Abstract
In this double-blind, cross-over, placebo-controlled, randomized study, two groups of eight healthy male volunteers were challenged with endotoxin (4 ng/kg) on two occasions, once in conjunction with placebo and once with granulocyte colony-stimulating factor (G-CSF; 5 μg/kg). In group 1, G-CSF was administered intravenously 2 hours before endotoxin challenge; in group 2, G-CSF was administered subcutaneously 24 hours before endotoxin challenge. In group 1, G-CSF significantly enhanced the release of tumor necrosis factor (TNF ), interleukin-6 (IL-6), IL-8, IL-1 receptor antagonist (IL-1ra), and soluble TNF receptors. In group 2, G-CSF significantly reduced IL-8 concentrations and modestly attenuated TNF and IL-6 levels. In this group, IL-1ra and soluble TNF receptors were enhanced by G-CSF pretreatment and lipopolysaccharide (LPS)-induced soluble TNF receptor release was further augmented, whereas LPS-induced IL-1ra concentrations remained unaltered. Both pretreatments with G-CSF increased LPS-induced peripheral neutrophilia; the expression of CD11b, CD18, and CD67; and the release of elastase and lactoferrin. Both pretreatments also downregulated neutrophil L-selectin expression and prevented the endotoxin-induced pulmonary neutrophil accumulation during the first 2 hours after endotoxin challenge. These data indicate that two different pretreatments with G-CSF result in differential effects on LPS-induced cytokine release but similar effects on LPS-induced neutrophil activation and changes in expression of cell surface molecules. Finally, regardless of the effects of G-CSF on LPS-induced cytokine release, G-CSF blocks LPS-induced pulmonary granulocyte accumulation.
NORMAL NUMBERS and function of neutrophils are necessary for a successful host defense against a variety of invading microbes.1 On the other hand, systemic inflammatory responses are associated with sequestration of activated neutrophils in organs, which may lead to tissue destruction through release of oxygen radicals and proteolytic enzymes.2-4 The mechanism of granulocyte adherence to the vascular endothelium has been well characterized and consists of selectin-mediated rolling,5-7 followed by adhesion dependent on interactions of β2 integrins and intercellular adhesion molecule-1 (ICAM-1).8 Chemotactic agents, such as interleukin-8 (IL-8) and platelet activating factor, are involved in subsequent trans-endothelial cell migration.9,10 The importance of these interactions is underscored by the finding that, in animal models of endotoxemia, interference with the neutrophil-endothelial interaction, by administration of integrin-targeted antibodies, ameliorated end-organ damage, in particular lung injury.11 Granulocyte colony-stimulating factor (G-CSF ) is an 18-kD glycoprotein that increases peripheral blood neutrophil counts by stimulating proliferation and shortening bone marrow transit time, thereby reducing the neutrophil storage pool.12 In addition, G-CSF improves phagocytosis, delays apoptosis, increases chemotaxis and cytotoxicity in vitro, and causes upregulation of the expression of CD11b, CD18, and CD67 by human granulocytes in vivo.13-17 In chemotherapy-treated patients, administration of pharmacologic doses of G-CSF reduced the severity and duration of neutropenia, febrile episodes, and incidents of infections.18-20 Preliminary data suggest that G-CSF treatment might also be beneficial in nonneutropenic patients with bacterial pneumonia or burn injury or after major trauma.21,22 Furthermore, several animal models of bacterial intra-abdominal and pneumonia sepsis have shown that G-CSF improves survival.23-25 This beneficial effect of G-CSF might result from a reduction in the proinflammatory cytokine, tumor necrosis factor-α (TNF-α),23 particularly because the survival benefit occurred before alterations in neutrophil counts were seen.25
Although pretreatment with G-CSF attenuated lung injury during lethal endotoxemia in nonneutropenic animals,26-28 a potential disadvantage of the clinical use of G-CSF in systemic inflammatory diseases is the potentiation of neutrophil-mediated organ damage.
Endotoxin administration to humans is a widely accepted model to study early inflammatory host responses to sepsis in humans and results in the release of cytokines and activation of leukocytes. Knowledge of the effects of G-CSF on lipopolysaccharide (LPS)-induced cytokine release in humans is limited. In recent studies, ex vivo stimulations of whole blood from G-CSF–treated humans showed enhanced IL-1 receptor antagonist (IL-1ra) and soluble TNF receptor (sTNFR) concentrations.29 In Salmonella abortus equi endotoxemia in humans, G-CSF administered 12 hours before endotoxin increased LPS-induced proinflammatory and anti-inflammatory cytokine release.30 To date, the effects of different timing of administration of G-CSF on cytokine release and neutrophil function in human endotoxemia in vivo have not been reported. We therefore designed a controlled, cross-over, placebo-controlled study of low-dose human endotoxemia to evaluate the effects of G-CSF administered 2 hours before LPS challenge or 24 hours before LPS challenge on endotoxin-induced cytokine release, circulating granulocyte and monocyte counts, granulocyte degranulation, and expression of adhesion molecules. Furthermore, endotoxin-induced granulocyte accumulation in the lungs and liver was quantitatively determined by radionuclide dynamic granuloscintigraphy.
MATERIALS AND METHODS
Study group.The study was approved by the research and ethical committees of the Academic Medical Center and written informed consent was obtained from all volunteers. A total of 16 healthy male subjects (mean age, 23 years; range, 19 to 32 years) was studied. Medical history, physical examination, and routine laboratory examination were unremarkable in all volunteers. The study subjects were nonsmokers and did not use any medication, and their ECG and chest x-ray results were normal. Volunteers were excluded from participation in the study if they had had any febrile disease in the 2 weeks preceding the study.
Study design.The study was designed as a double-blind, cross-over, randomized, placebo-controlled study in which all volunteers were treated with endotoxin on two occasions, with a wash-out period of 6 weeks. On one occasion the endotoxin treatment was combined with placebo and on the other occasion with G-CSF. The volunteers were randomized into two groups of eight subjects. Group 1 received either placebo or G-CSF treatment, administered intravenously, starting 2 hours before endotoxin; group 2 received either placebo or G-CSF treatment administered subcutaneously 24 hours before endotoxin administration. These two time points were based on results from a pilot study (Pajkrt et al, unpublished data) in which it was shown that, upon LPS stimulation of whole blood of G-CSF–treated volunteers, TNF-α production was reduced at 2 and 24 hours after G-CSF administration. The study drug G-CSF (Amgen, Thousand Oaks, CA) was provided as a pyrogen-free, colorless, clear fluid in a glass vial containing 300 μg/mL recombinant human G-CSF. To ensure high circulating G-CSF levels at the time of endotoxin administration, group 1 received the total dose of 5 μg/kg as a 30-minute infusion through an intravenous line; group 2 received 5 μg/kg G-CSF subcutaneously, the usual route of administration. The placebo solution was identical in appearance and contained isotonic saline.
The Escherichia coli endotoxin preparations used in this study, lot EC-5 (D. Hochstein, Bureau of Biologics, Food and Drug Administration, Bethesda, MD; group 1) and lot G (United States Pharmacopeia Convention Inc, Rockville, MD; group 2), were administered as an intravenous injection (4 ng/kg) over 1 minute31 2 hours after the start of G-CSF or placebo infusion (group 1) or 24 hours after subcutaneously administered G-CSF or placebo. In group 2, lot G was used because of lack of availability of lot EC-5. The two endotoxin preparations are both smooth forms of endotoxin from the same serotype and show similar endotoxin activity as measured by the Limulus amebocyte lysate assay (data not shown). Each group received the same endotoxin during the two treatment periods.
The volunteers were confined throughout the two study periods at a clinical research unit. During these periods, they were under continuous supervision of at least two physicians. Complete emergency and resuscitation equipment was immediately available. Heart rate was monitored continuously during the first 8 hours after endotoxin infusion, and blood pressure and oxygen saturation were monitored each for 30 minutes using a Dinamap device (Criticon, Tampa, FL) during the first 8 hours after endotoxin administration. Oral temperature and respiratory rate were assessed at the same time points. Adverse events were registered throughout the confinement periods by a clinical symptom score. Adverse events were scored by incidence and severity (1 as weakly, 2 as moderately, and 3 as severely present).
Sampling and assays.Blood was collected by separate venipunctures at −24 (group 2) or −2 and −1 (group 1) and subsequently at 0, 0.5, 1, 1.5, 2, 3, 4, 6, 10, and 20 hours relative to endotoxin administration. For leukocyte and differential counts, blood was collected in tubes containing EDTA (K3 ) (15%) and assessed by flow cytometry (Technicon H1 system; Technicon Instruments, Tarrytown, NY). Blood for cytokine assays was collected in vacutainer tubes (Becton Dickinson, Mountain View, CA); after clotting, samples were centrifuged at 2,000g for 20 minutes at room temperature and serum was stored at −20°C until assays were performed batchwise. Cytokine concentrations were determined using specific enzyme-linked immunosorbent assays (ELISAs): TNF, sTNFR I, and sTNFR II (Medgenix Diagnostic, Brussels, Belgium); G-CSF and IL-1ra (Quantikine; R&D Systems, Abingdon, UK); and IL-6 and IL-8 (Central Laboratory of the Netherlands Red Cross Blood Transfusion Service CLB, Amsterdam, The Netherlands). The ELISAs measure immunologic activity and may not represent biologically active concentrations.
Blood for elastase α1 -antitrypsin complexes (elastase) and lactoferrin assays was obtained in tubes containing 10 mmol/L EDTA, 10 mmol/L benzamidine hydrochloride hydrate 98%, and 100 μg/mL soybean trypsin inhibitor. Circulating levels of elastase and lactoferrin were measured by radioimmunoassay (RIA).32
FACScan analysis of granulocytes.Blood for flow cytometry was obtained at −24 (group 2) or −2 (group 1), 0, 1, 2, 4, and 6 hours relative to endotoxin challenge in EDTA (K3 ) (15%) tubes and immediately placed on ice. Erythrocytes were lysed with ice-cold isotonic NH4Cl solution (155 mmol/L NH4Cl, 10 mmol/L KHCO3 , 0.1 mmol/L EDTA, pH 7.4) for 20 minutes. Cells were centrifuged (600g for 10 minutes at 4°C) and residual erythrocytes were lysed for 5 minutes. The remaining cells were washed twice in cold phosphate-buffered saline (PBS) and subsequently fixed in ice-cold PBS containing 1% bovine serum albumin (BSA), 0.3 mmol/L EDTA, 0.01% sodium azide, and 0.1% paraformaldehyde at a final concentration of 5 × 106 cells/mL.
The antibodies used were directed against CD11b, CD18, CD33, and CD67 (CLB-mon-gran/1,B2, CLB-LFA-1/1,54, CLB-MD33.6, and CLB-B13.9, respectively; Central Laboratory of the Netherlands Red Cross Blood Transfusion Service CLB). Control IgG1 , IgG2 , and L-selectin (Leu-8) were obtained from Becton Dickinson. After the addition of the primary monoclonal antibody (MoAb) to the cell suspension, the cells were incubated for 30 minutes at 4°C and washed twice in cold PBS containing 1% BSA, 0.3 mmol/L EDTA, and 0.01% sodium azide. Subsequently, R-phycoerythrin (RPE)-conjugated F(ab′)2 fragments of rabbit-antimouse Igs (R 0439; Dako A/S, Glostrup, Denmark) were added and cells were incubated for another 30 minutes at 4°C. After two washes, granulocytes were gated by forward scatter and side scatter using a FACScan (Becton Dickinson) and 10,000 cells were counted. After subtracting control IgG fluorescence, specific antibody binding was expressed as mean fluorescence intensity (MFI). Data are provided as the percentage changes from pretreatment values obtained at either 24 hours (group 2) or 2 hours (group 1) before endotoxin challenge (baseline).
Radiolabeling of granulocytes.All procedures were performed in a laminar flow chamber using sterile, pyrogen-free glassware and instruments. Five hours before the endotoxin administration, 100 mL of venous blood was collected from each volunteer into 20% (vol/vol) acid citrate dextrose (3.2%) and 6% (vol/vol) Hydroxyethyl Ether Starch (Fresenius, ‘s Hertogenbosch, The Netherlands). After sedimentation at room temperature for about 45 minutes, the supernatant, which was composed of leukocyte-rich plasma, was centrifuged at 200g for 10 minutes and the pellet was resuspended in 3 mL autologous plasma. The granulocytes were isolated by Percoll density gradient centrifugation (Pharmacia, Woerden, The Netherlands) at 200g for 30 minutes. The pellet, containing pure granulocytes, was suspended in 10 mL with saline and centrifuged at 200g for 10 minutes to remove the Percoll and was labeled with 925 MBq 99mTc-HMPAO (hexamethylpropylene-amineoxime; Amersham, ‘s Hertogenbosch, The Netherlands) as described.33 After an incubation time of 20 minutes, the labeled granulocytes were centrifuged at 200g for 10 minutes to remove free activity. The remaining pellet of 99mTc-HMPAO–labeled granulocytes was brought up to a volume of 2 mL with saline and administered intravenously 2 hours (group 1) and 1 hour before (group 2) LPS injection. The final total dose administered varied from 110 to 540 MBq.
Dynamic granuloscintigrams.Scintigraphic images in group 1 were continuously recorded from 5 minutes before endotoxin administration until 120 minutes after endotoxin administration. In group 2, granuloscintigrams were performed only in the second hour after endotoxin administration. This limitation in scanning was based on the results from group 1. The study of group 2 was designed after completing group 1 and, as could be deduced from the granuloscintigrams from group 1, no granulocyte accumulation was observed in either lungs, liver, or spleen during the first hour after LPS challenge. Dynamic anterior views from the thorax/upper abdomen were obtained with a single-head large field-of-view gamma camera (Diacam, Siemens; Gammasonics Inc, Hoffman Estates, IL) fitted with a low-energy collimator. Images were digitized and stored (Hermes; Nuclear Diagnostics, Hägersten, Sweden) in a 128 × 128 matrix. The evaluation included time-activity curves derived from regions of interest over the lungs, liver, and spleen. Granulocyte recruitment to the lungs, liver, and spleen by means of the mean activity was expressed as a decay-corrected percentage of the pre-endotoxin values starting at 100%.
Statistical analysis.Data are given as the mean ± SEM. Differences between placebo and G-CSF treatment groups were tested by analysis of variance (ANOVA) for repeated measures using SPSS for Windows (SPSS Inc, Chicago, IL). Mean differences in endotoxin-induced adverse events (headache, chills, nausea, and general malaise) were calculated using the clinical symptom scores and are depicted in a descriptive fashion. Changes of variables in time were analyzed using one-way ANOVA. A double-sided P value <.05 was considered significant. P values indicate differences between treatment groups, unless otherwise stated.
RESULTS
Clinical symptoms and signs.Injection of the endotoxin preparations caused similar flu-like symptoms such as headache, chills, nausea, vomiting, and fever. Administration of G-CSF did not induce any side effects. All volunteers were symptom-free within 20 hours after endotoxin challenge. In group 1, G-CSF treatment enhanced the endotoxin-induced chills and headache, whereas in group 2, G-CSF pretreatment had no effect on LPS-induced chills and headache. As shown in Fig 1, G-CSF pretreatment increased the LPS-induced increase in heart rate (peak levels: group 1, 98 ± 4 v 101 ± 4 beats/min, P < .001; group 2, 93 ± 4 v 98 ± 45 beats/min, P = .048). In both groups, the LPS-induced increase in temperature was enhanced by G-CSF, although not significantly. G-CSF had no effect on LPS-elicited changes in mean arterial pressure.
Mean (± SEM) heart rate (A), temperature (B), and mean arterial pressure (C) after endotoxin administration (4 ng/kg) in humans. Placebo (○) or G-CSF (5 μg/kg; •) was administered 2 hours before endotoxin challenge (group 1) or 24 hours before endotoxin administration (group 2).
Mean (± SEM) heart rate (A), temperature (B), and mean arterial pressure (C) after endotoxin administration (4 ng/kg) in humans. Placebo (○) or G-CSF (5 μg/kg; •) was administered 2 hours before endotoxin challenge (group 1) or 24 hours before endotoxin administration (group 2).
Cytokines.In group 1, administration of G-CSF caused an increase in mean serum G-CSF levels to 229.50 ± 16.33 ng/mL 1 hour after G-CSF administration, decreasing to baseline values thereafter (P < .001). In group 2, G-CSF concentrations of 0.65 ± 0.06 ng/mL were detected just before LPS challenge, further increasing to 4.37 ± 1.00 ng/mL at 4 hours after LPS challenge (P = .75; Fig 2A). As shown in Fig 2B, a 2-hour pretreatment with G-CSF significantly augmented LPS-induced TNF levels from peak concentrations of 3.22 ± 0.83 to 8.74 ± 2.16 ng/mL (P < .001); in group 2, G-CSF pretreatment attenuated LPS-induced TNF concentrations from peak levels of 11.16 ± 8.10 to 5.16 ± 1.74 ng/mL (P = .6). In group 1, LPS-induced IL-6 levels were increased by G-CSF from peak levels of 5.00 ± 1.95 to 20.94 ± 4.82 ng/mL (group 1, P < .001); in group 2, G-CSF pretreatment reduced LPS-induced IL-6 levels from peak concentrations of 8.11 ± 6.22 to 2.65 ± 0.74 ng/mL (P = .4; Fig 2C). In group 1, G-CSF pretreatment enhanced peak levels of IL-8 of 1.74 ± 0.30 ng/mL (endotoxin/placebo) to 4.70 ± 0.79 ng/mL (P < .001); in group 2, G-CSF pretreatment significantly reduced IL-8 peak levels from 3.16 ± 1.30 ng/mL (endotoxin/placebo) to 1.48 ± 0.48 ng/mL (P = .002; Fig 2D).
Mean (± SEM) G-CSF (A), TNF (B), IL-6 (C), and IL-8 (D) concentrations after endotoxin administration (4 ng/kg) in humans. Placebo (○) or G-CSF (5 μg/kg; •) was administered 2 hours before endotoxin challenge (group 1) or 24 hours before endotoxin administration (group 2).
Mean (± SEM) G-CSF (A), TNF (B), IL-6 (C), and IL-8 (D) concentrations after endotoxin administration (4 ng/kg) in humans. Placebo (○) or G-CSF (5 μg/kg; •) was administered 2 hours before endotoxin challenge (group 1) or 24 hours before endotoxin administration (group 2).
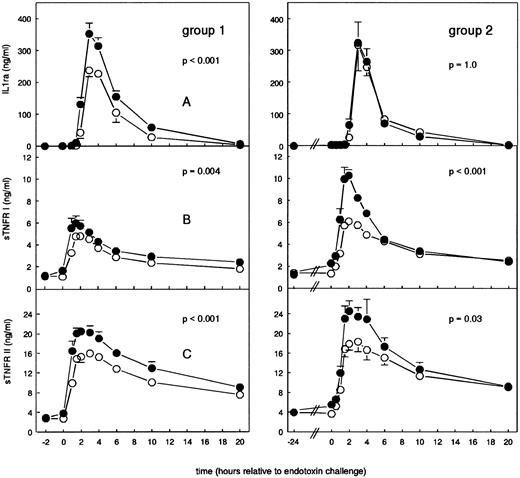
As a 2-hour pretreatment, G-CSF increased LPS-elicited IL-1ra levels from 238.00 ± 20.21 to 353.00 ± 32.64 ng/mL (P < .001; Fig 3A); a 24-hour pretreatment with G-CSF increased IL-1ra concentrations from 0.15 ± 0.03 ng/mL (t = −24 hours) to 2.04 ± 0.33 ng/mL (t = 0 hours; P = .001 in time), but had no effect on LPS-induced increases in IL-1ra concentrations (P = 1.0). Endotoxin-induced peak levels of sTNFR I were significantly enhanced by both pretreatments with G-CSF: group 1, from 4.79 ± 0.46 to 5.74 ± 0.51 ng/mL (P = .004); and group 2, from 6.12 ± 0.31 to 10.30 ± 0.50 ng/mL (P < .001). Furthermore, the 24-hour pretreatment with G-CSF, per se, increased sTNFR I levels from 1.36 ± 0.05 to 2.28 ± 0.11 ng/mL at t = 0 hours (P < .001 in time). LPS-induced increases in sTNFR II levels were also significantly increased by both pretreatments with G-CSF: group 1, from 16.01 ± 0.60 to 20.49 ± 0.92 ng/mL (P < .001); and group 2, from 18.36 ± 2.02 to 24.60 ± 2.06 ng/mL (P < .001). The 24-hour pretreatment with G-CSF increased sTNFR II levels from 4.04 ± 0.24 ng/mL (t = −24 hours) to 5.58 ± 0.40 ng/mL (t = 0 hours; P = .001 in time).
Mean (± SEM) IL-1ra (A), sTNFR I (B), and sTNFR II (C) concentrations after endotoxin administration (4 ng/kg) in humans. Placebo (○) or G-CSF (5 μg/kg; •) was administered 2 hours before endotoxin challenge (group 1) or 24 hours before endotoxin administration (group 2).
Mean (± SEM) IL-1ra (A), sTNFR I (B), and sTNFR II (C) concentrations after endotoxin administration (4 ng/kg) in humans. Placebo (○) or G-CSF (5 μg/kg; •) was administered 2 hours before endotoxin challenge (group 1) or 24 hours before endotoxin administration (group 2).
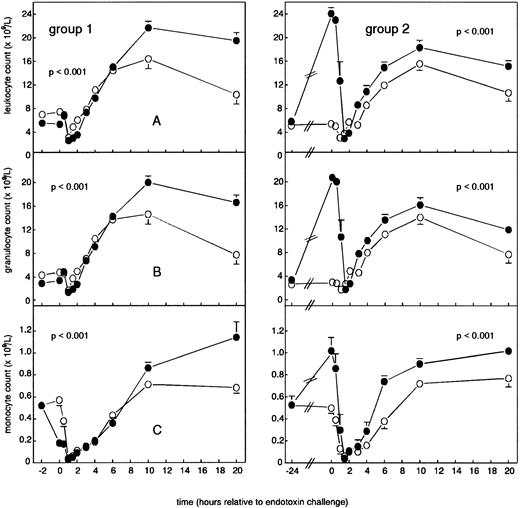
Leukocyte counts.As shown in Fig 4A, a 2-hour pretreatment with G-CSF enhanced both LPS-induced leukopenia (from nadir levels of 3.1 ± 0.4 × 109/L to 2.6 ± 0.1 × 109/L) and leukocytosis (from peak levels of 16.4 ± 1.7 × 109/L to 21.7 ± 1.1 × 109/L; P < .001). In group 2, G-CSF pretreatment increased leukocyte concentrations to 24.1 ± 1.0 × 109/L at t = 0 hours (P < .001 in time) and again enhanced LPS-induced leukopenia (from nadir concentrations of 3.1 ± 0.4 × 109/L to 2.9 ± 0.7 × 109/L) and leukocytosis (from peak concentrations of 15.6 ± 1.1 × 109/L to 18.3 ± 1.2 × 109/L; P < .001). Changes in neutrophil counts mimicked changes in total leukocyte counts, as shown in Fig 4B. In group 1, G-CSF decreased the LPS-induced reduction in monocyte counts (from mean levels of 0.04 ± 0.01 × 109/L to 0.03 ± 0.01 × 109/L) and augmented the subsequent increase (from peak concentrations of 0.71 ± 0.03 × 109/L to 1.14 ± 0.14 × 109/L; P < .001). In group 2, G-CSF pretreatment increased monocyte counts to 1.02 ± 0.12 × 109/L at t = 0 hours (P < .001 in time) and enhanced LPS-induced monopenia (from mean levels of 0.06 ± 0.02 × 109/L to 0.04 ± 0.01 × 109/L) and monocytosis (from peak levels of 0.77 ± 0.08 × 109/L to 1.02 ± 0.04 × 109/L; P < .001; Fig 4C). Neither treatment regimen had any effect on endotoxin-induced changes in lymphocyte counts (data not shown).
Mean (± SEM) leukocyte counts in human endotoxemia. Total leukocyte counts (A), neutrophils (B), and monocytes (C). Placebo (○) or G-CSF (5 μg/kg; •) was administered 2 hours -b e f o r e e n d o t o x i n c h a l l e n g e -(group 1) or 24 hours before endotoxin challenge (group 2).
Mean (± SEM) leukocyte counts in human endotoxemia. Total leukocyte counts (A), neutrophils (B), and monocytes (C). Placebo (○) or G-CSF (5 μg/kg; •) was administered 2 hours -b e f o r e e n d o t o x i n c h a l l e n g e -(group 1) or 24 hours before endotoxin challenge (group 2).
Neutrophil degranulation and expression of cell surface markers.Figure 5A shows that, in both groups, G-CSF pretreatment induced release of elastase before endotoxin administration (142.8 ± 36.7 ng/mL in group 1 and 168.0 ± 8.4 ng/mL in group 2; P = .001 in time) and augmented the LPS-induced mean peak elastase release (in group 1, from 195.1 ± 22.3 to 347.9 ± 23.8 ng/mL, P < .001; and in group 2, from 278.3 ± 33.8 to 399.6 ± 23.8 ng/mL, P = .013). G-CSF pretreatment resulted in an increase of lactoferrin concentrations at t = 0 hours (644.5 ± 172.7 ng/mL in group 1 and 226.14 ± 19.1 ng/mL in group 2; P < .001 in time) and further boosted endotoxin-induced release (in group 1, from 645.9 ± 112.1 to 2,328.8 ± 371.3 ng/mL; and in group 2, from 539.7 ± 74.2 to 1,573.3 ± 174.0 ng/mL; P < .001 for each; Fig 5B).
The effect of G-CSF (5 μg/kg) on mean (± SEM) elastase (A) and lactoferrin (B) concentrations in human endotoxemia. Placebo (○) or G-CSF (•) was administered 2 hours before endotoxin challenge (group 1) or 24 hours before endotoxin challenge (group 2).
The effect of G-CSF (5 μg/kg) on mean (± SEM) elastase (A) and lactoferrin (B) concentrations in human endotoxemia. Placebo (○) or G-CSF (•) was administered 2 hours before endotoxin challenge (group 1) or 24 hours before endotoxin challenge (group 2).
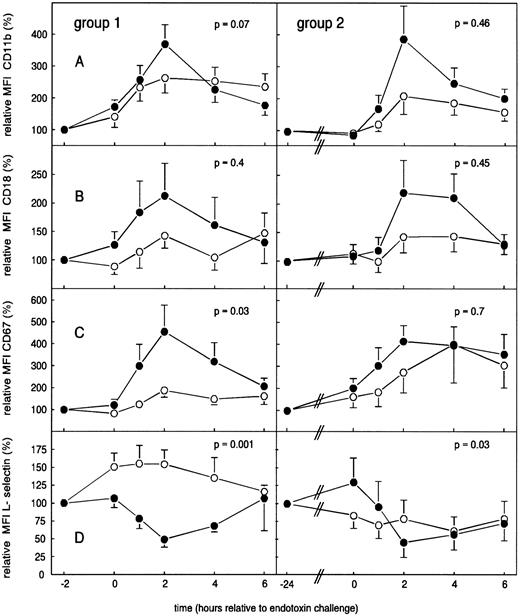
As shown in Fig 6A and B, a 2-hour pretreatment with G-CSF significantly augmented LPS-induced increases of neutrophil CD11b from 262.8% ± 46.5% to 364.9% ± 61.2% (P = .07) and modestly augmented LPS-induced increases in CD18, from 142.8% ± 21.9% to 212.6% ± 56.7% (P = .4). In group 2, G-CSF also enhanced, although not significantly, LPS-induced increases of neutrophil CD11b and CD18 expression, from 208.7% ± 57.6% to 386.5% ± 103.9% (CD11b) and from 143.4% ± 27.6% to 219.5% ± 56.6% (CD18; P = .46 and P = .45, respectively). In both groups, G-CSF pretreatment enhanced LPS-elicited increases in CD67 expression on neutrophils from 187.9% ± 33.1% to 455.0% ± 123.1% (group 1, P = .03) and from 400.8% ± 176.0% to 414.1% ± 71.9% (group 2, P = .7; Fig 6C). Neutrophil L-selectin expression showed a variable, nonsignificant change after endotoxin administration. G-CSF pretreatment resulted in a significant downregulation of L-selectin during the first 2 hours after endotoxin administration, reaching a nadir at t = 2 hours of 49.1% ± 10.9% (group 1, P = .001) and of 45.4% ± 20.9% (group 1, P = .03; Fig 6D).
Mean (± SEM) relative MFI of CD11b (A), CD18 (B), CD67 (C), and L-selectin (D) in human endotoxemia. Placebo (○) or G-CSF (•) was administered 2 hours before endotoxin challenge (group 1) or 24 hours before endotoxin challenge (group 2).
Mean (± SEM) relative MFI of CD11b (A), CD18 (B), CD67 (C), and L-selectin (D) in human endotoxemia. Placebo (○) or G-CSF (•) was administered 2 hours before endotoxin challenge (group 1) or 24 hours before endotoxin challenge (group 2).
Dynamic granuloscintigrams.Figure 7 shows that endotoxin administration caused an increase in radioactivity in the lungs up to 120.7% ± 10.3% (group 1) and 117.9% ± 12.6% (group 2; P < .001 in time for each). This increase of radiolabeled granulocytes into the lungs was completely blocked by both G-CSF treatment regimens (group 1, P < .001; group 2, P = .007; Fig 7A). In group 1, accumulation of radiolabeled granulocytes in the liver peaked after endotoxin administration to 114.7% ± 4.0% (P = .027 in time) and was significantly reduced by G-CSF pretreatment (P = .011). In group 2, only a modest increase in granulocyte accumulation in the liver was observed to 108.6% ± 4.1% (P = .3). In this group, G-CSF pretreatment modestly enhanced LPS-induced increase in radioactivity in the liver region (P = .27; Fig 7B). No accumulation in the spleen region was observed after endotoxin administration (data not shown).
Mean (± SEM) of relative radioactivity in lungs (A) and liver (B) after endotoxin challenge in humans (4 ng/kg). Placebo (○) or G-CSF (•) was administered 2 hours before endotoxin challenge (group 1) or 24 hours before endotoxin challenge (group 2).
Mean (± SEM) of relative radioactivity in lungs (A) and liver (B) after endotoxin challenge in humans (4 ng/kg). Placebo (○) or G-CSF (•) was administered 2 hours before endotoxin challenge (group 1) or 24 hours before endotoxin challenge (group 2).
DISCUSSION
The results of this study show that G-CSF exerts different effects on cytokine release in human endotoxemia when administered intravenously 2 hours and subcutaneously 24 hours before endotoxin. Administered 2 hours before LPS challenge, G-CSF boosted the release of TNF, IL-6, IL-8, IL-1ra, and sTNFR I and II, whereas G-CSF, when administered 24 hours before endotoxin, significantly reduced the release of IL-8 and modestly attenuated systemic TNF and IL-6 levels. Furthermore, the 24-hour pretreatment importantly induces IL-1ra and sTNF receptor release and further enhanced LPS-induced increase in sTNFR I and II, but did not enhance LPS-induced IL-1ra concentrations. Despite these diverse effects on cytokine release, both G-CSF treatment regimens augmented neutrophil activation and increased granulocyte adhesion molecule expression. Nonetheless, G-CSF completely blocked the LPS-induced granulocyte accumulation in lungs when administered 2 hours before LPS challenge, and also blocked accumulation in the liver.
Endotoxin administration to humans elicits a systemic inflammatory response, characterized by release of inflammatory cytokines and activation of neutrophils,34,35 and is often used as a model to study early host responses to sepsis. However, the inflammatory responses seen after endotoxin injection into healthy volunteers may largely differ from the inflammatory responses seen in septic patients. It should be noted that the endotoxin administered in group 1 and group 2 differed (lot EC-5 and lot G, respectively), which may explain, at least in part, why, in general, the inflammatory responses observed in group 2 during endotoxemia were more pronounced. However, because of the cross-over design of the study, the effects of different timing of G-CSF administration can be compared. The effects of G-CSF on endotoxin-induced cytokine release are in accordance with previous animal studies of infection and sepsis,23-25 which showed that G-CSF improved survival rate and attenuated the release of TNF-α.23 Furthermore, a human study showed that, upon ex vivo stimulation with LPS of whole blood drawn 24 hours after G-CSF administration, increased IL-1ra and sTNFR I and II levels were observed in the G-CSF–treated group in comparison with placebo-treated subjects.29 In another study of human endotoxemia, G-CSF, when administered 12 hours before LPS challenge, increased the release of both proinflammatory and anti-inflammatory cytokines.30 In our study, we clearly showed that, by delaying LPS challenge after G-CSF, an excessive proinflammatory host response can be altered into a more anti-inflammatory response. However, although these effects of G-CSF on cytokine release in human endotoxemia seem clear, the biologic relevance of these observations needs to be evaluated in patients with acute infection and sepsis.
To ensure high G-CSF concentrations at the time of LPS challenge in group 1, G-CSF was administered intravenously, whereas in group 2 the usual route of administration was used. An explanation for the different effect of G-CSF on cytokine release in human endotoxemia might therefore be that, in group 1, high systemic G-CSF levels were found at the time of LPS challenge, whereas in group 2, only modest elevation in G-CSF concentrations were found at the time of LPS challenge. However, the precise mechanism of how G-CSF increases or reduces cytokine release in human endotoxemia needs to be elucidated.
Administration of G-CSF to healthy volunteers mimicked the effect of endotoxin on neutrophils and resulted in the release of elastase and lactoferrin and upregulation of the membrane expression of CD11b, CD64, and CD67.12,17,36 The data from the present study indicate that G-CSF, when administered either 2 or 24 hours before endotoxin challenge, amplified endotoxin-induced neutrophil activation. This effect of G-CSF was, at least in part, a direct consequence of stimulation of neutrophils by G-CSF, because both G-CSF pretreatments caused the release of elastase and lactoferrin, even before administration of endotoxin, which is in accordance with previously reported data.12,17 IL-8 can increase CD11b/CD18 expression in vitro,37 but it seems unlikely that the enhancement of upregulation of CD11b and CD18 by G-CSF during endotoxemia is mediated by IL-8, for in group 2, reduced IL-8 levels were found in conjunction with enhanced CD11b/CD18 expression on neutrophils. However, increased β2 integrin expression may have resulted from enhanced elastase release that was caused by both G-CSF treatment regimens38 and that preceded β2 integrin upregulation.
Both G-CSF and endotoxin are known to influence the numbers of circulating neutrophils and monocytes.12 31 In human volunteers, endotoxin causes a decrease in leukocyte counts followed by a leukocytosis. After endotoxin challenge, granulocytes accumulated in the lung and liver region, peaking at 90 minutes (group 1) and 105 minutes (group 2) after endotoxin challenge. It is not clear why no significant granulocyte accumulation in the liver was observed in group 2. Surprisingly, although G-CSF pretreatment did not prevent endotoxin-induced early neutropenia, it completely prevented the endotoxin-induced accumulation of neutrophils in the lungs within 2 hours after LPS challenge. These findings underscore the fact that low circulating neutrophil numbers do not automatically indicate increased neutrophil accumulation in specific organs, such as lungs and liver. The blockage of neutrophil accumulation in the lungs occurred under otherwise proadhesive conditions, ie, in the presence of increased CD11b and CD18 expression on neutrophils. The Tc-radiolabeling technique used in this study does not allow discrimination between neutrophil retention as a consequence of aspecific trapping, specific adhesion, or transendothelial transmigration. A potential confounding factor in interpreting the granuloscintiscan data is the fact that both endotoxin and G-CSF alter the neutrophil population by causing rapid mobilization of young forms from the bone marrow. Because the neutrophil labeling occurred ex vivo and before endotoxin challenge, the scintigraphic assessments did not take these changes into account. On the other hand, the observed blockage of granulocyte accumulation occurred early (within 2 hours) after endotoxin challenge and thus is not expected to be importantly affected by increased amounts of newly released neutrophils from the bone marrow. This was confirmed by unaltered CD33 expression on neutrophils during either G-CSF or endotoxin treatment (data not shown).
L-selectin that is constitutively present on the neutrophil membrane is necessary for initial neutrophil-endothelial cell interaction that results in rolling of neutrophils on endothelium.39,40 Leukocyte recruitment to inflammatory sites is markedly impaired in L-selectin–deficient mice and after specific L-selectin blockade in normal rabbits.41,42 Although activation of granulocytes caused shedding of L-selectin in vitro and in animal studies,43 in our study no decrease in L-selectin expression was observed during endotoxemia. It should be noted that the amount of endotoxin used in healthy volunteers is much lower than is commonly used in in vitro or in animal models. It has been shown by others44 that G-CSF can cause downregulation of L-selectin in vivo. In this study, pretreatment with G-CSF and subsequent endotoxin challenge caused a loss of neutrophil L-selectin expression during the initial 2 hours after endotoxin challenge. In view of the known role of L-selectin in pulmonary recruitment of neutrophils, the early G-CSF–induced downregulation of L-selectin is a likely contribution to the prevention of pulmonary neutrophil retention by G-CSF pretreatment.
In conclusion, we state that G-CSF, administered at different time points before endotoxin challenge, exerts differential effects on cytokine release in humans in vivo. Administered 2 hours before endotoxin, G-CSF augmented LPS-induced inflammatory cytokine responses, whereas, when administered 24 hours before LPS challenge, G-CSF attenuated the endotoxin-induced proinflammatory state and induced a more anti-inflammatory response. Furthermore, G-CSF pretreatment enhanced endotoxin-induced neutrophil activation and adhesion molecule expression and increased shedding of neutrophil L-selectin during human endotoxemia. Regardless of the effects of G-CSF on cytokine release and neutrophil activation, G-CSF prevents the accumulation of granulocytes in the lungs during the first 2 hours after endotoxin treatment.
ACKNOWLEDGMENT
The authors thank C.E. Hack and A.J.M. Eerenberg from the Central Laboratory of the Netherlands Red Cross Blood Transfusion Service for the measurements of elastase/α1 -antitrypsin complexes and lactoferrin concentrations and N.C.J.J. Ladiges for performing the FACScan analyses and M. Sala for assisting with the cytokine measurements. The authors are grateful to the nursing staff of the Clinical Research Unit for their excellent practical skills.
Supported by Amgen (Thousand Oaks, CA). T.v.d.P. is a fellow of the Royal Netherlands Academy of Arts and Sciences.
Address reprint requests to Dasja Pajkrt, MD, Laboratory of Experimental Internal Medicine, Academic Medical Center G2-105b, Meibergdreef 9, 1105 AZ Amsterdam, The Netherlands.





This feature is available to Subscribers Only
Sign In or Create an Account Close Modal