Key Points
In transplant-eligible patients with MM, MRD by NGF and MS achieves similar prognostic value in single time point assessments and kinetics.
The minimally invasive nature of MRD monitoring by MS represents a breakthrough in highly sensitive serial response assessment in MM.
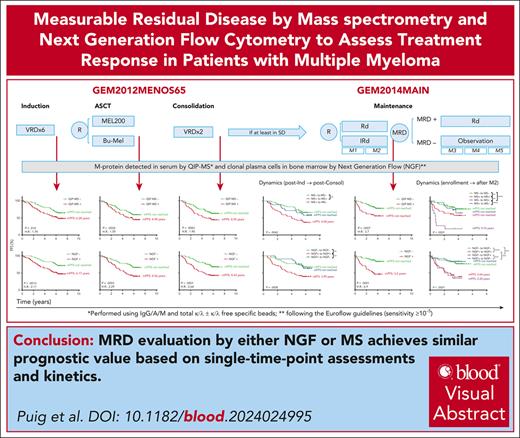
Visual Abstract
Quantitative immunoprecipitation mass spectrometry (QIP-MS) allows the identification of the M-protein in patients with multiple myeloma (MM) otherwise in complete response, and could be considered suitable for measurable residual disease (MRD) evaluation in peripheral blood. In the context of the GEM2012MENOS65 and GEM2014MAIN trials, we compared the performance of QIP-MS in serum with next-generation flow (NGF) cytometry in bone marrow to assess MRD in paired samples obtained postinduction, transplant, consolidation and after 24 cycles of maintenance. At each time point, both NGF and QIP-MS were able to segregate 2 groups of patients with significantly different progression-free survival; when the evolution of the results obtained with either method was considered, maintaining or converting to MRD negativity was associated with longer survival, significantly better when compared with sustaining or converting to MRD positivity. Reemergence of MRD by QIP-MS was associated with high risk of imminent clinical progression. In conclusion, MRD evaluation by NGF and MS achieves similar prognostic value based in single time point assessments and kinetics. Thus, the minimally invasive nature of MRD monitoring by MS represents a breakthrough in highly sensitive response assessment in MM. The trials were registered at www.clinicaltrials.gov as #NCT01916252 (GEM2012MENOS65) and at EudraCT as #2012-005683-10; and as #NCT02406144 (GEM2014MAIN) and at EudraCT as 2014-00055410.
Introduction
Both quantitative immunoprecipitation mass spectrometry (QIP-MS) from the EXENT system (The Binding Site, part of Thermo Fisher Scientific, Birmingham, UK) and MASS-FIX, an alternative method developed by the Mayo Clinic, also based on the analysis of reduced immunoglobulin fragments and commercially available, have outperformed serum immunofixation electrophoresis (sIFE) in detecting the M-protein in patients with multiple myeloma (MM).1,2 Being able to detect persistent disease beyond complete response (CR), MS could be compared with next-generation flow (NGF) or next-generation sequencing (NGS) as a complementary method to evaluate the presence of measurable residual disease (MRD) in peripheral blood. The present study aims to compare QIP-MS with NGF to assess MRD in patients enrolled in the GEM2012MENOS65 (NCT01916252) and GEM2014MAIN (NCT02406144) clinical trials. Achieving and maintaining MRD negativity in bone marrow (BM) is the category of response so far associated with the best outcome in MM but the dynamics of MRD have also shown to modulate the risk of progression. Thus, we have also investigated the influence of MRD dynamics by QIP-MS and NGF in the patients’ outcome.3,4
Study design
Details regarding patients’ enrollment and treatment schemas can be found elsewhere.5,6 Briefly, newly diagnosed patients with MM included in GEM2012MENOS65 received bortezomib, lenalidomide, and dexamethasone as induction and consolidation and an autologous stem cell transplantation (ASCT); in the GEM2014MAIN, patients were randomized to receive lenalidomide and dexamethasone plus or minus ixazomib for 2 or 5 years, according to the MRD status after the first 2. GEM2012MENOS65 included MRD evaluation by NGF post-induction, after ASCT, and at the end of treatment (postconsolidation) and GEM2014 yearly for 5 years. Serum samples were obtained at the time points of MRD evaluation and were retrospectively analyzed by QIP-MS using the EXENT Immunoglobulin Isotype GAM assay, as previously described, but excluding the free light chains.1 MRD was evaluated following the guidelines of the International Myeloma Working Group (IMWG) and negativity was defined with a limit of detection of 2 × 10−6.7,8
Each study site's independent ethics committee reviewed and approved the protocols, amendments, and informed consent forms.
Results and discussion
First, we confirmed the higher sensitivity of EXENT to detect the M-protein compared with conventional methods at all the time points analyzed (Figure 1A) and that, among cases in CR and stringentCR (sCR), the status by QIP-MS was able to segregate 2 cohorts with significantly different progression-free survival (PFS) (supplemental Figure 1B, available on the Blood website). An alternative clonotypic method that requires a baseline sample with M-protein with ≥0.2 g/L did not discriminate patients with different outcomes among cases in stringent sCR, although only 40 cases were analyzed.9 Other clonotypic approaches target a specific peptide of the M-protein deduced from DNA or RNA sequencing of the patient plasma cell clone and therefore require a BM aspiration during active disease. These assays have shown potential of being more analytically sensitive than matrix-assisted laser desorption/ionization time-of-flight (MALDI-TOF)–based methods, but the procedure is laborious, technically complex, and time-consuming and its clinical value has yet to be stablished.10
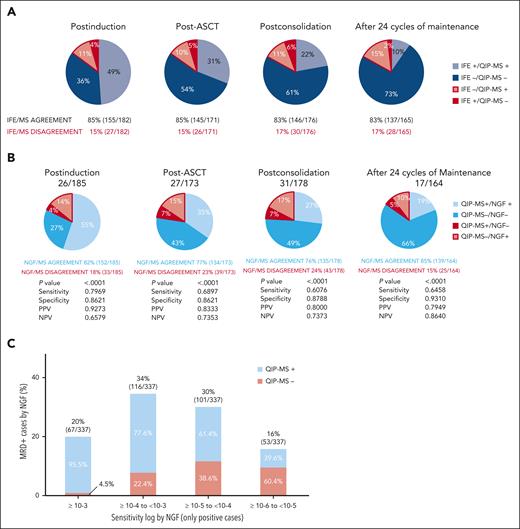
Analysis of the combined results of QIP-MS and NGF or SPEP/IFE (and free light chains). (A) Concordances and discordances between QIP-MS and NGF at each preestablished treatment time point. P value has been calculated by Fisher exact test. (B) Concordances and discordances between QIP-MS and SPEP/IFE and free light chains at each preestablished treatment time point. (C) Concordances and discordances between QIP-MS and NGF by log range of MRD independently of the treatment time point. The percentages inside the bars have been calculated considering each of them independently.
Analysis of the combined results of QIP-MS and NGF or SPEP/IFE (and free light chains). (A) Concordances and discordances between QIP-MS and NGF at each preestablished treatment time point. P value has been calculated by Fisher exact test. (B) Concordances and discordances between QIP-MS and SPEP/IFE and free light chains at each preestablished treatment time point. (C) Concordances and discordances between QIP-MS and NGF by log range of MRD independently of the treatment time point. The percentages inside the bars have been calculated considering each of them independently.
Focusing then on MRD, QIP-MS identified the M-spike in 59% of cases (109/185) postinduction, 42% (72/173) post-ASCT, 34% (60/178) postconsolidation, and 24% (39/164) at the end of treatment, whereas NGF was positive in 69% (128/185), 50% (87/173), 44% (79/178), and 29% (48/164) of cases, respectively. Thus, NGF detected MRD more frequently than QIP-MS, both during active treatment and maintenance.
Analysis of the combined results of both methods showed that they were concordant in 82% of cases postinduction, 77% post-ASCT, 76% postconsolidation, and 85% after 2 years of maintenance (Figure 1B). Evaluation of discordances showed that they were mostly attributable to increasingly higher cases NGF+/MS− (14% postinduction, 15% post-ASCT, 17% postconsolidation, and 10% at the end of the treatment). Trying to understand these NGF+/MS− results, we pooled together all NGF+ cases (n = 337) and analyzed the percentage of NGF+/QIP-MS− cases by log range of MRD, independently of the time point of extraction. Now, we found a clear increase in these NGF+/MS− cases at the lowest MRD ranges being 4.5% at ≥10−3, 22.4% between ≥10−4 and <10−3, 38.6% between ≥10−5 and <10−4, and 60.4% between ≥10−6 and <10−5 NGF+ cases (Figure 1C). The discordant rate at this latter level of sensitivity could represent a limitation for using QIP-MS as an alternative method for evaluating MRD; however, only 16% of the samples were NGF+ at this log level and 10−5 is the required limit of MRD detection as per the IMWG guidelines. In contrast, the number of NGF−/QIP-MS+ cases was low (n = 39), remained almost stable throughout (7 postinduction, 12 post-ASCT, 12 postconsolidation, and 8 after treatment completion), and were obtained from a total of 29 patients with MM (16 IgG, 10 IgA, and 3 light chain) from whom 8 (27.5%) have already relapsed. Besides the IgG clearance, these discordances can also be explained by patchy infiltration, extramedullary disease, and/or hemodiluted BM samples. A similar percentage of agreement (83%) between MS and NGS as alternative BM-based method of MRD evaluation was reported by Derman et al in 60 paired samples obtained after 18 cycles of carfilzomib, lenalidomide and dexamethasone (KRd); in contrast to our results, discordant cases were all attributable to NGS-/MS+ cases with the only NGS+/MALDI-TOF MS− case obtained from an NGS assay with limit of detection <10−6.11 When considering NGF as a reference, the estimated negative predictive value (NPV) of MS was found to be 65% postinduction, 73% post-ASCT, 73% postconsolidation, and 86% after 2 years of maintenance (Figure 1B). Using NGF as the gold standard and a comparable MS-based method of disease evaluation (MASS-FIX), the NPV reported by Dispenzieri et al in the STAMINA trial among patients in CR and sCR postinduction, premaintenance, and after 1 year of maintenance was 83%, 90%, and 95%, respectively.2
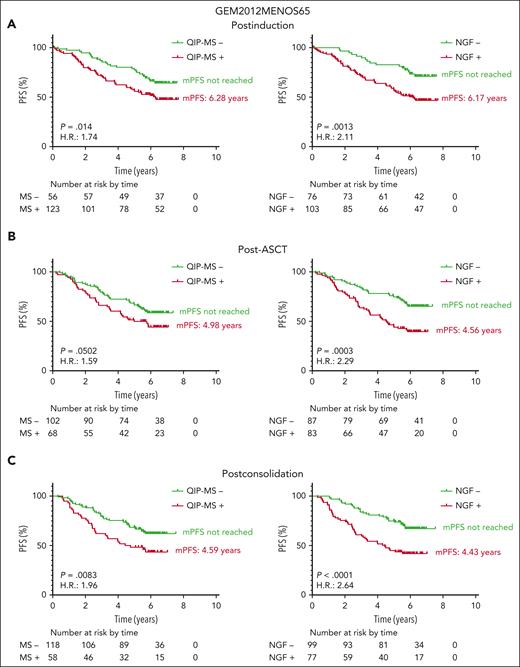
We then analyzed the clinical impact in terms of PFS of MRD by NGF and QIP-MS in paired samples from 179, 170, 176, and 164 patients at postinduction, post-ASCT, postconsolidation, and after 24 cycles of maintenance, respectively. As shown in Figure 2, both methods were equally able to identify 2 groups with significantly different PFS, although P values and hazard ratio clearly favored NGF in all cases. The median PFS (mPFS) when testing positive for NGF or QIP-MS was similar in each time point, except after 24 cycles of maintenance, where QIP-MS–positive patients have not reached yet the mPFS (despite a just slightly different progression rate of 44% [17/39] by MS vs 52% [25/48] by NGF). Importantly, the mPFS for both NGF- and MS-negative patients has not been reached at any of the time points analyzed. Varios studies performed as ours in transplant-eligible patients with MM and involving >1000 samples have also shown the clinical impact of the analysis of residual disease by MS at different moments throughout the treatment. 2,11,12 Although in a limited number of samples, Derman et al also found that liquid chromatography-MS status after 18 cycles of KRd appeared to be a superior predictor of PFS compared with NGS or MALDI-TOF-MS status.11
Progression free survival (PFS) according to the results of QIP-MS and NGF at the 4 time points analyzed. Landmark analyses of PFS of patients included in GEM12MENOS65 and GEM2014MAIN based on MRD status by QIP-MS (left panels) or by NGF (right panels) at postinduction (A), post-ASCT (B), postconsolidation (C), and after 2 years of maintenance (D).
Progression free survival (PFS) according to the results of QIP-MS and NGF at the 4 time points analyzed. Landmark analyses of PFS of patients included in GEM12MENOS65 and GEM2014MAIN based on MRD status by QIP-MS (left panels) or by NGF (right panels) at postinduction (A), post-ASCT (B), postconsolidation (C), and after 2 years of maintenance (D).
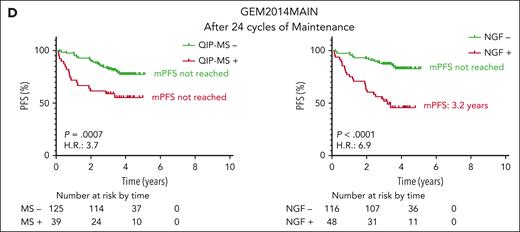
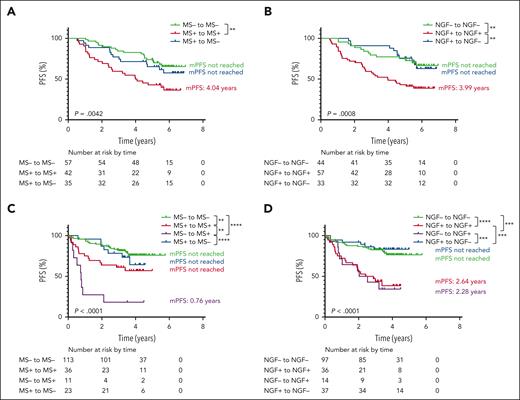
During intensive treatment within the GEM2012MENOS65 trial, QIP-MS and NGF dynamics were analyzed in 134 patients with available samples after induction, ASCT, and consolidation. Sustained MS or NGF positivity at the 3 time points was associated with a significantly shorter PFS (mPFS, 4.04 years, P = .0042 and 3.9 years, P = .0008, respectively), compared with patients remaining negative or converting from positive to negative along the treatment (Figure 3A-B). MS and NGF dynamics were also analyzed in the 183 patients enrolled in GEM2014MAIN clinical trial with at least 2 samples available, including enrollment and after 1 and 2 years of maintenance. Interestingly, converting from negative to positive by QIP-MS (n = 11) was associated with a poor outcome (mPFS, 0.76 years), whereas conversion to positive by NGF (n = 14) did not significantly differ from sustained positivity (Figure 3C-D). Of the 14 patients converting from NGF negative to positive, 7 had NGF levels between 10−5 and 10−6. This finding suggests that although a conversion at MS level probably reflects a relatively high tumor regrowth, the same does not apply for NGF conversion from negative to positive at a level of 10−5 or 10−6 and probably impending relapses will not occur until MRD by NGF at 10−4 or 10−3 emerges. Similarly, analyzing the time points before and after 1 year of maintenance or observation, Mai et al found the best outcome in the 28 patients converting from MS positive to negative and the worst in those 6 converting from MS negative to positive with a mPFS of only 0.6 years.12
Progression free survival (PFS) according to the dynamics of the results of QIP-MS and NGF at the 4 time points analyzed. Landmark analyses of PFS from postconsolidation to biochemical/clinical progression or death of patients included in GEM12MENOS65 trial based on QIP-MS (A) or NGF (B) dynamics from postinduction to postconsolidation. Landmark analyses of PFS from 24 cycles of maintenance to biochemical/clinical progression or death of patients included in GEM14MAIN trial based on QIP-MS (C) or NGF (D) dynamics from enrollment to 24 cycles of maintenance.
Progression free survival (PFS) according to the dynamics of the results of QIP-MS and NGF at the 4 time points analyzed. Landmark analyses of PFS from postconsolidation to biochemical/clinical progression or death of patients included in GEM12MENOS65 trial based on QIP-MS (A) or NGF (B) dynamics from postinduction to postconsolidation. Landmark analyses of PFS from 24 cycles of maintenance to biochemical/clinical progression or death of patients included in GEM14MAIN trial based on QIP-MS (C) or NGF (D) dynamics from enrollment to 24 cycles of maintenance.
In this cohort of newly diagnosed transplant-eligible patients with MM, MRD assessment, by either QIP-MS or NGF, at the 4 prespecified time points is associated with a similar and significant clinical value. Analysis of dynamics shows that, whereas during intensive treatment the condition to aim should be to achieve sustained MRD negativity, during maintenance, the most definitive factor seems to be the conversion from negative to positive by QIP-MS, since meaning an imminent clinical progression. These data and others support the use of QIP-MS–based monitoring during maintenance or observation to anticipate progressive disease. The MS approach here investigated represents a noninvasive strategy that would facilitate frequent evaluations of the kinetics of response complementary to the periodic BM evaluations, particulary in the context of MRD treatment adapted clinical trials, such as the GEM12-14.
Acknowledgments
The authors thank all the investigators and centers participating in the GEM (Grupo Español de Mieloma)/PETHEMA (Programa para el studio de la Terapeutica en Hemopatías Malignas) cooperative study group (list of investigators in the Supplemental Appendix). The authors acknowledge Alfonso de Santiago and Carmen Carrero for the supportive administration of PETHEMA and Roberto Maldonado and Arturo Touchard for data management. The authors thank the patients and their families, and the physicians, nurses, study coordinators, and research staff for participation in the trial.
This study was supported by the Leukemia and Lymphoma Society (grant 6660-23)
Authorship
Contribution: N.P., C.A., M.-T.C., and M.-V.M. conceived the analysis; N.P., M.-T.C., M.-V.M., L.R., J.B., J.F.S.-M., and J.J.L. designed the analysis protocol; N.P., C.A., and M.-T.C. analyzed the mass spectrometry data and the M-protein kinetics; N.P., C.A., M.-T.C., and M.-V.M. analyzed and interpreted data; N.P., C.A., and M.-T.C. performed statistical analysis; N.P., M.-T.C., C.A., and M.-V.M. wrote the manuscript; and all authors provided study material or patients and reviewed and approved the manuscript.
Conflict-of-interest disclosure: N.P. has received honoraria from Amgen, Celgene, Janssen, Takeda, and The Binding Site; has served in a consulting or advisory role for Amgen, Celgene, Janssen, and Takeda; has served on a speakers' bureau for Celgene; has received research funding from Celgene, Janssen, Amgen, and Takeda; and has received travel, accommodations, and expenses from Amgen, Celgene, Janssen, and Takeda. B.P. has received consultancy, honoraria, research funding, and speaker's bureau for Amgen, Bristol Myers Squibb, Celgene, Janssen, Novartis, Roche, and Sanofi; has received unrestricted grants from Celgene, EngMab, and Takeda; and has served in a consultancy for Celgene, Janssen, and Sanofi. M.-T.C. has received honoraria from Janssen, Celgene, and AbbVie. J.M.-L. has served in a consultancy and speaker's bureau and received honoraria and research funding for Amgen, Astellas, Bristol Myers Squibb, Janssen, Novartis, Roche, and Sanofi; and has received unrestricted grants from Bristol Myers Squibb. A.O. has served in a consultancy and speaker’s bureau for Celgene and Amgen and consultancy for Janssen, Sanofi, and GlaxoSmithKline (GSK). R.R. has received honoraria or served as an advisory role for Becton-Dickinson, Sanofi, and The Binding Site. J.d.l.R. has served as a consultant and provided expert testimony within the past 2 years for Amgen, Celgene, GSK, Takeda, Janssen, and Sanofi. V.G.-C. has received honoraria from Janssen and Celgene; has received research funding from Janssen (BECA SEHH-JANSSEN ESTANCIAS DE FORMACIÓN EN EL EXTRANJERO 2016-2017); and has served in a consulting or advisory role for Prothena and Janssen. J.-M.M. has served in a consultancy or advisory role for Novartis, Gilead, Roche, Sanofi, Jazz, and Takeda. L.R. has received honoraria from Janssen, Celgene, Amgen, and Takeda. J. Bladé has received honoraria for Janssen, Celgene, Takeda, Amgen, and Oncopeptides. J.F.S.-M. has served in a consultancy or advisory role for AbbVie, Amgen, Bristol Myers Squibb, Celgene, GSK, Janssen, Karyopharm, MSD, Novartis, Roche, Sanofi, SecuraBio, and Takeda. J.-J.L. has served in a consulting or advisory role for Celgene, Takeda, Amgen, Janssen, and Sanofi; and has received travel accommodations and expenses for Celgene. M.-V.M. has received honoraria and membership on an entity's Board of Directors or advisory committees for Janssen, Celgene, Takeda, Amgen, Adaptive, GSK, Sanofi, and Oncopeptides; and has received honoraria from membership in Board of Directors or advisory committees for AbbVie, Roche, Pfizer, Regeneron, and Seattle Genetics. The remaining authors declared no competing financial interests.
Correspondence: María-Victoria Mateos, Hospital Universitario de Salamanca, Paseo de la Transición Española, s/n, CP 37007, Salamanca, Spain; email: mvmateos@usal.es.
References
Author notes
N.P. and C.A. contributed equally to this work as first authors.
B.P. and M.-V.M. contributed equally to this work as last authors.
The online version of this article contains a data supplement.
There is a Blood Commentary on this article in this issue.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal