Abstract
Transplantation of marrow from unrelated donors was investigated in patients with Philadelphia chromosome-positive (Ph1+) acute lymphoblastic leukemia (ALL) who lacked a suitable family donor. Eighteen patients underwent transplantation at our center between 1988 and 1995. The median patient age was 25 years (range, 1.7 to 51 years). Seven patients were in first complete remission, 1 in second remission, 3 in first relapse, and the remaining 7 had more advanced or chemotherapy refractory leukemia at transplant. All patients were conditioned with cyclophosphamide and total body irradiation followed by marrow transplants from closely HLA-matched, unrelated volunteers. Posttransplant graft-versus-host disease (GVHD) prophylaxis included methotrexate with either cyclosporine or FK506. Graft failure was not observed. Severe (grades III-IV) GVHD appeared in 6 of 17 evaluable patients and chronic extensive GVHD in 7 of 13 patients at risk. Five patients had recurrent ALL after transplantation and another 4 died from causes other than leukemia. Six patients transplanted in first remission, 2 in first relapse, and 1 in second remission remain alive and leukemia-free at a median follow-up of 17 months (range, 9 to 73 months). The probability of leukemia-free survival at 2 years is 49% ± 12%. These data indicate that unrelated donor marrow transplantation is an effective treatment option for patients with early stage Ph1+ ALL without a family match and suggest that in such patients an unrelated donor search should be initiated as soon as possible after diagnosis.
THE PHILADELPHIA chromosome (Ph1 ) is detectable in 2% to 5% of children and in approximately 25% of adults with acute lymphoblastic leukemia (ALL).1 The probability of achieving a complete remission (CR) with current chemotherapy in Ph1+ ALL is approximately 70%, but most patients relapse and leukemia-free survival is less than 15% at 5 years.1 Allogeneic marrow transplant from related donors can cure a substantial proportion of patients with Ph1+ ALL.2-5 Results with unrelated donor marrow transplants in series of patients with this disease have not been published. We report the outcome for 18 patients, showing that it is possible to achieve sustained leukemia-free survival in patients with early disease.
PATIENTS AND METHODS
Between September 1979 and December 1995, 114 patients with primary ALL received unrelated donor marrow transplants at the Fred Hutchinson Cancer Research Center (FHCRC). All patients had been diagnosed and initially treated at institutions other than the FHCRC. The Ph1 chromosome was present at diagnosis in 16 patients and was detected after relapse before transplantation in 1 other patient. In 1 additional patient with unsuccessful cytogenetic study, the bcr-abl rearrangement was detected at diagnosis. Characteristics of these 18 patients receiving unrelated donor marrow transplants between November 1988 and October 1995 are summarized in Table 1. Seventeen patients had B-lineage CD10+ ALL and one had a CD10− disease. All patients received intensive chemotherapy regimens for high-risk ALL before transplantation.
