Key Points
Limited data are available on HRQoL of patients with AML treated with decitabine.
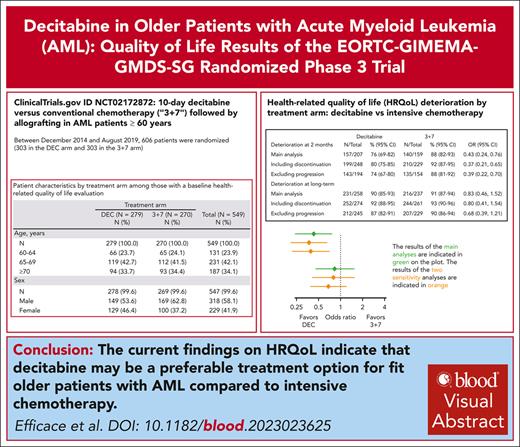
Current HRQoL findings suggest that decitabine, may be preferable to intensive chemotherapy in fit older patients with AML.
Visual Abstract
We hypothesized that fit older patients with acute myeloid leukemia (AML) treated with decitabine (DEC) would report better health-related quality of life (HRQoL) outcomes than those receiving intensive chemotherapy (IC). We conducted a phase 3 randomized trial to compare DEC (10-day schedule) with IC (3+7) in older fit patients with AML. HRQoL was a secondary end point, and it was assessed with the European Organisation for Research and Treatment of Cancer Quality of Life Questionnaire Core 30 (EORTC QLQ-C30) in conjunction with its elderly module (EORTC QLQ-ELD14). The following scales were a priori selected for defining the primary end point: physical and role functioning, fatigue, pain, and burden of illness. HRQoL was assessed at baseline, at regeneration from cycle 2, and at 6 and 12 months after randomization, and also before allogeneic hematopoietic stem cell transplantation (allo-HSCT) and 100 days after transplantation. Overall, 606 patients underwent randomization. At 2 months, the risk of HRQoL deterioration was lower in the DEC arm than in the 3+7 arm; 76% (95% confidence interval [CI], 69-82) vs 88% (95% CI, 82-93); odds ratio, 0.43 (95% CI, 0.24-0.76; P = .003). No statistically significant HRQoL differences were observed between treatment arms at the long-term evaluation combining assessments at 6 and 12 months. HRQoL deteriorations between baseline and after allo-HSCT were observed in both arms. However, these deteriorations were not clinically meaningful in patients randomized to DEC, whereas this was the case for those in the 3+7 arm, in 4 of 5 primary HRQoL scales. Our HRQoL findings suggest that lower-intensity treatment with DEC may be preferable to current standard IC (3+7) in fit older patients with AML. This trial was registered at www.clinicaltrials.gov as #NCT02172872.
Introduction
Acute myeloid leukemia (AML) is a disease that typically affects older persons, with a median age of 68 years at the time of diagnosis.1 Although major advances have been made in the treatment of younger patients with AML over the last decades,2 the 5-year survival rates for those aged ≥65 years remain poor.3
Treatment options for older patients with AML have historically been very limited until the introduction of low-intensity doses and schedules of the hypomethylating agents (HMAs) decitabine (DEC) and azacitidine.4,5 In more recent years, further advances have been observed for elderly patients who are unfit for intensive chemotherapy (IC). Based on findings from 2 pivotal randomized controlled trials (RCTs),6,7 the US Food and Drug Administration approved venetoclax in combination with HMAs or low-dose cytarabine, thereby defining a new standard of care for this population. The inclusion of health-related quality of life (HRQoL) as secondary end points in these RCTs has been critical to demonstrate a longer preservation of functioning and overall health status in patients treated with this novel regimen.8
Furthermore, clinical decision-making for older patients with AML who are considered fit for IC at clinical presentation remains a major challenge,9 and these patients have poor survival unless they are consolidated with an allogeneic hematopoietic stem cell transplantation (allo-HSCT).10 The current standard bridging approach to allo-HSCT is IC, which may not be well tolerated by many patients who are then forced to discontinue therapy, hence limiting the option of the potentially curative value of allo-HSCT.
Therefore, an international phase 3 RCT in fit older patients with AML was performed to compare IC (3+7) vs DEC followed by allo-HSCT, which revealed similar survival and comparable allo-HSCT rates between treatment groups.11 Briefly, the hazard ratio for death was 1.04 (95% confidence interval [CI], 0.86-1.26; P = .68). The remission rates achieved as a part of the protocol treatment were 48% (95% CI, 42-54) for DEC and 61% (95% CI, 56-67) for 3+7. Overall remission rates including response to postprotocol treatments before allografting were 60% (95% CI, 55-66) in the DEC and 67% (95% CI, 61-72) in the 3+7 arms, and hospital stays were shorter in the DEC than in the 3+7 arm.11 Given the importance of relying on evidence-based HRQoL information to optimize patient-centered care in the AML setting,3 and cognizant of the high value placed on HRQoL by patients with AML,12 we included it as a secondary end point in the study protocol of this RCT.11
We aimed to generate patient-reported outcome data that could better inform risk-benefit assessment in this setting and hypothesized that patients receiving DEC would experience better HRQoL outcomes owing to the lower-intensity regimens of HMAs compared with 3+7.
Patients and methods
Study design and patients
We conducted a prospective, multinational open-label, phase 3 randomized trial: the EORTC-1301 AML21 study. Eligibility criteria and full treatment procedures are reported elsewhere.11 In brief, patients with confirmed newly diagnosed AML aged ≥60 years, considered eligible for standard IC were randomized to DEC at a dose of 20 mg/m2 × 10 days vs IC, that is, conventional induction chemotherapy, daunorubicin 60 mg/m2 × 3 days and cytarabine 200 mg/m2 × 7 days (“3+7”), followed by 1 to 3 additional chemotherapy cycles. All patients, irrespective of their genetic risk profile, having an HLA-matched donor, and attaining at least disease stabilization after ≥1 treatment cycle, were encouraged to undergo an allo-HSCT. Overall survival was the primary study end point and HRQoL a secondary end point.
This study involved 54 centers, across 9 European countries, from 3 groups: European Organisation for Research and Treatment of Cancer (EORTC), Gruppo Italiano Malattie EMatologiche dell'Adulto, and German Myelodysplastic Syndromes Study Group. The study was approved by all ethics committees of each participating centers. The trial was conducted in accordance with the Declaration of Helsinki and with good clinical practice as defined by the International Conference on Harmonization. All patients provided written informed consent. The study was designed by the academic authors and the legal sponsor was EORTC. The study was registered at www.clinicaltrials.gov as #NCT02172872.
Randomization
Registration was done centrally at the EORTC headquarters (Brussels, Belgium). Eligible patients were randomly assigned (1:1) to receive DEC or 3+7. The randomization, based on a minimization technique, was stratified by AML type (de novo vs secondary), age (60-64 vs 65-69 vs ≥70 years) and site. The study was open-label.
Procedures for HRQoL assessment and reporting of study results
The EORTC Quality of Life Questionnaire-Core 30 (EORTC QLQ-C30; version 3)13 in conjunction with its elderly module (EORTC QLQ-ELD14)14 was used to assess HRQoL. The EORTC QLQ-C30 consists of 5 functioning scales: physical, role, emotional, cognitive, and social; and 9 symptom scales: fatigue, nausea/vomiting, pain, dyspnea, sleep disturbance, appetite loss, constipation, diarrhea, and financial difficulties; and the global health status/QoL scale. The items were scaled and scored using the recommended EORTC procedures.15 Standardized scores range from 0 to 100, with higher scores representing a higher level of functioning and health status/QoL or higher level of symptoms. This questionnaire is 1 of the most frequently used HRQoL measures in cancer RCTs in general16 and also specifically in AML studies.17 The QLQ-ELD14 was selected having being developed in an international setting to cover key HRQoL aspects relevant for older patients with cancer.14 It consists of the following 2 functional scales: maintaining purpose and family support; it also has 5 symptom scales: mobility, worries about others, future worries, burden of illness, and joint stiffness. Standardized scores range from 0 to 100, with higher scores on the functional scales indicating better functioning and higher scores on the symptom scales indicating more severe problems.
The following 5 scales were a priori selected for defining the primary HRQoL end point: physical and role functioning, fatigue, pain (EORTC QLQ-C30), and burden of illness (QLQ-ELD14). This selection was based on clinical relevance for our study population.
Questionnaires were completed by patients (paper version) at the hospital when patients came for a scheduled visit according to the EORTC guidelines for administration of questionnaires.18 Baseline questionnaires were filled before start of protocol treatment. Subsequent questionnaires were filled out at regeneration cycles 2 to 3 (in between end of cycle 2 and start of cycle 3) and at 6 and 12 months after randomization. For those who received an allo-HSCT, HRQoL was also assessed before starting the conditioning and at day +100 after transplantation. HRQoL data were collected regardless of the patient’s progression status. The HRQoL findings of this study are reported in accordance with the CONSORT-PRO (Consolidated Standards of Reporting Trials—Patient-Reported Outcomes) extension guidelines.19
Outcomes
The main end point was HRQoL deterioration, defined as the occurrence of any of the following events: (1) deterioration (of at least 10 points) in fatigue, pain, burden of illness, physical functioning, or role functioning relative to baseline; (2) death before the HRQoL evaluation; and (3) disease progression before the HRQoL evaluation. Two versions of this end point used in the analysis were short-term and long-term HRQoL deterioration. The short-term HRQoL deterioration was based on the HRQoL evaluation ∼2 months after the randomization. The long-term HRQoL deterioration was defined as HRQoL deterioration either at 6 months or at 12 months after randomization. When evaluating differences in group means, a difference of at least 10 points was considered as clinically meaningful for all HRQoL scales.20
Statistical methods
The study was initially planned to define the primary end point HRQoL deterioration as a deterioration at any of the 5 evaluation time points (regeneration from cycle 2, at 6 months, at 12 months, before allo-HSCT, and 100 days after allo-HSCT). However, because only a proportion of patients underwent allo-HSCT and because not all patients proceeded to cycle 2, compliance checks revealed a prohibitively low compliance for many of the 5 evaluation time points. Therefore, the evaluations at 6 and 12 months were analyzed separately, as an indicator of the long-term HRQoL. For the purpose of analysis, a new time point was introduced at about 2 months after randomization which included questionnaires administered after cycle 2 and prior to allo-HSCT. This time point was analyzed separately as an indicator of the HRQoL during the treatment. Finally, the time points before and 100 days after an allo-HSCT were analyzed separately in the cohort of patients who received transplantation. The time windows for all evaluation time points used in the analyses are available in supplemental Table 1, available on the Blood website.
The exact test was used to compare short-term and long-term HRQoL deterioration between the treatment arms. A logistic regression model without covariates was used to estimate the odds ratio (OR). Exact CIs were used for proportions. CIs based on the t-distribution of group means and differences in group means were used. The variance of the difference in group means was estimated using the Satterthwaite method. Patients without a baseline questionnaire were excluded from all analyses. In the main analysis of HRQoL deterioration, alive and progression-free patients at the time of the HRQoL evaluation and without a HRQoL questionnaire for the time points of interest were excluded.
We used a number of strategies to deal with the problem of missing values. First, because patients with a disease progression were expected to have a low HRQoL and a low compliance to HRQoL evaluations potentially introducing a bias in the analysis, progressions were treated as indicating a deterioration in the main end point HRQoL deterioration. Two sensitivity analyses were performed by modifying the definition of the end point HRQoL deterioration. First, the definition was modified by counting patients who discontinued the treatment as having a deterioration, which was expected to significantly reduce the number of patients with a missing value. Second, the definition of deterioration used in the primary analysis was changed by not counting patients who had a disease progression as having a deterioration. In addition, we also performed sensitivity analyses using multiple imputation, in which we imputed missing values using HRQoL at remaining time points, treatment arm, age, sex, performance status at the time of evaluation, and remission status at the time of evaluation (details are described in supplemental Material). Because these covariates were expected to represent most important predictors of HRQoL in the trial, the analysis using multiple imputation was expected to significantly reduce the risk of bias due to missing values. The analysis was performed in SAS version 9.4 (SAS Institute, Inc, Cary, NC).
Results
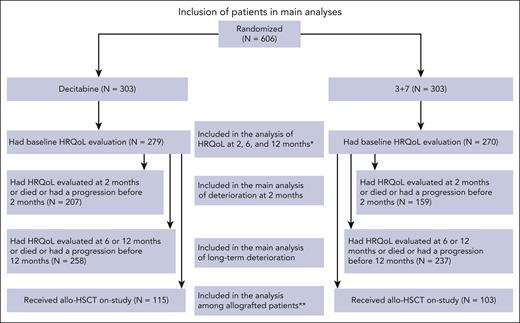
Between December 2014 and August 2019, 606 patients were randomized (303 in the DEC arm, and 303 in the 3+7 arm). The flowchart of patients included in the HRQoL analysis is reported in Figure 1.
Inclusion of patients in main analyses. ∗The number of patients with a HRQoL evaluation at 2, 6, and 12 months is available in the supplemental Data. ∗∗The number of patients with a HRQoL evaluation prior to and post–allo-HSCT is available in the supplemental Data. allo-HSCT, allogeneic hematopoietic stem cell transplantation; HRQoL, health-related quality of life; N, number of patients.
Inclusion of patients in main analyses. ∗The number of patients with a HRQoL evaluation at 2, 6, and 12 months is available in the supplemental Data. ∗∗The number of patients with a HRQoL evaluation prior to and post–allo-HSCT is available in the supplemental Data. allo-HSCT, allogeneic hematopoietic stem cell transplantation; HRQoL, health-related quality of life; N, number of patients.
Compliance at baseline was, overall, 91% (n = 549), and 92% (n = 279) and 89% (n = 270) in the DEC and 3+7 arm, respectively. Baseline demographics and clinical characteristics of patients included in the HRQoL analysis were well balanced between the 2 arms (Table 1). Baseline characteristics of patients with (n = 549) and without (n = 57) baseline HRQoL questionnaires were similar (supplemental Table 2). Baseline scores of the HRQoL primary scales were well balanced between groups (Table 2), as were the scores for all other scales (supplemental Table 3). Among patients with baseline HRQoL data available, the median time on protocol treatment was 90 days (range, 2-1287) in the DEC and 64 days (range, 1-307) in the 3+7 arm. In the same group of patients, the median number of cycles was 3 (range, 1-41) in the DEC and 2 (range, 1-4) in the 3+7 arm.
Patient characteristics by treatment arm among those with a baseline HRQoL evaluation
| . | Treatment arm . | . | |
|---|---|---|---|
| DEC (n = 279) . | 3+7 (n = 270) . | Total (N = 549) . | |
| n (%) . | n (%) . | n (%) . | |
| Age, y | |||
| n | 279 (100.0) | 270 (100.0) | 549 (100.0) |
| 60-64 | 66 (23.7) | 65 (24.1) | 131 (23.9) |
| 65-69 | 119 (42.7) | 112 (41.5) | 231 (42.1) |
| ≥70 | 94 (33.7) | 93 (34.4) | 187 (34.1) |
| Sex | |||
| n | 278 (99.6) | 269 (99.6) | 547 (99.6) |
| Male | 149 (53.6) | 169 (62.8) | 318 (58.1) |
| Female | 129 (46.4) | 100 (37.2) | 229 (41.9) |
| ECOG performance status | |||
| n | 279 (100.0) | 270 (100.0) | 549 (100.0) |
| 0 | 144 (51.6) | 146 (54.1) | 290 (52.8) |
| 1 | 112 (40.1) | 103 (38.1) | 215 (39.2) |
| 2 | 23 (8.2) | 21 (7.8) | 44 (8.0) |
| Baseline HSCT comorbidity index | |||
| n | 277 (99.3) | 267 (98.9) | 544 (99.1) |
| 0-1 | 154 (55.6) | 157 (58.8) | 311 (57.2) |
| 2 | 35 (12.6) | 28 (10.5) | 63 (11.6) |
| ≥3 | 88 (31.8) | 82 (30.7) | 170 (31.3) |
| AML type at baseline | |||
| n | 278 (99.6) | 268 (99.3) | 546 (99.5) |
| De novo AML | 196 (70.5) | 196 (73.1) | 392 (71.8) |
| Secondary AML | 82 (29.5) | 72 (26.9) | 154 (28.2) |
| ELN 2017 risk group | |||
| n | 248 (88.9) | 247 (91.5) | 495 (90.2) |
| Favorable | 64 (25.8) | 42 (17.0) | 106 (21.4) |
| Intermediate | 112 (45.2) | 119 (48.2) | 231 (46.7) |
| Adverse | 72 (29.0) | 86 (34.8) | 158 (31.9) |
| . | Treatment arm . | . | |
|---|---|---|---|
| DEC (n = 279) . | 3+7 (n = 270) . | Total (N = 549) . | |
| n (%) . | n (%) . | n (%) . | |
| Age, y | |||
| n | 279 (100.0) | 270 (100.0) | 549 (100.0) |
| 60-64 | 66 (23.7) | 65 (24.1) | 131 (23.9) |
| 65-69 | 119 (42.7) | 112 (41.5) | 231 (42.1) |
| ≥70 | 94 (33.7) | 93 (34.4) | 187 (34.1) |
| Sex | |||
| n | 278 (99.6) | 269 (99.6) | 547 (99.6) |
| Male | 149 (53.6) | 169 (62.8) | 318 (58.1) |
| Female | 129 (46.4) | 100 (37.2) | 229 (41.9) |
| ECOG performance status | |||
| n | 279 (100.0) | 270 (100.0) | 549 (100.0) |
| 0 | 144 (51.6) | 146 (54.1) | 290 (52.8) |
| 1 | 112 (40.1) | 103 (38.1) | 215 (39.2) |
| 2 | 23 (8.2) | 21 (7.8) | 44 (8.0) |
| Baseline HSCT comorbidity index | |||
| n | 277 (99.3) | 267 (98.9) | 544 (99.1) |
| 0-1 | 154 (55.6) | 157 (58.8) | 311 (57.2) |
| 2 | 35 (12.6) | 28 (10.5) | 63 (11.6) |
| ≥3 | 88 (31.8) | 82 (30.7) | 170 (31.3) |
| AML type at baseline | |||
| n | 278 (99.6) | 268 (99.3) | 546 (99.5) |
| De novo AML | 196 (70.5) | 196 (73.1) | 392 (71.8) |
| Secondary AML | 82 (29.5) | 72 (26.9) | 154 (28.2) |
| ELN 2017 risk group | |||
| n | 248 (88.9) | 247 (91.5) | 495 (90.2) |
| Favorable | 64 (25.8) | 42 (17.0) | 106 (21.4) |
| Intermediate | 112 (45.2) | 119 (48.2) | 231 (46.7) |
| Adverse | 72 (29.0) | 86 (34.8) | 158 (31.9) |
ECOG, Eastern Cooperative Oncology Group; ELN, European LeukemiaNet.
Baseline scores of primary HRQoL scales in all patients (left side) and in those who received allo-HSCT (right side) by treatment arm
| . | Treatment arm . | Treatment arm . | ||
|---|---|---|---|---|
| DEC (n = 279) . | 3+7 (n = 270) . | DEC (n = 115) . | 3+7 (n = 103) . | |
| EORTC QLQ-C30 | ||||
| Physical functioning | ||||
| n | 278 | 267 | 114 | 101 |
| Mean (SD) | 75.38 (23.86) | 78.61 (21.98) | 77.38 (22.91) | 78.89 (23.27) |
| Role functioning | ||||
| n | 276 | 268 | 112 | 103 |
| Mean (SD) | 65.34 (33.58) | 69.34 (31.51) | 68.75 (32.89) | 68.93 (32.84) |
| Fatigue | ||||
| n | 277 | 269 | 113 | 103 |
| Mean (SD) | 41.86 (30.31) | 39.45 (28.87) | 38.84 (31.81) | 38.46 (29.79) |
| Pain | ||||
| n | 278 | 270 | 114 | 103 |
| Mean (SD) | 17.99 (25.93) | 17.65 (26.79) | 15.06 (25.47) | 17.64 (27.10) |
| EORTC QLQ-ELD14 | ||||
| Burden of illness | ||||
| n | 245 | 242 | 100 | 97 |
| Mean (SD) | 54.90 (26.59) | 54.06 (28.78) | 55.00 (26.11) | 54.98 (29.38) |
| . | Treatment arm . | Treatment arm . | ||
|---|---|---|---|---|
| DEC (n = 279) . | 3+7 (n = 270) . | DEC (n = 115) . | 3+7 (n = 103) . | |
| EORTC QLQ-C30 | ||||
| Physical functioning | ||||
| n | 278 | 267 | 114 | 101 |
| Mean (SD) | 75.38 (23.86) | 78.61 (21.98) | 77.38 (22.91) | 78.89 (23.27) |
| Role functioning | ||||
| n | 276 | 268 | 112 | 103 |
| Mean (SD) | 65.34 (33.58) | 69.34 (31.51) | 68.75 (32.89) | 68.93 (32.84) |
| Fatigue | ||||
| n | 277 | 269 | 113 | 103 |
| Mean (SD) | 41.86 (30.31) | 39.45 (28.87) | 38.84 (31.81) | 38.46 (29.79) |
| Pain | ||||
| n | 278 | 270 | 114 | 103 |
| Mean (SD) | 17.99 (25.93) | 17.65 (26.79) | 15.06 (25.47) | 17.64 (27.10) |
| EORTC QLQ-ELD14 | ||||
| Burden of illness | ||||
| n | 245 | 242 | 100 | 97 |
| Mean (SD) | 54.90 (26.59) | 54.06 (28.78) | 55.00 (26.11) | 54.98 (29.38) |
SD, standard deviation.
Overall compliance was 57% (311/550), 57% (272/475), and 64% (229/360) at 2, 6, and 12 months, respectively. At 2 months, a significantly higher compliance rate was observed in the DEC arm (63%, 175/279) than in the 3+7 arm (50%, 136/271; supplemental Table 4).
Primary HRQoL analysis in the overall population
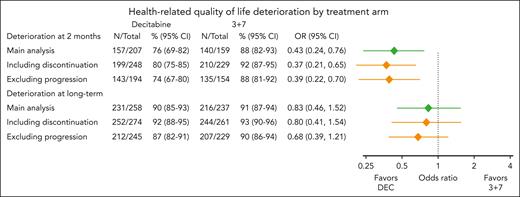
At 2 months, patients from the DEC arm had a statistically significant lower risk of HRQoL deterioration than those from the 3+7 arm (76% [95% CI, 69-82] vs 88% [95% CI, 82-93]; OR, 0.43 [95% CI, 0.24-0.76]; P = .003). The risk of HRQoL deterioration at long-term was not significantly different between arms. Sensitivity analyses counting treatment discontinuation before HRQoL assessment as an event or excluding disease progression confirmed the findings and are reported in Figure 2. A distribution of the end points composing deterioration is available in supplemental Table 5 (at 2 months) and supplemental Table 6 (long term).
HRQoL deterioration by treatment arm. Results of the main analyses are indicated in green on the plot. Results of the two sensitivity analyses, 1 modifying the definition of deterioration by counting patients who discontinued the treatment as having a deterioration and 1 modifying the definition of deterioration by not counting patients who had a disease progression as having a deterioration are indicated in orange. CI, confidence interval; DEC, decitabine; N, number of patients with a deterioration; OR, odds ratio of a deterioration.
HRQoL deterioration by treatment arm. Results of the main analyses are indicated in green on the plot. Results of the two sensitivity analyses, 1 modifying the definition of deterioration by counting patients who discontinued the treatment as having a deterioration and 1 modifying the definition of deterioration by not counting patients who had a disease progression as having a deterioration are indicated in orange. CI, confidence interval; DEC, decitabine; N, number of patients with a deterioration; OR, odds ratio of a deterioration.
Additional HRQoL analyses in the overall population
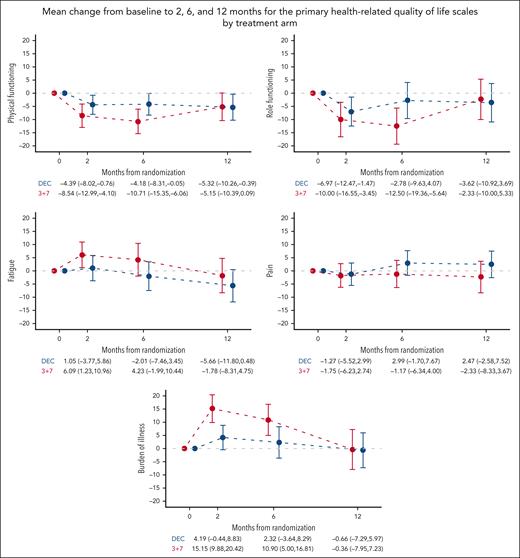
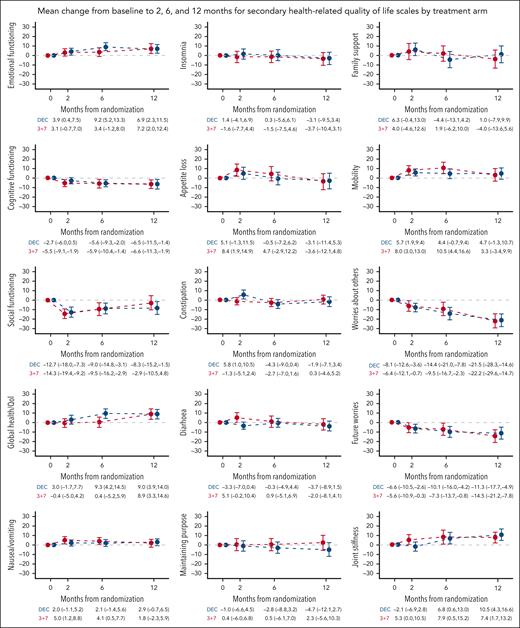
Investigation of average changes from baseline to subsequent assessments showed a lower burden of illness in the DEC arm than in the 3+7 arm (difference [Δ] = −11.0 [95% CI, −17.9 to −4.0]; P = .004) at 2 months. No statistically significant differences were observed between arms in other primary HRQoL scales (supplemental Table 7). Mean changes from baseline at 2, 6, and 12 months for the 5 primary scales are depicted in Figure 3. In both arms, there appeared to be a HRQoL deterioration at 2 and 6 months and a resumption to baseline levels at 12 months. At 6 months, deterioration in the 3+7 arm was clinically meaningful for physical and role functioning and burden of illness. Deterioration in the DEC arm was not clinically meaningful at any time point. Mean changes from baseline of all other HRQoL scales are reported in Figure 4.
Mean change from baseline to 2, 6, and 12 months for the primary HRQoL scales by treatment arm. Mean change from baseline and 95% CIs are displayed. Higher scores in fatigue, pain, and burden of illness scales indicate higher severity, while higher scores in physical and role functioning indicate better functioning. DEC, decitabine.
Mean change from baseline to 2, 6, and 12 months for the primary HRQoL scales by treatment arm. Mean change from baseline and 95% CIs are displayed. Higher scores in fatigue, pain, and burden of illness scales indicate higher severity, while higher scores in physical and role functioning indicate better functioning. DEC, decitabine.
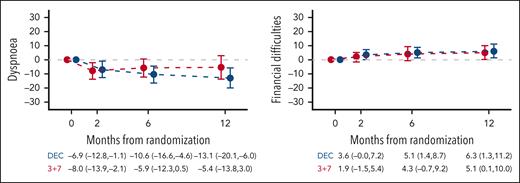
Mean change from baseline to 2, 6, and 12 months for secondary HRQoL scales by treatment arm. Mean change from baseline and 95% CIs are displayed. Higher scores in emotional, cognitive and social functioning, global health/quality of life, maintaining purpose, and family support indicate better functioning/quality of life. Higher scores in fatigue, nausea/vomiting, dyspnea, insomnia, appetite loss, constipation, diarrhea, financial difficulties, mobility, worries about others, future worries, and joint stiffness indicate higher severity of symptoms/problems. DEC, decitabine.
Mean change from baseline to 2, 6, and 12 months for secondary HRQoL scales by treatment arm. Mean change from baseline and 95% CIs are displayed. Higher scores in emotional, cognitive and social functioning, global health/quality of life, maintaining purpose, and family support indicate better functioning/quality of life. Higher scores in fatigue, nausea/vomiting, dyspnea, insomnia, appetite loss, constipation, diarrhea, financial difficulties, mobility, worries about others, future worries, and joint stiffness indicate higher severity of symptoms/problems. DEC, decitabine.
Descriptive HRQoL analyses among patients who received allo-HSCT
Overall, 240 patients received an allo-HSCT as a part of the study protocol, 122 in the DEC and 118 in the 3+7 arm. Of 240 patients, 218 (91%) had a baseline HRQoL assessment, and compliance before and after allo-HSCT was 68% (163/240) and 65% (138/211), respectively. There were no significant differences in HRQoL compliance rates between the 2 arms at any time point (supplemental Table 8). Sociodemographic and clinical characteristics of patients who received transplantation, before the procedure (supplemental Table 9), and donor and conditioning regimen characteristics were well balanced between the treatment arms (supplemental Table 10). Graft-versus-host disease was reported for 80 (70%) patients from the DEC and 65 (63%) patients from the 3+7 arm among those with available baseline HRQoL assessment.
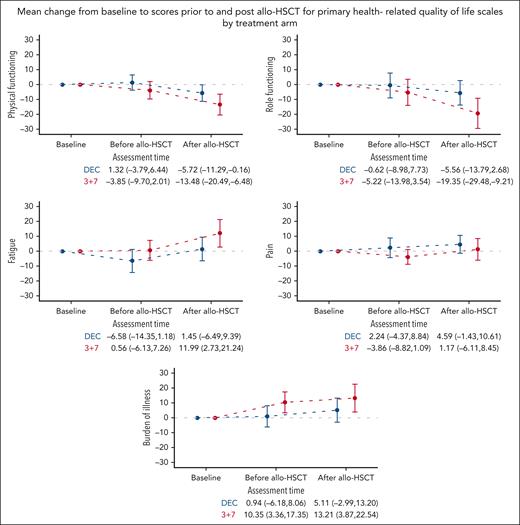
Baseline scores of the HRQoL primary scales were also well balanced between the treatment arms (Table 2). Patients from the 3+7 arm reported a clinically meaningful deterioration after allo-HSCT, relative to baseline, regarding physical functioning (Δ = −13.5 [95% CI, −20.5 to −6.5]), role functioning (Δ = −19.4 [95% CI, −29.5 to −9.2]), fatigue (Δ = 12.0 [95% CI, 2.7-21.2]), and burden of illness (Δ = 13.2 [95% CI, 3.9-22.5]). However, among patients from the DEC arm, no clinically meaningful deteriorations after allo-HSCT were observed for any of the 5 primary HRQoL scales (Figure 5).
Mean change from baseline to scores before and after allo-HSCT for primary HRQoL scales by treatment arm. Mean change from baseline and 95% CIs are displayed. Higher scores in fatigue, pain, and burden of illness scales indicate higher severity, while higher scores in physical and role functioning indicate better functioning. Allo-HSCT, allogeneic hematopoietic stem cell transplantation; DEC, decitabine.
Mean change from baseline to scores before and after allo-HSCT for primary HRQoL scales by treatment arm. Mean change from baseline and 95% CIs are displayed. Higher scores in fatigue, pain, and burden of illness scales indicate higher severity, while higher scores in physical and role functioning indicate better functioning. Allo-HSCT, allogeneic hematopoietic stem cell transplantation; DEC, decitabine.
Missingness mechanism and supportive analyses
Inspection of reasons for missing HRQoL data indicated that this information was mainly not documented (596/1129 [53%]) or was because of administrative failure (456/1129 [40%]). Investigation of missingness mechanism indicated an association with poorer performance and remission status. However, it was independent of age, sex, and European LeukemiaNet risk stratification. In the subgroup of patients who received allo-HSCT, more frequent missing data were associated with a poorer performance status. Sensitivity analyses, using multiple imputations, in the overall group and in the subgroup of patients who received allo-HSCT yielded similar results to the main analyses, confirming robustness of the findings (data not shown).
Adjusting for European LeukemiaNet risk group, the effect of treatment arm on the risk of HRQoL deterioration at 2 months was similar to that in the unadjusted analysis (OR, 0.45 [95% CI, 0.25-0.83]; P = .011).
Discussion
In this large international RCT we observed, at 2 months, a significantly lower risk of HRQoL deterioration in patients from the DEC arm but no difference in the long term. This short-term benefit finding has major implications, because it suggests that the induction with DEC may be preferrable to standard IC in fit older patients with AML. Together with the clinical efficacy and safety findings of this RCT,11 our HRQoL results provide novel information that will help both physicians and older patients with AML to make more informed decisions when considering treatment options. Of note, of the primary HRQoL scales of the study, the burden of illness from the QLQ-ELD14 (a dedicated questionnaire for elderly patients) was the key scale by indicating a statistically significant difference between arms in the short term. This may also suggest the importance of this specific scale in future AML trials with older patients.
Given the paucity of RCTs including older patients with AML in the setting of transplantation,21 it is difficult to compare our findings with those of other studies, and this is particularly true when considering HRQoL data of patients receiving DEC. With regard to HMAs, in general, we note that HRQoL was assessed in a previous RCT of newly diagnosed patients (aged ≥65 years) with AML comparing azacitidine vs conventional care regimens, and authors observed that HRQoL domains generally improved over the 9 treatment cycles in both arms.5 However, it is difficult to compare it with our findings given the few details provided about the HRQoL assessment methodology and the descriptive nature of the analysis. The differences between the treatment arms regarding HRQoL at a long-term follow-up were not statistically significant. At 12 months, there were negligible HRQoL differences relative to their baseline values in each arm. This finding may suggest that, once off treatment, patients treated with 3+7 recover well, which is partly in line with the sparse evidence in this area. A recent review22 summarizing HRQoL findings of studies comparing intensive vs lower-intensity therapies in older adults with AML, noted that HRQoL may initially worsen with IC but then improve to the levels observed with lower-intensity therapies.
Another finding, from our descriptive analysis was that patients treated with DEC did not experience a clinically meaningful deterioration in any of the 5 prespecified primary HRQoL scales after allo-HSCT, whereas this was the case for patients treated with 3+7. Relative to their baseline HRQoL, patients in this arm reported clinically relevant worse scores for physical and role functioning, as well as fatigue and burden of illness. A possible explanation is that this may reflect the higher cumulative burden of therapy experienced by patients treated with 3+7 before allo-HSCT because no large differences between the treatment arms were apparent regarding the characteristics of patients at the time of allografting, conditioning regimen use, donor characteristics, or graft-versus-host disease rates. The number of patients who are consolidated with an allo-HSCT in patients aged ≥60 years with hematologic malignancies has increased over the past decade.23 Therefore, the HRQoL trajectories after allo-HSCT by type of bridging therapy observed in our study provide novel insights on expected outcomes after transplantation.
Our study has limitations. Although the baseline HRQoL compliance was optimal, we observed a decline over time, which necessitated a change in analysis approach. Missing HRQoL data are a known challenge in international cancer trials,24 and this is particularly true when involving vulnerable patients with an acute disease as the participants included in this RCT. In order to deal with this limitation, the definition of the primary end point included disease progression, because patients with disease progression were expected to have a poor HRQoL and a poor compliance to HRQoL evaluations. Moreover, we performed several sensitivity analyses dealing with missing values, which showed results similar to the main analyses. Also, we cannot rule out the possibility that a specific HRQoL measure developed for patients with AML25 could have provided further insights in addition to the data we reported. However, our selection was driven by the international nature of the study (ie, requiring linguistically validated questionnaires for all participating centers) and the need to describe specific aspects relevant to elderly patients with cancer, which are comprehensively covered by the well-validated EORTC QLQ-ELD14 questionnaire.14
This study also has notable strengths. To the best of our knowledge, this is the first international RCT reporting comparative HRQoL data in fit older patients with AML treated with either 3+7 or HMAs. Second, the large sample including >600 patients from several countries, lends further credence to the generalizability of our findings. Third, considering the importance of moving toward a more patient-centered drug development process in oncology26 and the historical lack of HRQoL data from AML clinical trials,27-29 our findings bridge an important gap in this area of research. Indeed, owing to the number of novel drugs approved since 2017,2 HRQoL data should become even more critical to thoroughly inform clinical decisions. Recent guidelines by the American Society of Hematology have also pointed to the importance of patient-reported outcome research in the setting of newly diagnosed AML in older adults.30 Finally, our results were reported in accordance with the highest quality standards for reporting patient-reported outcomes from clinical trials.19
In summary, together with the efficacy results of this RCT,11 our HRQoL findings suggest that use of lower-intensity DEC may be preferable to the current standard of care in the frontline setting for fit older patients with AML.
Acknowledgments
The authors thank all team members of the European Organisation for Research and Treatment of Cancer (EORTC) Headquarters who have not been included among the coauthor list of this publication and who contributed to the study’s success (Sandrine Marreaud, Kin Jip Cheung, Liv Meert, Safaa Ramadan, Delphine Sartori, and Maarten Caspers) and Francesco Sparano from the Gruppo Italiano Malattie EMatologiche dell'Adulto Central Office. The authors also thank the patients and their families for participating in this study providing their invaluable contribution.
The authors are grateful to Janssen Pharmaceuticals for supporting this study. This work was also supported by grants from the Dutch Cancer Society (RUG 11072, to G.H.) and the José Carreras Leukemia Foundation (AH06-01, to A.N.).
Authorship
Contribution: F.E., M. Lübbert, G.H., M.K., C.C., and S.S. conceptualized and designed the study; and all authors contributed to writing the manuscript, approved the final manuscripts, and are accountable for all aspects of the work.
Conflict-of-interest disclosure: M. Lübbert received research support to his institution from Janssen and the European Organisation for Research and Treatment of Cancer (EORTC); is on the advisory boards for AbbVie, Astex, Janssen-Cilag, Otsuka, and Syros; and is currently working in an ongoing trial with a study drug provided by Cheplapharm. M.K. received research funding from Merck, Bristol Myers Squibb, Pierre Fabre, Janssen, and Immunocore. S.F. received personal funding from Bristol Myers Squibb and Celgene. A.G. received a grant for study conduct and drug supply for Dacogen from Janssen Pharma Educational. F.E. had a consultancy or advisory role for AbbVie, Incyte, Syros, Novartis, and Jazz Pharmaceuticals outside the submitted work. F.B. received payments to his institution from Incyte Biosciences, Takeda, ExCellThera, and MaaT Pharma. S.S. received funding to his institution from Janssen Pharmaceuticals. A.C. reports honoraria (consultancy, advisory role, or travel support) from AbbVie, Astellas, Janssen, Jazz, Celgene, Gilead, Pfizer, Incyte, and Amgen, outside the submitted work. M. Luppi received consulting fees from Jazz Pharma, Incyte, Grifols, AbbVie, Roche, and Novartis. R.F. served on the speakers’ bureau for Amgen, Novartis, Incyte, and AbbVie, outside the submitted work. A.V. received research funding from Jazz Pharmaceuticals; and reports consultancy for Servier, AstraZeneca, Pfizer, Kyte-Gilead, AbbVie, Johnson & Johnson, Astellas, Astex, Otzuka, Stemline Menarini, Bristol Myers Squibb, Glycostem, and Novartis. G.G. received consulting and speakers bureau fees from AbbVie, AstraZeneca, BeiGene, Hikma, Incyte, Janssen, and Lilly, outside the submitted work. The remaining authors declare no competing financial interests.
Correspondence: Fabio Efficace, Health Outcomes Research Unit Italian Group for Adult Hematologic Diseases, GIMEMA Data Center, Via Casilina 5, 00182 Rome, Italy; email: f.efficace@gimema.it.
References
Author notes
G.H. and M. Lübbert contributed equally to this study.
Presented, in part, as an oral presentation at the 64th annual meeting of the American Society of Hematology, New Orleans, LA, 10-13 December 2022.
EORTC supports developing greater knowledge to improve diagnostics, treatments, survival, and quality of life. Data requestors are invited to submit a research proposal according to the EORTC data sharing policy, by using the online form. Detailed information is available at: https://eortc.org.
The online version of this article contains a data supplement.
There is a Blood Commentary on this article in this issue.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal