| E-8 | − | NM | 0.47 | 0.38 | 0.8 | 770 |
| E-9 | − | NM | 0.34 | 0.52 | 1.5 | 1,600 |
| E-10 | ++ | +++ | 0.31 | 6.55 | 21.2 | 5,610 |
| E-m | − | ± | 0.52 | 6.86 | 13.1 | 6,670 |
| Clone . | β-Globin mRNA . | Epo-Dependent Growth . | ||||
|---|---|---|---|---|---|---|
| . | −Epo . | +Epo . | Cells/mL ×10−5 . | Fold Increase . | 125I-Epo Binding . | |
| . | . | . | d 0 . | d 2 . | . | (cpm/3 × 106 cells) . |
| G-1 | ++ | ++ | 1.2 | 6.83 | 5.7 | 2,740 |
| G-2 | + | + | 1.2 | 8.66 | 7.2 | 7,250 |
| G-3 | − | − | 1.2 | 11.6 | 9.7 | 5,740 |
| G-4 | +++ | +++ | 1.2 | 14.3 | 11.9 | 5,380 |
| E-1 | − | ± | 0.44 | 5.23 | 11.9 | 6,540 |
| E-2 | − | − | 0.41 | 9.88 | 23.9 | 8,540 |
| E-3 | ++ | +++ | 0.35 | 8.63 | 24.3 | 6,920 |
| E-4 | − | − | 0.46 | 9.55 | 21.0 | 15,600 |
| E-5 | − | NM | 0.44 | 0.50 | 1.1 | 830 |
| E-6 | − | − | 0.48 | 1.70 | 3.5 | 2,160 |
| E-7 | ± | +++ | 0.46 | 5.63 | 12.2 | 5,930 |
| E-8 | − | NM | 0.47 | 0.38 | 0.8 | 770 |
| E-9 | − | NM | 0.34 | 0.52 | 1.5 | 1,600 |
| E-10 | ++ | +++ | 0.31 | 6.55 | 21.2 | 5,610 |
| E-m | − | ± | 0.52 | 6.86 | 13.1 | 6,670 |
Relative levels of β-globin mRNA (−, ±, +, ++, and +++) in clones before and after exposure to Epo were determined by the intensity of the Northern blots shown in Figs 1 and 3. The β-globin mRNA content in clones E-5, E-8, and E-9 after Epo exposure was not measurable due to the absence of cell growth. Epo-induced cell growth was expressed as the fold increase in the cell density from day 0 (d 0) to day 2 (d 2) in the presence of Epo. Epo binding was measured as described in the Materials and Methods. The values for Friend erythroleukemia and Ba/F3 cells were 7,660 and 440 cpm/3 × 106 cells, respectively.
Abbreviation: NM, not measurable.
Levels of EpoR mRNA in G418r clones of Ba/F3 cells transfected with the EpoR expression plasmid. Note that exogenously derived EpoR mRNA in G418r clones was larger in size than endogenous EpoR mRNA present in Friend erythroleukemia cells. V-1 is a G418r clone transfected with the p75/15 vector lacking EpoR cDNA.
Levels of EpoR mRNA in G418r clones of Ba/F3 cells transfected with the EpoR expression plasmid. Note that exogenously derived EpoR mRNA in G418r clones was larger in size than endogenous EpoR mRNA present in Friend erythroleukemia cells. V-1 is a G418r clone transfected with the p75/15 vector lacking EpoR cDNA.
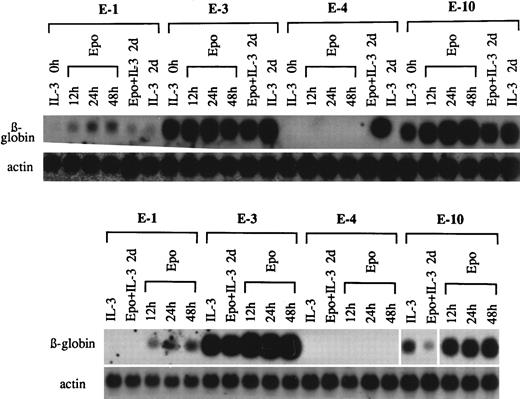
Previous reports by others are inconsistent in the time course of appearance of globin mRNA in EpoR+ Ba/F3 cells after exposure to Epo.9 10 Using clones exhibiting various amounts of globin mRNA and various sensitivities to Epo, the time courses of globin mRNA induction were measured (Fig 3, top panel). In clones E-1 and E-10, the induction was measurable at 12 hours and plateaued at 24 hours. Although costimulation with Epo and IL-3 after the washing of cells was effective in reducing globin mRNA levels in some of the clones (Fig 1), simple addition of Epo to cells grown in IL-3–containing medium did not reduce the globin mRNA content (compare Epo + IL-3 2 days with IL-3 2 days).
Effect of pretreatment with Epo and IL-3 on responsiveness to the differentiation inducing action of Epo. (Top panel) G418r clones E-1, E-3, E-4, and E-10 in IL-3–containing medium (IL-3 0 hours) were washed and exposed to Epo for 12, 24, and 48 hours. Epo added to cells without washing (Epo + IL-3 2 days) was compared with untreated cultures (IL-3 2 days). (Bottom panel) The same clones in IL-3–containing medium (IL-3) washed and pretreated with Epo and IL-3 for 2 days (Epo + IL-3 2 days). Cells were then washed again and exposed to Epo for 12, 24, and 48 hours.
Effect of pretreatment with Epo and IL-3 on responsiveness to the differentiation inducing action of Epo. (Top panel) G418r clones E-1, E-3, E-4, and E-10 in IL-3–containing medium (IL-3 0 hours) were washed and exposed to Epo for 12, 24, and 48 hours. Epo added to cells without washing (Epo + IL-3 2 days) was compared with untreated cultures (IL-3 2 days). (Bottom panel) The same clones in IL-3–containing medium (IL-3) washed and pretreated with Epo and IL-3 for 2 days (Epo + IL-3 2 days). Cells were then washed again and exposed to Epo for 12, 24, and 48 hours.
To examine the effects of pretreatment with Epo and IL-3 on the restoration of responsiveness to Epo (Fig 3, bottom panel), the same set of clones E-1, E-3, E-4, and E-10 in IL-3–containing medium (IL-3 0 hours) were washed and exposed to Epo and IL-3 for 2 days (Epo + IL-3 2 days). This treatment significantly reduced the amount of globin mRNA in clone E-10, but not appreciably in clone E-3. Cells were again washed and exposed to Epo alone for 12, 24, and 48 hours. Clone E-10 showed the greatest response to Epo, because the initial level was lowered by the pretreatment with Epo and IL-3. The pretreatment did not modify the sensitivity of clones E-1 and E-4 to Epo.
The time course of β-globin mRNA induction by Epo was determined in the Epo-responsive clone E-7 (Fig 4). An increase in globin mRNA was detected at 9 hours and the level plateaued at 24 hours. Thus, the kinetics of the increase in globin mRNA in clone 7 were similar to those in other clones such as E-1 and E-10. These results indicate that it takes at least 9 hours for the differentiation signal from the activated EpoR to be processed to the nucleus to turn on the globin gene. The kinetics of induction of globin mRNA by Epo shown in this investigation are significantly faster than those reported by others.9 10 Figure 4 also shows that simultaneous stimulation of clone E-7 with Epo and IL-3 not only completely abolished the induction of globin mRNA but also reduced the background level of this message. The phenotype of clone E-7, as well as that of other clones, was stable, with the measured inducibility of globin mRNA by Epo and the globin mRNA content remaining relatively constant over a period of months.
Time course of accumulation of β-globin mRNA induced by Epo and inhibition of the accumulation by IL-3 in clone E-7. Cells were washed and exposed to either Epo alone or Epo and IL-3 at the indicated times.
Time course of accumulation of β-globin mRNA induced by Epo and inhibition of the accumulation by IL-3 in clone E-7. Cells were washed and exposed to either Epo alone or Epo and IL-3 at the indicated times.
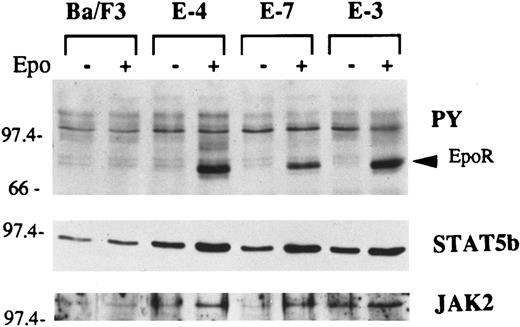
Whether clones with different sensitivities to Epo differ in their capacity to express the signal transduction pathways known to be activated by Epo was investigated in several Ba/F3 clones. These included E-4, which was representative of the clones resistant to the induction of globin mRNA by Epo; the Epo-sensitive clone E-7; and E-3, which was representative of the clones with a constitutively high level of globin mRNA. These three clones were not stimulated or stimulated with Epo for 10 minutes. Cell lysates were prepared and immunoprecipitated with antiphosphotyrosine antibody. The immune complexes were resolved on a nitrocellulose membrane. The blot was successively probed with antiphosphotyrosine, anti-STAT5b, and anti-JAK2 antibodies (Fig 5). The most prominent component in Epo-stimulated cells detected by the antiphosphotyrosine probe is tyrosine-phosphorylated EpoR.30 Tyrosine phosphorylation of STAT5b and JAK2 was also stimulated by Epo in EpoR+ clones. Overall, no gross difference in Epo-induced tyrosine phosphorylation of these signal molecules was noticeable in the three different representative clones. An EMSA using the STAT5 consensus sequence and nuclear extracts from Epo-treated clones, on the other hand, showed that E-7 contained a distinctive binding component in addition to that shared by the other two clones (Fig 6).
Analysis of the phosphotyrosyl signal proteins before and after stimulation with Epo in clones with differing sensitivities to Epo. Cells were washed and either not stimulated or stimulated with Epo for 10 minutes. Cell lysates were immunoprecipitated with antiphosphotyrosine antibody 4G10 (PY). The immune complex was resolved by PAGE and transferred onto a nitrocellulose membrane. The blot was successively probed with antibodies against phosphotyrosine, STAT5b, and JAK2.
Analysis of the phosphotyrosyl signal proteins before and after stimulation with Epo in clones with differing sensitivities to Epo. Cells were washed and either not stimulated or stimulated with Epo for 10 minutes. Cell lysates were immunoprecipitated with antiphosphotyrosine antibody 4G10 (PY). The immune complex was resolved by PAGE and transferred onto a nitrocellulose membrane. The blot was successively probed with antibodies against phosphotyrosine, STAT5b, and JAK2.
An EMSA using the STAT5 consensus sequence and nuclear extracts from clones with differing sensitivities to Epo. Cells were washed and either not stimulated or stimulated with Epo for 10 minutes. Nuclear extracts incubated with 32P-labeled STAT5 consensus oligonucleotide were analyzed by 5% PAGE.
An EMSA using the STAT5 consensus sequence and nuclear extracts from clones with differing sensitivities to Epo. Cells were washed and either not stimulated or stimulated with Epo for 10 minutes. Nuclear extracts incubated with 32P-labeled STAT5 consensus oligonucleotide were analyzed by 5% PAGE.
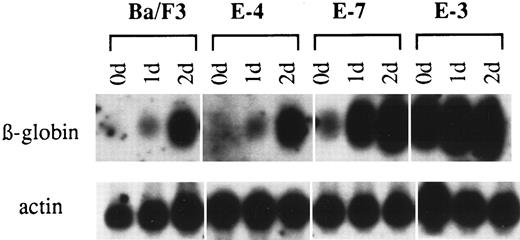
Because accumulation of β-globin mRNA is also induced in Ba/F3 cells by chemical initiators of differentiation such as sodium butyrate,29 it was of interest to determine the sensitivity of the three representative clones to sodium butyrate (Fig 7). Clone E-4 was similar to Ba/F3 cells in its sensitivity to sodium butyrate, indicating that the differentiation pathway used by the chemical inducer is not blocked by the resistance of clone E-4 to Epo. In contrast, accumulation of globin mRNA by sodium butyrate was enhanced in clone E-7 and globin mRNA levels were almost saturated in clone E-3 even before exposure to sodium butyrate.
Induction of differentiation by sodium butyrate in clones with differing sensitivities to Epo. Clones were exposed to sodium butyrate at a concentration of 1 mmol/L for 0, 1, and 2 days and total RNA was hybridized with β-globin and actin probes.
Induction of differentiation by sodium butyrate in clones with differing sensitivities to Epo. Clones were exposed to sodium butyrate at a concentration of 1 mmol/L for 0, 1, and 2 days and total RNA was hybridized with β-globin and actin probes.
DISCUSSION
In this report, we describe the variability observed in the levels of βmajor-globin mRNA in clones of IL-3–dependent Ba/F3 cells transfected with the EpoR. These clones were derived by selection with G418 followed by exposure to Epo to ascertain the degree of induction of globin mRNA by the cytokine. In the absence of exposure to Epo, 45% (5/11) of the EpoR+ clones were positive for globin mRNA expression. Forty-five percent (5/11) of the clones contained low levels of globin mRNA; surprisingly, these clones did not accumulate globin mRNA when they were exposed to Epo. The expected sensitivity to the growth and differentiation inducing actions of Epo was observed in only one clone (1/11).
Several possibilities exist to explain the high levels of β-globin mRNA present in clones selected with G418 in the absence of exposure to Epo. The EpoR may be activated by trace amounts of bovine Epo present in the FBS. This explanation assumes that the level of Epo present in FBS is sufficient to transmit a differentiation signal. However, burst-forming unit-erythroid (BFU-E) and colony-forming unit-erythroid (CFU-E) levels never increase to substantial levels in the absence of the exogenous addition of Epo to cultured bone marrow cells and the concentration of Epo present in FBS would be expected to be exceedingly low. Nonetheless, Carroll et al13 have reported that the lower the concentration of Epo in the medium, the higher the degree of differentiation in EpoR+ Ba/F3 cells. However, this possibility appears to be limited by the assumption that Epo would be required to cause differentiation in the presence of IL-3. It is also conceivable that the function of the EpoR may be modulated by a factor(s) other than Epo. Steel cell factor, for example, is known to phosphorylate the EpoR,31 and fetal calf serum has been reported to contain activities that induce hemoglobin.32 Other possibilities are that EpoR, at a relatively high level of expression under the control of a strong promoter, may undergo spontaneous activation or that the formation of a ligand-independent EpoR dimer is sufficient to elicit a differentiation signal. In contrast to several possible explanations for the ligand-independent activation of the globin gene, the mechanism by which 45% of the clones only respond to the growth-promoting action of Epo and exhibit resistance to the differentiation inducing action of the cytokine is difficult to explain.
To define the domain of the EpoR necessary for the induction of differentiation, we have constructed several mutated receptors and used a protocol in which clones were selected by growth in Epo-containing semisolid medium instead of by resistance to G418.18 In this protocol, 100% of the clones were homogeneously positive for β-globin mRNA expression; thus, quantification of the capacity of the clones transfected with the various constructs to undergo growth and differentiation was possible. From these investigations, an inverse relationship was found between the levels of the EpoR and globin mRNA in each construct, as well as an inverse relationship between the growth-promoting and differentiation-inducing capacities of the constructs. Thus, although experiments were conducted with a single clone of Ba/F3 cells, cells may have diverged into heterogeneous populations in a stochastic fashion, with G418-selected clones representing the entire population and Epo-selected clones representing a subpopulation that is more Epo responsive. Because all of the studies on the ability of the EpoR to induce β-globin mRNA expression in Ba/F3 cells reported thus far9-15 have relied on the selection of clones through their resistance to G418, it is possible that the expression of globin mRNA in the absence of ligand and the resistance of some clones to Epo were observed in these studies and not reported or not taken into consideration in the interpretation of the results.
One of the puzzling aspects in the induction of differentiation by Epo is the modulation by IL-3. IL-3 has been consistently reported to counteract the differentiation-inducing action of lineage-specific cytokines.13,16,26-28 In agreement with these reports, in our studies, IL-3 competely abrogated the induction of globin mRNA by Epo in clone E-7 (Fig 4). The coexposure of cells to Epo and IL-3 was also effective in lowering the level of β-globin mRNA in E-7 and other clones. However, β-globin mRNA accumulation in clones such as G-1, G-2, G-4, E-3, and E-10 in the absence of ligand stimulation occurred in the presence of IL-3. Moreover, once a high level of β-globin mRNA was induced in EpoR+ Ba/F3 cells, the level was maintained, even after cells were switched to IL-3–containing medium for several months10 (also confirmed in this laboratory). Taken together, it appears that IL-3 per se is not inhibitory for globin gene expression, but that the copresence of Epo and IL-3 is necessary for IL-3 to exert its suppressive effect.
Previous reports concerning the induction by Epo of β-globin mRNA in EpoR+ Ba/F3 cells showed significant variability in the time course of appearance of the globin message.9,10 Thus, in one study, the induction of β-globin mRNA by Epo first occurred at day 3 and continued to progress until day 8.10 In another report, the kinetics of appearance of globin mRNA were variable in various clones, ranging from day 1 to day 3.9 In the present investigation, the induction of β-globin mRNA was consistently detectable at 9 to 12 hours and plateaued at 24 hours in the various clones, regardless of the degree of response. The time course observed in our studies fell within the range reported for the J2E cell line, in which β-globin mRNA induction was detected as early as 6 hours.7 The kinetic data shown in this report have important implications for the mechanism by which the differentiation signal is processed from the receptor to the nucleus to activate functional genes and indicate the utility of the globin gene promoter in studies of the specific transcription factors involved.
Investigations by others14 as well as by us18 localize the domain required for the induction of differentiation to the membrane proximal 100 amino acids of the cytoplasmic portion of the EpoR. This area coincides with the domain essential for induction of growth and activation of JAK2 and STAT5 (reviewed by Watowich et al17 ). Thus, it is unknown whether the JAK-STAT pathway controls both growth and differentiation or whether an as yet unidentified pathway regulates the differentiation pathway. Although many reports have implicated the JAK-STAT pathway in the control of growth, this pathway is also clearly involved in specialized cellular functions. Thus, interferons that are largely antiproliferative use the JAK-STAT pathway.33 The STAT5 binding site is also located in the promoter region of the casein gene, which is activated by prolactin.34 Recently, a role for STAT5 activation in Epo-induced hemoglobin accumulation has been shown.35 Our finding that the Epo-sensitive clone E-7 is different from others in the STAT binding component may be one such indication. However, there is a discrepancy in the time between the activation of the STAT protein and the activation of the functional gene. Thus, the appearance of the activated STAT protein after stimulation with Epo peaks in about 10 minutes in the nucleus,36 whereas activation of the functional gene takes place at a much later time (at least 9 hours in Ba/F3 cells). These findings show that other events occur in the gap between these phenomena.
Transfection of a chimera consisting of the extracellular domain of the EpoR and the intracellular domain of the IL-3 receptor β (IL-3Rβ) into Ba/F3 cells has led to a controversial interpretation as to which domain of the EpoR specifies erythroid differentiation and as to whether the intracellular domain of IL-3Rβ has the ability to transmit an erythroid-specific differentiation signal.11,12 15 Because signals induced by different cytokines are redundant in many cases, it is possible that nonphysiologic receptors could serve as surrogates for physiologic receptors, if they can properly relay signals to cellular machinery that are intrinsic to a specific cell lineage. Conversely, some prototypical chemical inducers of differentiation can induce both myeloid and erythroid differentiation; thus, a common biochemical mechanism may be involved in inducing the differentiation of different cellular lineages.
The potential limitations, as well as the versatility, of IL-3–dependent Ba/F3 cells as a model of Epo-induced erythroid differentiation are shown in the present report. Clonal variability, including the presence of β-globin mRNA in the absence of Epo stimulation, and the sensitivity and resistance to Epo in inducing the globin gene present difficulties in using this cell system for assessing Epo-induced differentiation. However, conversely, the attainment of stable clones that respond to Epo in different ways presents advantages as well. One clone responds to Epo by both growth and differentiation. Other clones respond to Epo only by growth. Both types of clones are sensitive to the differentiation inducing property of sodium butyrate. These clones offer useful models to dissect signal transduction pathways for induction of cell proliferation and differentiation by the physiologic cytokine Epo and by nonphysiologic chemical inducers.
ACKNOWLEDGMENT
The authors are grateful to Dr Alan D'Andrea and Dr Harvey Lodish for the EpoR expression plasmid pXM-EpoR and the murine Ba/F3 bone marrow cell line, respectively. We also thank the R.W. Johnson Pharmaceutical Research Institute for providing human recombinant Epo.
Address reprint requests to Alan C. Sartorelli, PhD, Department of Pharmacology, Yale University School of Medicine, 333 Cedar St, New Haven, CT 06520.



This feature is available to Subscribers Only
Sign In or Create an Account Close Modal