Characteristics of Ph1+ ALL
| UPN . | WBC . | CD10 . | CD13 . | CD33 . | CD34 . | Ph+ (sp) . | Additional Abnormal Cytogenetics . | Courses to 1st CR . | CR 1 duration (mo) . | Extramed. Leukemia . |
|---|---|---|---|---|---|---|---|---|---|---|
| . | (×109/L) . | . | . | . | . | . | . | . | . | . |
| 5191 | 3 | + | − | − | − | + (NA) | −19, +22 | 1 | 9 | No |
| 7361 | 20 | + | − | − | − | + (p210) | No | 1 | 1 | CNS |
| 8124 | 4 | + | − | + | + | + (p210) | No | 2 | 3 | No |
| 9115 | 18 | + | − | − | − | + (NA) | −2, −13, −16 | 2 | 4 | No |
| 9222 | 38 | + | − | − | + | + (p190, p210) | No | 1 | 4 | No |
| 8278 | NA | + | − | − | − | + (NA) | No | 1 | 8 | No |
| 9861 | 16 | − | − | − | + | + (NA) | NA | 1 | 6 | No |
| 9833 | 30 | + | − | − | + | + (p190) | 9 p− | 1 | 4 | No |
| 7958 | 333 | + | − | − | − | + (p210) | No | 1 | 3 | CNS |
| 9635 | 10 | + | − | − | − | + (p210) | No | 1 | 10 | No |
| 4637 | 335 | + | − | − | − | + (NA) | −7, 9q− | 4 | 31 | No |
| 8765 | 85 | + | − | − | − | + (p190) | +12 | 1 | 7 | CNS |
| 6642 | 171 | + | − | − | − | + (p190) | 11q−, 13q−, −17, −20 | 2 | 6 | CNS |
| 8650 | NA | + | − | − | − | + (NA) | t1; 19 | 1 | 13 | CNS |
| 6056 | 93 | + | − | − | − | + (p190, p210) | 20q− | 1 | 3 | CNS |
| 5942 | 35 | + | + | + | − | + (p190, p210) | No | 1 | 2 | No |
| 4838 | 48 | + | − | − | − | + (NA) | 9p− | 1 | 4 | No |
| 7841 | 288 | + | − | + | + | + (NA) | t1; 15 | NA | NA | No |
| UPN . | WBC . | CD10 . | CD13 . | CD33 . | CD34 . | Ph+ (sp) . | Additional Abnormal Cytogenetics . | Courses to 1st CR . | CR 1 duration (mo) . | Extramed. Leukemia . |
|---|---|---|---|---|---|---|---|---|---|---|
| . | (×109/L) . | . | . | . | . | . | . | . | . | . |
| 5191 | 3 | + | − | − | − | + (NA) | −19, +22 | 1 | 9 | No |
| 7361 | 20 | + | − | − | − | + (p210) | No | 1 | 1 | CNS |
| 8124 | 4 | + | − | + | + | + (p210) | No | 2 | 3 | No |
| 9115 | 18 | + | − | − | − | + (NA) | −2, −13, −16 | 2 | 4 | No |
| 9222 | 38 | + | − | − | + | + (p190, p210) | No | 1 | 4 | No |
| 8278 | NA | + | − | − | − | + (NA) | No | 1 | 8 | No |
| 9861 | 16 | − | − | − | + | + (NA) | NA | 1 | 6 | No |
| 9833 | 30 | + | − | − | + | + (p190) | 9 p− | 1 | 4 | No |
| 7958 | 333 | + | − | − | − | + (p210) | No | 1 | 3 | CNS |
| 9635 | 10 | + | − | − | − | + (p210) | No | 1 | 10 | No |
| 4637 | 335 | + | − | − | − | + (NA) | −7, 9q− | 4 | 31 | No |
| 8765 | 85 | + | − | − | − | + (p190) | +12 | 1 | 7 | CNS |
| 6642 | 171 | + | − | − | − | + (p190) | 11q−, 13q−, −17, −20 | 2 | 6 | CNS |
| 8650 | NA | + | − | − | − | + (NA) | t1; 19 | 1 | 13 | CNS |
| 6056 | 93 | + | − | − | − | + (p190, p210) | 20q− | 1 | 3 | CNS |
| 5942 | 35 | + | + | + | − | + (p190, p210) | No | 1 | 2 | No |
| 4838 | 48 | + | − | − | − | + (NA) | 9p− | 1 | 4 | No |
| 7841 | 288 | + | − | + | + | + (NA) | t1; 15 | NA | NA | No |
Abbreviations: Additional Abnormal Cytogenetics, cytogenetics abnormalities in addition to the Ph+ clone; CNS, central nervous system; CR, complete remission; Extramed. Leukemia, extramedullary leukemia (at anytime pre-BMT); NA, not available; Ph+, Philadelphia chromosome-positive; sp, BCR/abl species by PCR; WBC, white blood cell count.
Characteristics and Outcome of 18 Patients Receiving Marrow Transplants From Unrelated Donors for Treatment of Ph1+ ALL
| UPN . | Age (y) . | Gender (Pt/D) . | Search Start to BMT (mo) . | Donor HLA-Mismatching . | Disease Stage at BMT . | GVHD . | Relapse (mo) . | Survival (mo) . | |
|---|---|---|---|---|---|---|---|---|---|
| . | . | . | . | . | . | Acute . | Chronic . | . | . |
| 5191 | 25 | M/M | 8.9 | None | 1st Remission | III | Extensive | No | >73.0 |
| 7361 | 47 | F/F | 3.6 | None | 1st Remission | II | Extensive | No | >41.5 |
| 8124 | 26 | M/F | 2.5 | None | 1st Remission | II | No | No | >24.7 |
| 9115 | 3 | F/M | 4.8 | None | 1st Remission | III | Extensive | No | >17.1 |
| 9222 | 1.7 | M/F | 3.8 | None | 1st Remission | II | Extensive | No | >16.5 |
| 8278 | 43 | F/F | 6.1 | None | 1st Remission | I | Extensive | No | 9.6 |
| 9861 | 51 | M/M | 4.5 | None | 1st Remission | I | No | No | >9.3 |
| 9833 | 34 | M/M | 2.9 | None | 2nd Remission | I | Limited | No | >9.0 |
| 7958 | 20 | M/M | 4.0 | DRB1* | 1st Relapse† | II | Extensive | No | >31.4 |
| 9635 | 29 | M/M | 3.5 | None | 1st Relapse‡ | II | Extensive | No | >12.6 |
| 4637 | 43 | M/M | 2.6 | None | 1st Relapse | II | NA | 1.9 | 4.0 |
| 8765 | 47 | F/F | 5.9 | Aρ | 2nd Relapse | I | No | 4.5 | 5.2 |
| 6642 | 8 | F/M | 4.9 | B* | 2nd Relapse | IV | NA | No | 2.0 |
| 8650 | 20 | M/M | 4.1 | DRB1ρ | 2nd Relapse | NA | NA | No | 0.1 |
| 6056 | 19 | F/F | 3.0 | DRB1* | 3rd Relapse | III | NA | 3.2 | 3.7 |
| 5942 | 35 | F/M | 5.6 | B* | 3rd Relapse | IV | NA | No | 2.5 |
| 4838 | 21 | F/M | 11.7 | None | 4th Relapse | III | No | 3.9 | 4.2 |
| 7841 | 25 | M/M | 8.3 | DRB1* | Induction failure | II | No | 3.6 | 4.9 |
| UPN . | Age (y) . | Gender (Pt/D) . | Search Start to BMT (mo) . | Donor HLA-Mismatching . | Disease Stage at BMT . | GVHD . | Relapse (mo) . | Survival (mo) . | |
|---|---|---|---|---|---|---|---|---|---|
| . | . | . | . | . | . | Acute . | Chronic . | . | . |
| 5191 | 25 | M/M | 8.9 | None | 1st Remission | III | Extensive | No | >73.0 |
| 7361 | 47 | F/F | 3.6 | None | 1st Remission | II | Extensive | No | >41.5 |
| 8124 | 26 | M/F | 2.5 | None | 1st Remission | II | No | No | >24.7 |
| 9115 | 3 | F/M | 4.8 | None | 1st Remission | III | Extensive | No | >17.1 |
| 9222 | 1.7 | M/F | 3.8 | None | 1st Remission | II | Extensive | No | >16.5 |
| 8278 | 43 | F/F | 6.1 | None | 1st Remission | I | Extensive | No | 9.6 |
| 9861 | 51 | M/M | 4.5 | None | 1st Remission | I | No | No | >9.3 |
| 9833 | 34 | M/M | 2.9 | None | 2nd Remission | I | Limited | No | >9.0 |
| 7958 | 20 | M/M | 4.0 | DRB1* | 1st Relapse† | II | Extensive | No | >31.4 |
| 9635 | 29 | M/M | 3.5 | None | 1st Relapse‡ | II | Extensive | No | >12.6 |
| 4637 | 43 | M/M | 2.6 | None | 1st Relapse | II | NA | 1.9 | 4.0 |
| 8765 | 47 | F/F | 5.9 | Aρ | 2nd Relapse | I | No | 4.5 | 5.2 |
| 6642 | 8 | F/M | 4.9 | B* | 2nd Relapse | IV | NA | No | 2.0 |
| 8650 | 20 | M/M | 4.1 | DRB1ρ | 2nd Relapse | NA | NA | No | 0.1 |
| 6056 | 19 | F/F | 3.0 | DRB1* | 3rd Relapse | III | NA | 3.2 | 3.7 |
| 5942 | 35 | F/M | 5.6 | B* | 3rd Relapse | IV | NA | No | 2.5 |
| 4838 | 21 | F/M | 11.7 | None | 4th Relapse | III | No | 3.9 | 4.2 |
| 7841 | 25 | M/M | 8.3 | DRB1* | Induction failure | II | No | 3.6 | 4.9 |
Abbreviations: BMT, bone marrow transplant; CR1, first complete remission; D, donor; NA, not applicable; Pt, patient; UPN, unique patient number.
Minor mismatch defined as a single disparity for HLA-A or HLA-B antigens belonging to the same cross-reactive group, or for a single DRB1 disparity for subtype alleles within the same DR specificity.
Transplanted in CNS relapse and marrow remission.
Transplanted with 11% blasts in the marrow. The blank line separates patients in complete remission at transplantation from those with primary refractory or relapsed leukemia.
ρ One-antigen mismatch defined as a single disparity not fullfilling the criteria for minor mismatch.
Pretransplant evaluation included analysis of banded chromosome preparations from unstimulated marrow.6 The presence of Ph1 chromosome was confirmed at the FHCRC in all patients transplanted in marrow relapse and in 2 patients transplanted during first remission. Since 1990, 13 cases were studied for both p210 bcr-abl and p190 bcr-abl fusion transcripts in marrow and/or peripheral blood according to the methods described elsewhere.7 Four of five patients studied in remission before transplantation had bcr-abl transcripts detected.
HLA-typing for all patients and donors was confirmed by the FHCRC Clinical Immunogenetics Laboratory. Histocompatibility criteria for donor selection have been previously published.8 Seven patients (39%) received marrow from donors with a single disparity at one locus (Table 2).
The Institutional Review Board of the FHCRC approved all treatment protocols. Conditioning for transplantation consisted of cyclophosphamide at 60 mg/kg, intravenously, on each of 2 successive days, and total body irradiation (TBI) administered from dual opposing 60Co sources to a total exposure of 13.2 Gy (11 fractions) in patients ≥18 years of age and of 14.4 Gy (12 fractions) in children. In addition, male patients received 4 Gy of testicular radiation. All patients received 2 doses of intrathecal methotrexate before the transplant and 4 to 6 additional doses after engraftment.
Marrow was transported at ambient temperature and was administered intravenously after completion of the conditioning regimen. The median number of nucleated marrow cells harvested, not corrected for potential contamination with peripheral blood cells, was 3.0 × 108/kg (range, 1.2 to 9.9 × 108/kg). Seven patients had a positive cytomegalovirus (CMV) serology pretransplant. The remaining 11 patients were CMV-seronegative and received marrow from CMV-seronegative donors. Acute graft-versus-host disease (GVHD) prophylaxis was cyclosporine and methotrexate for 12 cases,9 methotrexate and FK506 for 3,10 cyclosporine plus methotrexate and humanized monoclonal antibody against the interleukin-2α chain receptor (anti-Tac) for 1 patient,11 and T-cell depletion of the marrow (total depletion of CD4+ cells and partial depletion of CD8+ cells) plus cyclosporine and methotrexate posttransplant for 2 patients who received marrow from one-antigen major mismatch donor (study in progress).
Engraftment was evaluated in patients surviving at least 3 weeks after transplantation and was defined as the first of 3 consecutive days when the absolute neutrophil count (ANC) surpassed 0.5 × 109/L. Donor origin of engrafted cells was shown by in situ hybridization of Y-chromosome among patients whose donor was of opposite sex or by molecular study of the variable number of tandem repeats polymorphisms. Acute and chronic GVHD were diagnosed and graded according to standard criteria.12 13
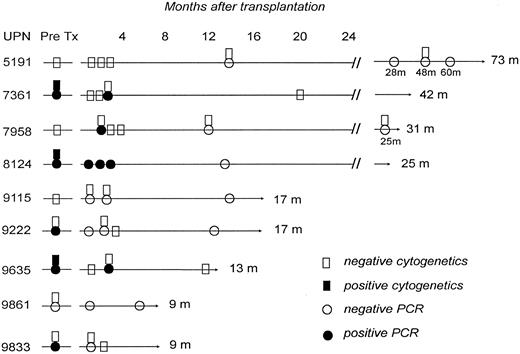
Results of cytogenetic and bcr-abl gene studies in 9 survivors in remission after an unrelated donor marrow transplant for Ph1+ ALL. m, months.
Results of cytogenetic and bcr-abl gene studies in 9 survivors in remission after an unrelated donor marrow transplant for Ph1+ ALL. m, months.
CR was defined as less than 5% blasts in the marrow and no extramedullary leukemia. The definitions of leukemia-free survival, nonleukemic death, and relapse have been reported elsewhere.14 Follow-up has been updated as of July 1, 1996.
RESULTS
All patients had myeloid reconstitution except 1 who died 3 days after transplant, and the median time to recover 0.5 × 109/L ANC was 23 days (range, 12 to 36 days). A self-sustained platelet count greater than 50 × 109/L was achieved in 13 patients at a median time of 26 days (range, 13 to 361 days) and in 4 patients it required more than 100 days. Chimerism studies performed between day 28 and day 80 after transplantation consistently showed full engraftment of donor cells in all 16 patients tested. All patients who engrafted developed acute GVHD at a median time of 18 days (range, 6 to 42 days) after transplant. The proportions of patients with grade II-IV and grade III-IV acute GVHD were 76% and 35%, respectively. Among 13 survivors in remission for more than 100 days, 8 (62%) developed chronic GVHD at a median of 130 days (range, 100 to 285 days), and 5 continued with immunosuppressive treatment at days 269, 278, 377, 496, and 2191 after transplantation, respectively. Only 2 of the survivors in remission have severe chronic GVHD as indicated by the development of scleroderma.
Overall, 4 patients (22%) died of causes other than leukemia. One patient died of cardiac failure on day +3. Two patients died of idiopathic pneumonia on days +60 and +90, respectively, and 1 other died of pulmonary aspergillosis on day +287.
Five of 10 (50%) patients transplanted during primary refractory or relapsed disease had leukemia recurrence at a median of 107 days (range, 56 to 135 days) after transplantation, as opposed to none of 8 who underwent transplantation during remission. All patients with recurrent malignancy after transplantation subsequently died. Tests for the bcr-abl gene rearrangement were negative in 7 of the 9 survivors (Fig 1). The remaining 2 patients are in cytogenetic and hematological remission 10 and 38 months after a positive polymerase chain reaction (PCR) test.
Nine patients remain alive in CR with a median follow-up of 17 months (range 9 to 73 months). The Kaplan-Meier estimate of leukemia-free survival is 49% ± 12% at 2 years. Six of the seven patients transplanted in first remission, 2 of 3 in first relapse, and the 1 in second remission are leukemia-free survivors. The Karnofsky scores of the 7 surviving adults are 100% in 3, 90% in 2, and 80% in 2 cases. One of the two latter patients has sclerodema and the other is only 9 months after transplant. One surviving child has a Lansky play score of 100% and another has a score of 60% because of extensive scleroderma.
DISCUSSION
Data from the International Bone Marrow Transplant Registry indicate that leukemia-free survival after allogeneic marrow transplantation from HLA-identical siblings for treatment of Ph1+ ALL is 31% at 2 years for all patients and 38% for patients transplanted during first remission.4 Combined data in 47 patients reported from nine centers are similar with 33% leukemia-free survival.2,3,15-17 Patients without an HLA-compatible family donor are frequently offered an autologous transplant. The data published to date show that 24 of 35 patients (69%) with Ph1+ ALL included in six studies relapsed or died in remission after autologous bone marrow transplantation (ABMT).3,5 15-18 Therefore, new approaches are needed in patients with this disease.
From our series, it appears that unrelated donor marrow transplantation is an effective therapy for patients with early stage Ph1+ ALL who lack a family match. Of note, none of 8 patients transplanted during remission had recurrent malignancy and 7 survive leukemia-free. It is unknown if the low relapse rate observed in patients transplanted during remission is the consequence of an increased graft-versus-leukemia effect after unrelated donor marrow transplantation, when compared with HLA-identical sibling transplants. The 9 patients in CR in this series are likely cured of malignancy, because most relapses after allogeneic marrow transplantation for Ph1+ ALL occur during the first 10 months,4 and the shortest follow-up of the survivors reported here is 9 months. In addition, most of our patients in sustained remission posttransplant have no bcr-abl transcripts detectable by a very sensitive reverse transcription-PCR assay.7 19
Although some degree of acute GVHD occurred in all the patients at risk, in most cases GVHD was not severe. In contrast, chronic GVHD was frequent among survivors in remission and more effective prophylaxis and treatment of this complication are needed to improve the quality of life.
Given the increase in the number of HLA-typed volunteer donors, the probability of finding a serologic-defined HLA-A, B, DR compatible unrelated donor for a Caucasian patient has increased to approximately 70% and the median search duration in the NMDP has declined to 4.1 months.20 Because the median remission duration for patients with Ph+ ALL is 8 to 9 months,1 transplantation should be feasible during first remission in most patients with an HLA-compatible unrelated donor. To facilitate the timelines of transplantation, HLA-typing should be performed at the time of diagnosis and in the absence of a family match, an unrelated donor search should be initiated immediately.
ACKNOWLEDGMENT
The authors thank Dr E. Donnall Thomas for the critical review of this manuscript and Dottie Thomas for her editorial assistance.
Supported in part by National Institutes of Health Grants No. CA 15704, CA 18029, CA 18221, and AI 33484. J.S. is a recipient of Grant No. PF94 37727364 from the Secretarı́a de Estado de Universidades e Investigación, Ministerio de Educación y Ciencia, Grant No. FIS 97/0626 from the Fondo de Investigación Sanitaria, Ministerio de Sanitad y Consumo, and a grant from Hospital Clı́nic, Barcelona, Spain.
Address reprint requests to Claudio Anasetti, MD, Clinical Research Division, Fred Hutchinson Cancer Research Center, 1124 Columbia St, Seattle, WA 98104-2092.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal