Abstract
Using a recently described serum-free culture system of purified human CD34+ progenitor cells, we show here a critical cooperation of flt3 ligand (FL) with transforming growth factor-β1 (TGF-β1) in the induction of in vitro dendritic cell/Langerhans cell (DC/LC) development. The addition of FL to serum-free cultures of CD34+ cells supplemented with TGF-β1, granulocyte-macrophage colony-stimulating factor, tumor necrosis factor α, and stem cell factor strongly increases both percentages (mean, 36% ± 5% v 64% ± 4%; P = .001) and total numbers (4.4- ± 0.8-fold) of CD1a+ dendritic cells. These in vitro-generated CD1a+ cells molecularly closely resemble a particular type of DC known as an epidermal Langerhans cell. Generation of DC under serum-free conditions was found to strictly require supplementation of culture medium with TGF-β1. Upon omission of TGF-β1, percentages of CD1a+ DC decreased (to mean, 10% ± 8%; P = .001) and, in turn, percentages of granulomonocytic cells (CD1a− cells that are lysozyme [LZ+]; myeloperoxidase [MPO+]; CD14+) increased approximately threefold (P < .05). Furthermore, in the absence of TGF-β1, FL consistently promotes generation of LZ+, MPO+, and CD14+ cells, but not of CD1a+ cells. Serum-free single-cell cultures set up under identical TGF-β1– and FL-supplemented culture conditions showed that high percentages of CD34+ cells (mean, 18% ± 2%; n = 4) give rise to day-10 DC colony formation. The majority of cells in these DC-containing colonies expressed the Langerhans cell/Birbeck granule specific marker molecule Lag. Without TGF-β1 supplementation, Lag+ colony formation is minimal and formation of monocyte/macrophage-containing colonies predominates. Total cloning efficiency in the absence and presence of TGF-β1 is virtually identical (mean, 41% ± 6% v 41% ± 4%). Thus, FL has the potential to strongly stimulate DC/LC generation, but has a strict requirement for TGF-β1 to show this costimulatory effect.
DENDRITIC CELLS (DC) comprise a connected system of bone marrow-derived leukocytes that are the primary antigen-presenting cells for the stimulation of naive T cells. DC develop from hematopoietic stem/progenitor cells and are thought to undergo discrete maturational stages. DC at intermediate differentiation stages can be found in various nonlymphoid tissues, in which they are highly specialized in capturing and processing antigens. Upon so far poorly characterized stimuli, these nonlymphoid DC seem to migrate to T-cell–rich zones of lymphoid organs and acquire a fully mature phenotype (reviewed in Steinman1 2 ).
Substantial progress in the characterization of cells of the DC system has only recently been gained by the demonstration that the cytokine combination granulocyte-macrophage colony-stimulating factor (GM-CSF ) plus tumor necrosis factor α (TNFα) can induce development of DC from purified CD34+ progenitor cells in vitro.3-5 At least two in vitro differentiation pathways for the development of DC seem to exist, and these differ from each other in their relationship to in vitro granulomonopoiesis.6,7 The one pathway is characterized by an intermediate monocytoid cell stage (CD14+/c-fms+/CD1a−) with limited proliferation potential and with maintained monocyte/macrophage and DC differentiation potential. The second differentiation pathway occurs via a distinct intermediate cell stage (CD14−/c-fms−/CD1a+) that possesses restricted DC differentiation potential. Birbeck granule (BG) -containing DC resembling epidermal Langerhans cells seem to only develop from this latter pathway.7 This phenotypic distinction is in line with the occurrence of two distinct DC colony types (ie, mixed monocyte/DC colonies and pure DC colonies, respectively) in GM-CSF– and TNFα-supplemented semisolid medium.5,8 A possible third pathway gives rise to simultaneous DC and lymphoid cells (B, T, and natural killer cells) but not to granulomonocytic cell development.9 The addition of the early acting cytokine stem cell factor (SCF; c-kit ligand) to the culture medium further boosts GM-CSF– plus TNFα-dependent DC development.10
The recently cloned flt3 ligand (FL) shows structural homology with SCF11-13 and signals via flt3 a member of the class III tyrosine kinase receptor family that also includes c-kit and c-fms.14,15c-kit and flt3 show similar expression patterns among subsets of CD34+ bone marrow progenitor cells, with the exception of flt3+/c-kit− pro-B cells.16 SCF and FL similarly induce in vitro proliferation of highly purified immature and committed myeloid colony-forming cells in synergism with a number of other growth factors17 18 and increase yields of CD1a+ DC19 in cultures of CD34+ cells.
FL seems to exceed other known cytokines in its capacity to stimulate cells of the DC system. It has been shown that, when administered subcutaneously to mice, FL dramatically increases the numbers of DC in peripheral blood, spleen, and lymph nodes.20 Furthermore, FL exceeds SCF and monocyte colony-stimulating factor (M-CSF ) in its costimulatory effects on monocyte colony-formation from purified CD34+ cells.21
We analyzed here the costimulatory effects of FL on highly purified CD34+ progenitor cells using a recently established serum-free culture system.22 We show that FL possesses dramatic costimulatory effects on DC development from CD34+ progenitors when added together with transforming growth factor-β1 (TGF-β1) plus GM-CSF, TNFα, and SCF to serum-free medium. However, FL strictly requires TGF-β1 costimulation for its DC growth-promoting effects. In the absence of TGF-β1, FL promotes in vitro generation of MPO+/LZ+ granulomonocytic cells but not DC.
MATERIALS AND METHODS
Antibodies.Murine monoclonal antibodies (MoAbs) of the following specificities were used in our study. Human myeloperoxidase (clone H-43-5), human lysozyme (clone LZ-1), and CD14 (clone MEM18) were obtained from An der Grub (Kaumberg, Austria). HLA-DP (clone B7/21), CD34 (clone HPCA2), CD11c (clone Leu12), CD80 (clone L307), and CD86 (clone IT2.2) were obtained from Becton Dickinson Immunocytometry Systems (San Jose, CA). Antibodies specific for CD1a (clone VIT6b), CDw65 (clone VIM2), and CD15 (clone VIMD5) were produced in our laboratory. Antibodies specific for CD11a (clone TS1/22), CD32 (clone IV.3), CD64 (clone 32.2), and HLA-DR (clone L243) were obtained from American Tissue Culture Collection (ATCC; Rockville, MD). CD68 MoAb Ki-M7 was purchased from Behring AG (Marburg, Germany). CD54 MoAb RRI/1.1.1 was purchased from Bender (Vienna, Austria). CD83 MoAb HB15a was kindly provided by Dr T.F. Tedder (Boston, MA). Lag antibody (clone Lag), specific for a 40-kD glycoprotein associated with BG,23 was kindly provided by Dr S. Imamura (Kyoto, Japan).
Immunofluorescence staining procedures.For membrane staining, 50 μL of isolated mononuclear cells (MNC; 107/mL) were incubated for 15 minutes at 0°C to 4°C with 20 μL of conjugated MoAb. Triple stainings were performed by first incubating cells with biotinylated and phycoerythrin-labeled antibodies and then washing twice and subsequently incubating with the second-step reagent streptavidin PerCP (Becton Dickinson Immunocytometry Systems). Afterwards, cells were submitted for intracellular staining.
For suspension stainings of the intracellular antigens, we used the commercially available reagent combination Fix&Perm from An der Grub according to the manufacturer's procedure. Briefly, cells are first fixed for 15 minutes at room temperature (50 μL of cells plus 100 μL of formaldehyde-based fixation medium). After one washing with phosphate-buffered saline (PBS), pH 7.2, cells are resuspended in 50 μL PBS and mixed with 100 μL permeabilization medium plus 20 μL fluorochrome-labeled antibody. After a further incubation for 15 minutes at room temperature, cells are washed again and analyzed by flow cytometry.
Indirect suspension stainings for the intracellular BG marker molecule Lag were performed as described previously22 and followed the fixation and permeabilization procedure described above. For immunofluorescence labeling of cytospin preparations, cells were fixed with 100% acetone for 5 minutes, washed twice in PBS/bovine serum albumin, and incubated with Ab Lag or LZ-1. After additional washing, cells were incubated with fluorescein isothiocyanate-conjugated sheep antimouse Ig Abs (SAM; An der Grub) and analyzed by immunofluorescence microscopy. In all surface and/or intracellular staining experiments, control stainings were performed using nonreactive MoAbs of the same isotype. The following control MoAbs were used: clone VIAP (IgG1), clone 4H1-A7 (IgG2a), and clone 1939-3G5 (IgM).
Flow cytometry and single-cell sorting.Flow cytometric analyses were performed using a FACScan flow cytometer (Becton Dickinson Immunocytometry Systems) equipped with a single laser emitting at 488 nm. Cell sorting was performed using a FACS Vantage flow cytometer (Becton Dickinson). After single-cell sorting, each well (96-well plates; Costar, Cambridge, MA) was examined individually by microscopy for the presence of single cells. Wells containing no cells or more than 1 cell were excluded from further analyses. The purity of the CD1a+ cell fractions obtained by sorting was determined by reanalysis and found to be greater than 95%.
Cord blood (CB) cells.CB samples were collected during normal full-term deliveries. MNC were isolated within 10 hours after collection using discontinuous Ficoll/Hypaque (Pharmacia, Uppsala, Sweden) density gradient centrifugation. CD34+ cells were isolated from CB MNC using the MACS CD34 Progenitor Cell Isolation Kit (Miltenyi Biotec, Bergisch Gladbach, Germany), according to the instructions of the manufacturer. The purity of the CD34+ population ranged from 87% to 98% (mean, 94%).
Culture of CD34+ CB cells.Culture of purified CD34+ CB cells was performed in 24-well plates (Costar; 1 to 3 × 104 cells in 1 mL/well) at 37°C in a humidified atmosphere and in the presence of 5% CO2 . The serum-free medium X-VIVO 15 (Bio Whittaker, Walkersville, MD) contained L-glutamine (2.5 mmol/L), penicillin (125 U/mL), and streptomycin (125 μg/mL). Cultures were supplemented with optimized concentrations of the following human cytokines: FL (100 ng/mL; kindly provided by S. Lyman, Immunex, Seattle, WA), TGF-β1 (0.5 ng/mL; purified from platelets; British Biotechnology, Abington, UK), rhTNFα (50 U/mL; Bender, Vienna, Austria), rhGM-CSF (100 ng/mL; Sandoz, Basel, Switzerland), and rhSCF (20 ng/mL; Amgen, Thousand Oaks, CA). Serum-free cultures supplemented with cytokines were controlled for endotoxin (lipopolysaccharide [LPS]) as described previously24 and found to contain less than 1 pg/mL LPS. Identical cell multiplication and differentiation patterns were observed in cultures initiated with 1 × 104 to 3 × 104 purified CD34+ cells (data not shown). Therefore, all cell numbers presented are normalized to 1 × 104 purified CD34+ cells seeded at culture initiation. Single-cell cultures were performed in 96-well plates (200 μL growth medium per well) using identical serum-free growth conditions as described above for bulk culture experiments. At least 90 single-cell cultures were analyzed for each growth condition in all individual experiments. The percentage of cloning efficiency was calculated using the following formula: % Cloning Efficiency = (No. of Single-Cell Cultures With Colony Growth)/ (No. of Single-Cell Cultures Set Up) × 100.
lsolation of T cells.T cells were purified from peripheral blood MNC (PBMNC) by negative immunomagnetic depletion as previously described.25 Briefly, Ficoll/Hypaque (Pharmacia, Uppsala, Sweden) isolated PBMNC were incubated with biotinylated MoAbs of the following specificities: CD14 (clone VIM13; generated in our laboratory), CD16 (clone 3G8; purchased from Caltag, San Francisco, CA), CD19 (clone HD37; kindly provided by Dr G. Moldenhauer, Heidelberg, Germany), HLA-DR (clone L243; obtained as hybridoma from ATCC), and CD33 (4D3; generated in our laboratory). They were then washed and incubated with streptavidin-coated MACS beads (Miltenyi Biotec). Unbound cells were eluted from the magnetic columns exactly as proposed by the manufacturer (Miltenyi Biotec). Purity of T cells exceeded 99% in all experiments.
Mixed leukocyte reactions (MLR).Stimulator cell fractions were irradiated with 30 Gy ([137Cs] source). Subsequently, graded numbers of these cells were mixed with a constant amount (5 × 104) of highly purified peripheral blood T cells and seeded into round-bottomed 96-well tissue culture plates (Costar). T cells were prepared as described above. Stimulation of responding T cells was monitored by measuring methyl-[3H]-thymidine incorporation (Amersham Life Science, Buckingham, UK) into newly synthesized DNA on day 5 of culture. The incorporated radioactivity was measured by a Top-Count microscintillation counter (Packard Instrument Co, Meriden, CT).
RESULTS
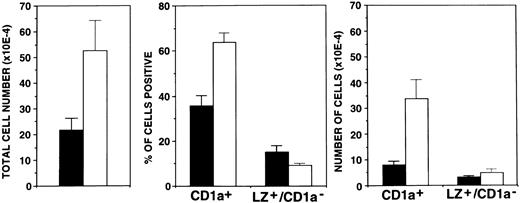
FL strongly enhances in vitro development of DC.We recently showed that the addition of TGF-β1 to GM-CSF plus TNFα and SCF supplemented culture medium allows the generation of large numbers of DC under serum-free conditions.22 The availability of such a system that allows the generation of DC under defined conditions enabled us to study the effects of other potentially important cytokines. FL is certainly such a candidate.20 We therefore cultured purified CD34+ cells under serum-free conditions in the presence of TGF-β1, GM-CSF, TNFα, and SCF with or without further FL supplementation. Cells were then analyzed after 10 days of culture. In line with our previous findings22 in the presence of TGF-β1, GM-CSF, TNFα, and SCF (Fig 1), substantial proportions (mean, 36% ± 5%) of cells acquired the DC marker molecule CD1a and only low percentages showed the granulomonocytic phenotype LZ+/CD1a− (15% ± 4%). Upon addition of FL to the cultures, DC development was even more pronounced. Percentages of CD1a+ cells substantially increased to 64% ± 4% (P = .001), whereas LZ+/CD1a− cells remained infrequent (9% ± 5%).
Effects of FL supplementation on total cell yields and the generation of CD1a+ cells. Ten thousand CD34+ CB cells were cultured for 10 days in serum-free medium in the presence of TGF-β1 plus GM-CSF, TNFα, and SCF as described in the Materials and Methods. Parallel cultures were further supplemented with FL. Bars represent mean values ± SEM of total cell numbers (left), percentages of cells positive (CD1a+ or LZ+/CD1a−; center), or total numbers of positive cells (right) observed in five experiments. The number of CD1a+ or LZ+/CD1a− cells was calculated from the percentage of cells showing the respective phenotype multiplied by the total number of cells in each culture. (▪) −FL; (□) +FL.
Effects of FL supplementation on total cell yields and the generation of CD1a+ cells. Ten thousand CD34+ CB cells were cultured for 10 days in serum-free medium in the presence of TGF-β1 plus GM-CSF, TNFα, and SCF as described in the Materials and Methods. Parallel cultures were further supplemented with FL. Bars represent mean values ± SEM of total cell numbers (left), percentages of cells positive (CD1a+ or LZ+/CD1a−; center), or total numbers of positive cells (right) observed in five experiments. The number of CD1a+ or LZ+/CD1a− cells was calculated from the percentage of cells showing the respective phenotype multiplied by the total number of cells in each culture. (▪) −FL; (□) +FL.
The effect of FL on in vitro CD1a+ cell generation is even more impressive when looking at total numbers of generated CD1a+ cells. As can be seen from Fig 1, total numbers of CD1a+ cells recovered per culture at day 10 increased from 8 × 104 in the absence of FL to 34 × 104 in its presence (P = .02). In contrast, total numbers of LZ+/CD1a− cells did not change significantly (Fig 1).
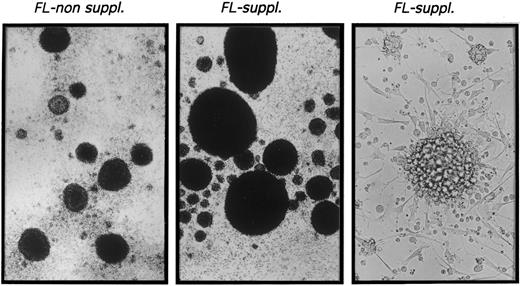
CD1a+ cells generated in the presence of FL possess phenotypic and functional characteristics of DC.The typical morphologic appearance of day-10 cultures is shown in Fig 2. Upon addition of FL to TGF-β1 plus GM-CSF, TNFα, and SCF supplemented serum-free cultures (FL suppl.), very large cell clusters are formed. Cluster development starts at days 3 to 4 of culture; at day 10, clusters are composed of cells that are weakly adherent to plastic and show long spiny processes as well as overlying clustered cells. Virtually identical cluster morphology was observed in the presence or absence of FL (data not shown).
Microscopic appearance of 10-day cultures. CD34+ CB cells were cultured in TGF-β1 plus GM-CSF, TNFα, and SCF supplemented serum-free medium (left) or in identical medium additionally containing FL (center and right) as described in the Materials and Methods. Representative phase contrast microscopic appearance of cells at low power (original magnification × 20 for left and center panels) or high power (original magnification × 40 for right panel) view is shown.
Microscopic appearance of 10-day cultures. CD34+ CB cells were cultured in TGF-β1 plus GM-CSF, TNFα, and SCF supplemented serum-free medium (left) or in identical medium additionally containing FL (center and right) as described in the Materials and Methods. Representative phase contrast microscopic appearance of cells at low power (original magnification × 20 for left and center panels) or high power (original magnification × 40 for right panel) view is shown.
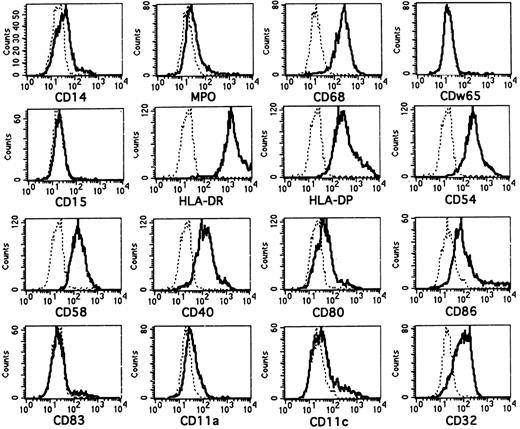
The molecular features of CD1a+ cells generated in FL-supplemented cultures are shown in Fig 3. Most CD1a+ cells generated in the presence of FL lack or express only at low levels intracellular LZ (see Fig 5B)26 or myeloperoxidase (MPO).27 They strongly coexpress the monocyte/macrophage/DC-associated lysosomal membrane glycoprotein CD68 (macrosialin)26 28 and are surface membrane CD14dim/−. They also lack expression of the granulomonocyte-associated molecules CDw65 and CD15 (Fig 3).
Phenotypic analysis of in vitro-generated CD1a+ cells. Purified CD34+ CB cells were cultured for 10 days in serum-free medium in the presence of FL plus TGF-β1, GM-CSF, TNFα, and SCF. They were then harvested and combined-stained for expression of CD1a and several informative molecules as indicated. Overlay diagrams show expression of the indicated molecules (lines) versus negative control (dotted lines) by gated CD1a+ cells. CD1a+ cells were gated in separate diagrams (CD1a v SSC, not shown). Histograms represent at least 3,000 gated CD1a+ cells.
Phenotypic analysis of in vitro-generated CD1a+ cells. Purified CD34+ CB cells were cultured for 10 days in serum-free medium in the presence of FL plus TGF-β1, GM-CSF, TNFα, and SCF. They were then harvested and combined-stained for expression of CD1a and several informative molecules as indicated. Overlay diagrams show expression of the indicated molecules (lines) versus negative control (dotted lines) by gated CD1a+ cells. CD1a+ cells were gated in separate diagrams (CD1a v SSC, not shown). Histograms represent at least 3,000 gated CD1a+ cells.
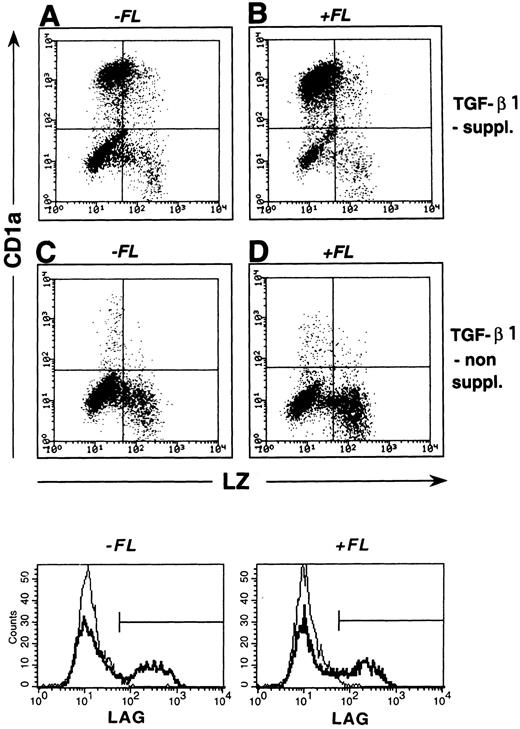
Correlated expression of CD1a and LZ by cells generated in the presence or absence of FL and/or TGF-β1. Purified CD34+ CB cells were cultured for 10 days in serum-free medium in the presence of the basic growth factor combination GM-CSF plus TNFα and SCF. Parallel cultures were further supplemented with TGF-β1 and/or FL at culture initiation (see the Materials and Methods). Day-10 cells were harvested and combined stained for intracellular LZ and surface-membrane CD1a expression (see the Materials and Methods). Representative diagrams show LZ (x-axis) versus CD1a (y-axis) expression by cells generated in the absence or presence of TGF-β1 and/or FL as indicated. Markers were set according to negative control stainings (see the Materials and Methods). Histograms shown in the lower part are cells obtained from identical culture conditions as described above that were submitted to indirect staining for the BG-associated molecule Lag (as described in the Materials and Methods). Overlay histograms represent Lag stainings of cells generated in TGF-β1 nonsupplemented (faint lines) and TGF-β1 supplemented (bold lines) cultures. Cultures without (left overlay histograms) or with FL supplementation (right overlay histograms) are shown. Flow cytometric analyses show at least 5,000 cells.
Correlated expression of CD1a and LZ by cells generated in the presence or absence of FL and/or TGF-β1. Purified CD34+ CB cells were cultured for 10 days in serum-free medium in the presence of the basic growth factor combination GM-CSF plus TNFα and SCF. Parallel cultures were further supplemented with TGF-β1 and/or FL at culture initiation (see the Materials and Methods). Day-10 cells were harvested and combined stained for intracellular LZ and surface-membrane CD1a expression (see the Materials and Methods). Representative diagrams show LZ (x-axis) versus CD1a (y-axis) expression by cells generated in the absence or presence of TGF-β1 and/or FL as indicated. Markers were set according to negative control stainings (see the Materials and Methods). Histograms shown in the lower part are cells obtained from identical culture conditions as described above that were submitted to indirect staining for the BG-associated molecule Lag (as described in the Materials and Methods). Overlay histograms represent Lag stainings of cells generated in TGF-β1 nonsupplemented (faint lines) and TGF-β1 supplemented (bold lines) cultures. Cultures without (left overlay histograms) or with FL supplementation (right overlay histograms) are shown. Flow cytometric analyses show at least 5,000 cells.
Virtually all CD1a+ cells are strongly positive for the major histocompatibility complex class II molecules HLA-DR/DP and express the costimulatory/accessory molecules CD54 (ICAM-1), CD58 (LFA-3), and CD40. In line with the phenotype of immature DC such as epidermal Langerhans cells, CD80 (B7.1) and CD86 (B7.2) expression is weak on most CD1a+ cells.29 (In the representative experiment shown, 29% of all CD1a+ cells show CD86 staining above isotype background control.) Furthermore, as also seen in LC, most CD1a+ cells express the FcγRII molecule CD324,30 and lack the mature DC-marker molecule CD8331,32 as well as the adhesion molecules LFA-1 (CD11a)30 and CD11c.30,33 34
CD1a+ cells generated in FL-supplemented cultures consistently showed weaker reactivity with CD86 antibody than those generated in FL nonsupplemented cultures (n = 3; average mean fluorescence intensity of 105 v 164, respectively). This might suggest but does not prove that FL supports a shift to an immature DC subset.
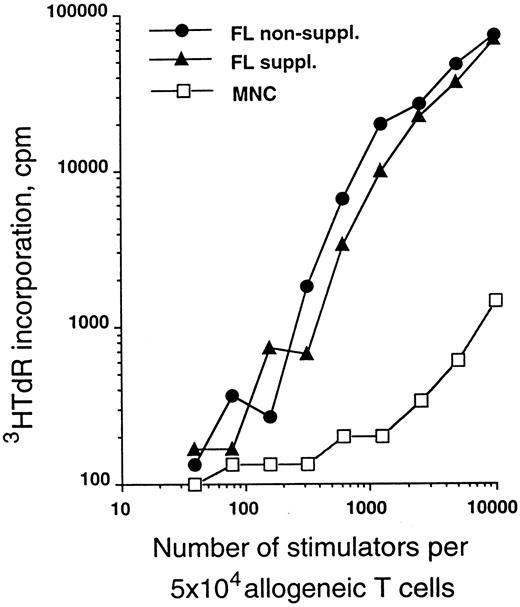
The allostimulatory capacity of the in vitro-generated cells was evalulated using highly purified T cells as responders. Figure 4 shows a relative comparison of CD34+ cells cultured in serum-free medium for 10 days in the presence of TGF-β1, GM-CSF, TNFα, and SCF with or without FL supplementation as described above. As can be seen from this representative experiment, cells generated in the presence of FL are of similar potency in the MLR as those generated in the absence of FL.
MLR-stimulatory capacity of generated cells. Purified CD34+ CB cells were cultured for 10 days in serum-free medium in the presence of TGF-β1, GM-CSF, TNFα, and SCF with or without FL (see the Materials and Methods). Graded numbers of cells obtained from these cultures were used to stimulate 5 × 104 purified allogeneic T cells (see the Materials and Methods) and were compared with adult PBMNC. Data are representative of five experiments.
MLR-stimulatory capacity of generated cells. Purified CD34+ CB cells were cultured for 10 days in serum-free medium in the presence of TGF-β1, GM-CSF, TNFα, and SCF with or without FL (see the Materials and Methods). Graded numbers of cells obtained from these cultures were used to stimulate 5 × 104 purified allogeneic T cells (see the Materials and Methods) and were compared with adult PBMNC. Data are representative of five experiments.
TGF-β1 supplementation of cultures is required for the DC growth-promoting effect of FL to occur.The experiments described above show that FL strongly promotes DC development when added to TGF-β1 plus GM-CSF–, TNFα-, and SCF-supplemented serum-free culture medium. TGF-β1 was recently found by us to be critical for DC development induced by GM-CSF plus TNFα and SCF under serum-free conditions.22 We therefore analyzed whether TGF-β1 is also required in FL supplemented cultures.
Figure 5A shows representative FACS profiles (surface CD1a v intracellular LZ) of cells generated in the presence or absence of TGF-β1. As can be seen, the majority of cells generated in the presence of both FL and TGF-β1 (Fig 5B) express CD1a and only few show granulomonocytic features (LZ+/CD1a−). However, in the absence of TGF-β1 supplementation (Fig 5D), a strikingly different staining pattern emerges. Percentages of cells expressing CD1a dramatically decrease (P = .001) and proportions of LZ+/CD1a− granulomonocytic cells significantly increase. Similar results were obtained in five independent experiments (Fig 6A; P < .001) and are further supported by stainings with two additional granulomonocytic marker molecules (MPO and CD14; P < .01).
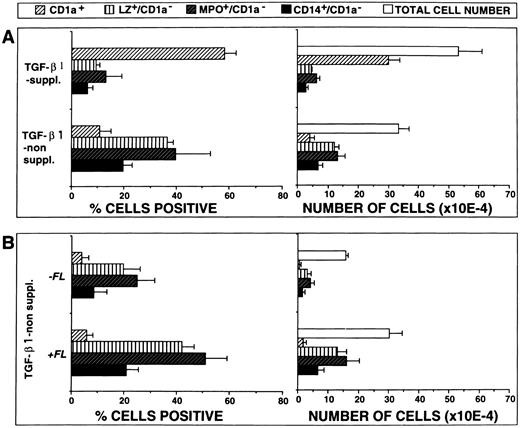
FL plus TGF-β1 cooperate in stimulating DC generation. (A) Effects of TGF-β1 on cell proliferation and differentiation. Ten thousand CD34+ CB cells were cultured for 10 days in serum-free medium supplemented with the cytokines FL plus GM-CSF, TNFα, and SCF, with or without TGF-β1, and combined analyzed for the expression of CD1a versus either LZ, MPO, or CD14, respectively, as described in the Materials and Methods. Bars represent mean the percentages ± SEM (left-hand side diagrams) and total numbers ± SEM (right-hand side diagrams), respectively, of cells with the indicated phenotypes observed in five experiments. The number of phenotypically defined cells was calculated from the percentage of cells showing the respective phenotype multiplied by the total number of cells in each culture. (B) Effects of FL costimulation in the absence of TGF-β1. Ten thousand CD34+ CB cells were cultured for 10 days in serum-free medium supplemented with the cytokines GM-CSF plus TNFα and SCF, with or without FL, and analyzed by flow cytometry as described above. Bars represent mean percentages ± SEM (left-hand side diagrams) and total numbers ± SEM (right-hand side diagrams), respectively, of cells with the indicated phenotypes observed in three experiments. The number of phenotypically defined cells was calculated from the percentage of cells showing the respective phenotype multiplied by the total number of cells in each culture.
FL plus TGF-β1 cooperate in stimulating DC generation. (A) Effects of TGF-β1 on cell proliferation and differentiation. Ten thousand CD34+ CB cells were cultured for 10 days in serum-free medium supplemented with the cytokines FL plus GM-CSF, TNFα, and SCF, with or without TGF-β1, and combined analyzed for the expression of CD1a versus either LZ, MPO, or CD14, respectively, as described in the Materials and Methods. Bars represent mean the percentages ± SEM (left-hand side diagrams) and total numbers ± SEM (right-hand side diagrams), respectively, of cells with the indicated phenotypes observed in five experiments. The number of phenotypically defined cells was calculated from the percentage of cells showing the respective phenotype multiplied by the total number of cells in each culture. (B) Effects of FL costimulation in the absence of TGF-β1. Ten thousand CD34+ CB cells were cultured for 10 days in serum-free medium supplemented with the cytokines GM-CSF plus TNFα and SCF, with or without FL, and analyzed by flow cytometry as described above. Bars represent mean percentages ± SEM (left-hand side diagrams) and total numbers ± SEM (right-hand side diagrams), respectively, of cells with the indicated phenotypes observed in three experiments. The number of phenotypically defined cells was calculated from the percentage of cells showing the respective phenotype multiplied by the total number of cells in each culture.
In addition, total yields of CD1a+ cells were significantly (P = .001) lower in the absence of TGF-β1 than in its presence, whereas the reverse was true for granulomonocytic cells (Fig 6A; P < .05).
In a separate series of experiments we analyzed the effects of FL on GM-CSF plus TNFα– and SCF but not TGF-β1–supplemented cultures (Fig 5C v D and Fig 6B). In three experiments, FL was found to consistently increase proportions and yields of granulomonocytic cells (LZ+/CD1a−, MPO+/CD1a−, and CD14+/CD1a−; Fig 6B). In contrast, the proportions/total numbers of CD1a+ cells remained unchanged at a low level. Also, morphologically, hardly any DC cluster formation was seen in TGF-β1 nonsupplemented cultures in the presence or absence of FL (data not shown). Thus, in TGF-β1 nonsupplemented cultures, FL promotes granulomonopoietic differentiation, but does not induce significant DC generation.
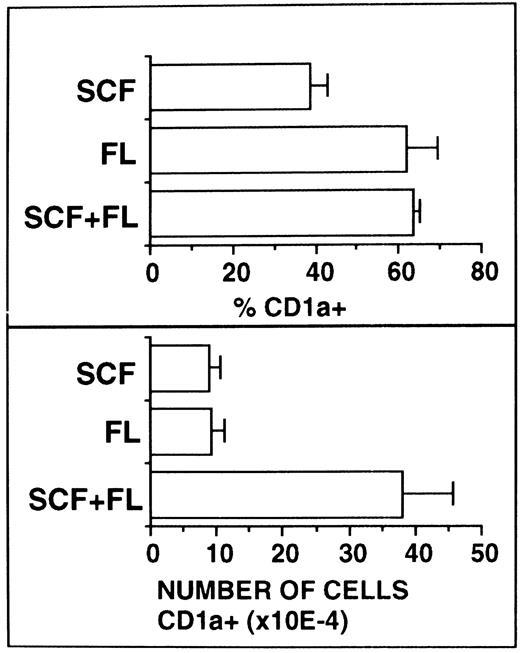
FL supplementation does not substitute for SCF supplementation.Because SCF and FL share molecular and functional features, we next analyzed whether these two cytokines show synergistic or redundant activity in our culture system. Serum-free cultures were supplemented with TGF-β1 plus GM-CSF and TNFα as a basic growth stimulus and the effects of additionally added SCF, FL, or SCF plus FL were analyzed. As can be seen from Fig 7, equally high percentages of CD1a+ cells were observed in FL-supplemented or FL plus SCF–supplemented cultures. In cultures with SCF alone, these values were significantly (P < .05) lower.
Comparative analysis of the effects of SCF and FL. Ten thousand CD34+ CB cells were cultured for 10 days in serum-free medium supplemented with the basic cytokine combination TGF-β1 plus GM-CSF and TNFα. This cytokine combination was further supplemented with SCF and/or FL as indicated. Cells were analyzed by flow cytometry for CD1a expression as described in the Materials and Methods. Bars represent mean percentages ± SEM (left-hand side diagrams) and total numbers ± SEM (right-hand side diagrams), respectively, of cells with the indicated phenotypes observed in four experiments. The number of phenotypically defined cells was calculated from the percentage of cells showing the respective phenotype multiplied by the total number of cells in each culture.
Comparative analysis of the effects of SCF and FL. Ten thousand CD34+ CB cells were cultured for 10 days in serum-free medium supplemented with the basic cytokine combination TGF-β1 plus GM-CSF and TNFα. This cytokine combination was further supplemented with SCF and/or FL as indicated. Cells were analyzed by flow cytometry for CD1a expression as described in the Materials and Methods. Bars represent mean percentages ± SEM (left-hand side diagrams) and total numbers ± SEM (right-hand side diagrams), respectively, of cells with the indicated phenotypes observed in four experiments. The number of phenotypically defined cells was calculated from the percentage of cells showing the respective phenotype multiplied by the total number of cells in each culture.
Total numbers of CD1a+ cells were found to be equivalent in SCF- or FL-supplemented cultures. However, upon combined addition of FL plus SCF to the cultures, total numbers of CD1a+ cells strongly increased (P < .05). In all experiments analyzed (n = 4), these increases were supraadditive in comparison to the values with either FL or SCF alone. Thus, synergism rather than redundancy seems to exist between SCF and FL in their capacity to stimulate DC growth.
LC/BG formation.We previously showed that TGF-β1 costimulation induces the generation of DC that morphologically resemble epidermal Langerhans cells and are characterized by bright cytoplasmic staining for the BG marker molecule Lag.22 In flow cytometric analyses, we observed that substantial percentages (15% ± 3%; n = 7) of all cultured cells in the presence of FL plus TGF-β1, GM-CSF, TNFα, and SCF strongly express Lag. Representative histograms showing Lag expression in cells generated under different culture conditions (with and without FL and/or TGF-β1) are presented in the lower part of Fig 5. As can be seen, similar percentages of cells generated in the presence of TGF-β1 without or with FL supplementation express Lag (bold lines, 21% and 19%, respectively). In contrast, less than 3% of cells generated in the absence of TGF-β1 under both conditions (FL-supplemented or nonsupplemented) express Lag (faint lines). As can be seen from Fig 8A, Lag antigen shows a granular cytoplasmic expression pattern that is particularly condensed at juxtanuclear sites. Development of Lag+ cells was strongly inhibited (to <3% Lag+ cells; n = 7; P < .05) upon omission of TGF-β1 from the culture medium.
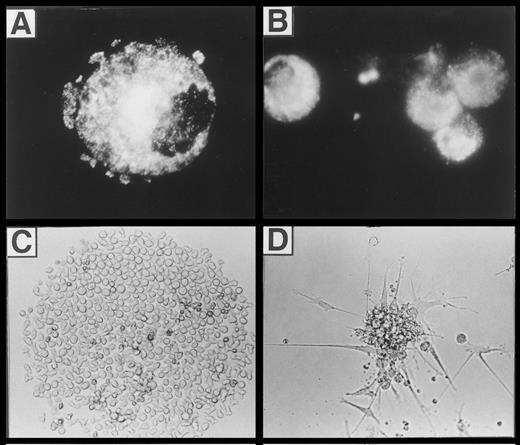
(A) Fluorescence microscopic appearance of Lag staining in a representative cell generated in the presence of FL plus TGF-β1, GM-CSF, TNFα, and SCF (see the Materials and Methods). (B, C, and D) Microscopic appearance of cells generated from singly seeded CD34+ cells. Purified CD34+ cells (obtained by immunomagnetic isolation, see the Materials and Methods) were sorted individually into 96-well plates using a FACSVantage and cultured in serum-free medium in the presence of FL, GM-CSF, TNFα, and SCF, with or without TGF-β1 for 10 days (see the Materials and Methods). The typical microscopic appearance of colonies is shown. (C) CFC classified as greater than 50 cells/colony (see also Fig 9) generated in the absence of TGF-β1. (D) DC colonies generated in the presence of TGF-β1 (see also Fig 9). (B) Immunofluorescence microphotography showing Lag expression of individually harvested CFU-DC.
(A) Fluorescence microscopic appearance of Lag staining in a representative cell generated in the presence of FL plus TGF-β1, GM-CSF, TNFα, and SCF (see the Materials and Methods). (B, C, and D) Microscopic appearance of cells generated from singly seeded CD34+ cells. Purified CD34+ cells (obtained by immunomagnetic isolation, see the Materials and Methods) were sorted individually into 96-well plates using a FACSVantage and cultured in serum-free medium in the presence of FL, GM-CSF, TNFα, and SCF, with or without TGF-β1 for 10 days (see the Materials and Methods). The typical microscopic appearance of colonies is shown. (C) CFC classified as greater than 50 cells/colony (see also Fig 9) generated in the absence of TGF-β1. (D) DC colonies generated in the presence of TGF-β1 (see also Fig 9). (B) Immunofluorescence microphotography showing Lag expression of individually harvested CFU-DC.
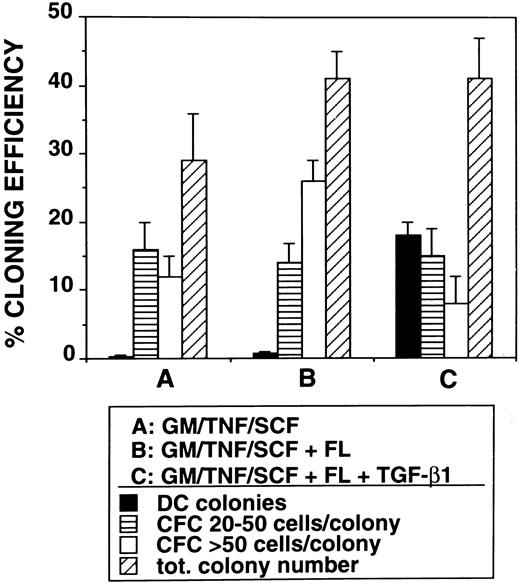
Induction of Lag+ DC colony growth.The observed highly efficient DC growth-promoting effect of FL together with TGF-β1, GM-CSF, TNFα, and SCF in bulk cultures prompted us to analyze DC growth also at the clonal level. Single CD34+ cells cultured in TGF-β1– and FL-supplemented serum-free medium gave rise to typical DC colony growth (colony-forming unit-DC [CFU-DC]) consisting of spiny plastic adherent cells and loosely attached overlying cells (compare Fig 8D with Fig 2). Cytospin preparations of individually harvested CFU-DC showed that between 63% and 91% (n = 9) of the cells included in these colonies express Lag (Fig 8B). In four independent experiments, we observed that between 14% and 24% (mean, 18% ± 2%) of singly seeded CD34+ cells developed into Lag+ CFU-DC after 10 days in the presence of FL plus TGF-β1 (Fig 9). In the absence of TGF-β1, CFU-DC growth was found to be markedly reduced (Fig 9). However, total numbers of colony-forming cells (CFC) were equivalent in FL-supplemented or FL plus TGF-β1–supplemented cultures. CFC classified as containing greater than 50 cells/colony and consistently showing monocyte/macrophage morphology (Fig 8C) were on average more frequently found in FL-supplemented cultures in the absence of TGF-β1 (Fig 9).
Assessment of the effects of TGF-β1 on colony formation by CD34+ cells. Purified CD34+ cells (obtained by immunomagnetic isolation, see the Materials and Methods) were sorted individually into 96-well plates using a FACSVantage and cultured in serum-free medium in the presence of GM-CSF, TNFα, and SCF or in medium additionally supplemented with FL or FL plus TGF-β1 for 10 days (see the Materials and Methods). Bars show percentage cloning efficiency for Lag+ dendritic cell colonies and small (20 to 50 cells/colony) or large (<50 cells/colony) colonies (CFC).
Assessment of the effects of TGF-β1 on colony formation by CD34+ cells. Purified CD34+ cells (obtained by immunomagnetic isolation, see the Materials and Methods) were sorted individually into 96-well plates using a FACSVantage and cultured in serum-free medium in the presence of GM-CSF, TNFα, and SCF or in medium additionally supplemented with FL or FL plus TGF-β1 for 10 days (see the Materials and Methods). Bars show percentage cloning efficiency for Lag+ dendritic cell colonies and small (20 to 50 cells/colony) or large (<50 cells/colony) colonies (CFC).
DISCUSSION
We show here, using a defined serum-free culture system, that the cytokine FL has profound effects on in vitro DC development. It amplifies DC generation 4.4- ± 0.8-fold when added to TGF-β1 plus GM-CSF, TNFα, and SCF supplemented bulk cultures of purified CD34+ progenitor cells. Moreover, substantial proportions (18% ± 2%) of singly seeded CD34+ cells give rise to DC-containing colonies (total cloning efficiency, 41% ± 4%) under these culture conditions.
We further demonstrate that this DC growth-promoting effect of FL is strictly dependent on the simultaneous presence in culture of TGF-β1. In the absence of TGF-β1, FL primarily promotes outgrowth of monocyte/macrophage-type cells.
The molecular features of DC generated with this cytokine combination (FL plus TGF-β1, GM-CSF, TNFα, and SCF ) strikingly resemble previously described features of freshly isolated epidermal LC.
In bulk cultures, up to 77% of cells generated strongly expressed CD1a and showed a surface marker profile (HLA-DR+, HLA-DP+, CD40+, CD54+, CD58+, CD32+, CD80dim, CD86dim, CD83−, CD11a−/dim, CD11c−/dim, CD14−/dim) reminiscent of LC rather than fully mature DC.29-31,33,34 LC features could also be shown at the intracellular level. Substantial proportions of generated cells contained the LC-specific BG-associated protein Lag.23 The vast majority of CD1a+ cells were negative for the granulomonocytic marker molecules MPO26,27 and LZ,26,35-37 but expressed the macrophage/DC marker CD68.28,37 38
In single-cell cultures, DC outgrowth of cultures could already be detected morphologically. These colonies grew out in 18% ± 2% of all single-cell cultures and were composed of underlying cells that loosely adhered to plastic and attached to these overlying clustered cells. Cytoplasmic staining of these colonies showed that they represent strongly enriched LC colonies. In all of these colonies, 63% to 91% of cells were Lag+.
Serum-free culture of CD34+ cells with the cytokine combination FL plus TGF-β1, GM-CSF, TNFα, and SCF thus allows a previously undescribed clonal outgrowth of LC like Lag+ DC from a substantial proportion of singly seeded progenitors. Morphologically, these colonies resemble previously described DC clusters generated from murine bone marrow39 and are reminiscent of mixed monocyte-DC clusters obtained in semisolid serum-containing cultures of human CD34+ progenitors.8
As indicated above, additional supplementation with TGF-β1 is absolutely required to achieve the DC growth-promoting effect of FL. In the absence of TGF-β1, FL promoted monocyte/macrophage development. This is most clearly demonstrated in single-cell colony experiments. Here, total cloning efficiency is about equal in the presence or absence of TGF-β1. FL plus GM-CSF, TNFα, and SCF induces colony development in 41% ± 4% of single-cell cultures. Further addition of TGF-β1 gives virtually identical results (total cloning efficiency, 41% ± 6%). However, substantial DC colony formation can only be observed when both FL and TGF-β1 are added together with GM-CSF plus TNFα and SCF to single-cell cultures of CD34+ cells (Fig 9). In the absence of TGF-β1, other colony types (containing monocytic cells but not DC) predominate. One is tempted to speculate, therefore, that FL in our culture system indiscriminately promotes monocyte and/or DC progenitor cell growth. In the presence of TGF-β1, differentiation is driven or allowed to drive along the DC axis. Such a scenario, ie, that TGF-β1 promotes DC/LC development at the expense of granulomonocyte differentiation, might also have in vivo relevance. It is consistent with the distribution of myelomonocytic cells and DC populations in vivo in TGF-β1 deficient mutant mice. TGF-β1−/− mice lack epidermal Langerhans cells,40 but show monocytosis as well as substantial granulocyte and macrophage tissue infiltration.41 The nature of this effect of TGF-β1 on LC differentiation has not been determined. However, we recently observed that TGF-β1, at least under in vitro culture conditions, seems to promote DC development by protecting DC progenitors from undergoing apoptosis rather than by stimulating their proliferation.42 Apart from this, TGF-β1 may also induce further differentiation and/or migration of Langerhans cell precursors.
The two structurally and functionally related cytokines FL and SCF seem to differ in their costimulatory capacity on in vitro DC generation. We show here that FL enhances DC development by both increasing numbers and percentages of CD1a+ DC. In contrast, SCF has previously been shown to increase numbers but not percentages of DC.10 The underlying mechanism of this enhancing effect of FL on DC generation remains to be determined. Among several possibilities, FL may costimulate recruitment of an additional subset included in the CD34+ population into DC development. Extra cell divisions of DC progenitors/precursors may also occur in response to FL costimulation. It is likely that FL predominantly acts on still common precursors for monocytic cells and DC, given the close developmental association of these two cell types and the fact that FL strongly promotes monopoietic cell growth.21 It is furthermore interesting to speculate that, in response to FL costimulation, a substantial percentage of generated CD1a+ cells may originate from bipotent monocytoid/DC precursors. Such a differentiation pathway involving intermediate stage cells with maintained monocyte/macrophage and DC developmental capacity has recently been identified in cultures of CD34+ bone marrow6 or cord blood cells.7
Although the majority of all cultured cells in the presence of FL plus TGF-β1 at day 10 express CD1a and an additional population of cells expresses the (pro)-monocye marker molecule LZ, approximately 25% of cells remained LZ−/CD1a−. Proportions of LZ−/CD1a− cells are particularly high in FL nonsupplemented cultures (approximately 45%). We further observed that, regardless of the presence or absence of FL, only low percentages of cells express the progenitor cell marker molecule CD34 (<5%) and less than 2% of cultured cells show lymphoid features as evidenced by absence of cytoplasmic CD3 or CD22 expression. However, most LZ−/CD1a− cells under both conditions express intracellular CD6826 43 and are HLA-DR+, suggesting that they may represent immature granulomonocytic cells and/or DC precursors.
The use of a serum-free in vitro protocol for obtaining large quantities of DC offers several advantages in the study of basic DC biology and in the practical application of DC for immunotherapy. DC generated in vitro from progenitor cells are not preexposed to antigens in the periphery. Furthermore, generation of these cells in serum-free medium precludes exposure to undefined foreign proteins such as occurring in fetal calf serum or allogeneic human serum/plasma during expansion. The immature differentiation stage of the generated DC may be of advantage because immature DC are particularly efficient in capturing and processing antigen (recently reviewed in Cella et al44 ). Further studies should analyze whether these immature DC generated in serum-free medium can be allowed to undergo further maturation for optimal stimulation of naive T cells.
ACKNOWLEDGMENT
The authors are grateful to Dr S. Imamura (Kyoto, Japan) for providing MoAb Lag. Furthermore, we thank A. Renner for his invaluable contribution in cell separation and flow sorting. We also thank Dr H. Stockinger (Vienna, Austria) for critically reading the manuscript and Dr G. Seidl (Vienna, Austria) for controlling cultures for endotoxin. Finally, we are indebted to all of the collaborating nurses and doctors of the gynecology departments at Sozialmedizinisches Zentrum Ost and Kaiser Franz Josef Spital (Vienna, Austria).
Supported by Fonds zur Förderung der wissenschaftlichen Forschung in Österreich.
Address reprint requests to Herbert Strobl, MD, Institute of Immunology-VIRCC at Sandoz Forschungsinstitut, University of Vienna, Brunnerstrasse 59, A-1235 Vienna, Austria.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal