Key Points
RAS/RAF mutations are present in up to 61% of patients with multiple myeloma.
Most of them are subclonal, in agreement with a secondary event.
Visual Abstract
Multiple myeloma is characterized by a huge heterogeneity at the molecular level. The RAS/RAF pathway is the most frequently mutated, in ∼50% of the patients. However, these mutations are frequently subclonal, suggesting a secondary event. Because these genes are part of our routine next-generation sequencing panel, we analyzed >10 000 patients with different plasma cell disorders to describe the RAS/RAF landscape. In this large cohort of patients, almost 61% of the patients presented a RAS/RAF mutation at diagnosis or relapse, but much lower frequencies occurred in presymptomatic cases. Of note, the mutations were different from that observed in solid tumors (higher proportions of Q61 mutations). In 29 patients with 2 different mutations, we were able to perform single-cell sequencing, showing that in most cases, mutations occurred in different subclones, suggesting an ongoing mutational process. These findings suggest that the RAS/RAF pathway is not an attractive target, both on therapeutic and residual disease assessment points of view.
Introduction
Multiple myeloma (MM) is a heterogeneous hematological malignancy at all levels (ie, clinical, prognostic, cytogenetic, and molecular).1 In contrast to non-Hodgkin lymphomas, it has not been possible (yet) to identify subentities that could require specific therapeutic approaches. If chromosomal changes identify clear high-risk factors, whole-exome or whole-genome sequencing has been so far disappointing in identifying molecular subgroups with a distinct evolution, except the classic TP53 mutations.2,3 Several thousands of MM tumors have been sequenced, enabling a clear picture of the mutational landscape.4-7 By frequency order, the most frequent mutations observed in MM are those involving KRAS (∼23%), NRAS (∼20%), DIS3 (∼11%), FAM46C (∼11%), TP53 (∼8%), BRAF (∼6%), and TRAF3 (∼5%). Because the RAS/RAF mutations are observed in approximately half of the patients, we have decided to explore them in a large set of patients (>10 000) analyzed in a routine clinical setting, using a targeted sequencing panel.8 We have also analyzed the BRAF V600E mutations known to activate the mitogen-activated protein kinase (MAPK) pathway.
These mutations are not specific to MM and are observed in a large spectrum of solid tumors, including pancreas, colon, lung, and melanoma.9 In those tumors, KRAS is much more frequently mutated than NRAS. RAS mutations are not random and frequently involve codons 12, 13, and 61. These mutations are known to constitutively activate the MAPK pathway, making the tumors intrinsically resistant to epidermal growth factor receptor inhibitors.
Study design
Bone marrow aspirates were obtained in 10 189 patients with plasma cell neoplasms: 1168 individuals with monoclonal gammopathy of undetermined significance, 922 individuals with smoldering MM (SMM), 489 patients with AL amyloidosis, 5934 patients at MM diagnosis, 247 patients with a primary plasma cell leukemia (PCL), and 1429 patients at relapse (supplementary Table, available on the Blood website). All patients signed an informed consent form, allowing research on their samples, and the study has been accepted by the Toulouse Ethics Committee. Myeloma cells were sorted using the Miltenyi technology with anti–CD138-coated beads. Only samples with a plasma cell purity >70% were kept for analysis. After DNA extraction, samples were sequenced with our published panel on an Illumina NextSeq 500 sequencing machine with a median 200× depth. This panel covers the entire KRAS, NRAS, and BRAF sequences. When extra plasma cells were present, they were frozen down in Cryostor for potential further single-cell analysis. Arbitrarily, the variant allelic fraction (VAF) defined clonality if ≥0.40. For patients with a plasma cell purity of <100%, clonality was adjusted by dividing the observed VAF by the purity percentage. Subclonality was defined for VAF between 0.10 and 0.39. In some cases with several different subclonal RAS mutations, and available frozen extra plasma cells, we performed single-cell sequencing experiments using the Mission Bio, Tapestri technology (South San Francisco, CA). On receipt, plasma cells were sorted from bone marrow with an automated magnetic sorting, targeting CD138+ cells (AutoMAcs and anti-CD138 beads from Miltenyi Biotec). For single-cell analyses, cells were frozen in fetal calf serum/dimethyl sulfoxide 10% solution and stored at −80°C. Cells were thawed with an optimized solution (NaCl 0.9%, citrate-dextrose solution, and human albumin; Sigma) and diluted to ∼1000 cells/μL in Cell Buffer (Mission Bio). A median of 150 000 cells per sample (range, 46 000-189 000) were encapsulated using the Tapestri instrument (Mission Bio), according to the Tapestri Single-Cell DNA Sequencing V2 User Guide (Mission Bio). Briefly, single cells were individually partitioned into subnanoliter droplets. Barcoding beads and polymerase chain reaction reagents were introduced using the Mission Bio Tapestri Instrument and DNA Cartridge. Cell lysis, protease digestion, cell barcoding, and targeted amplification using multiplexed polymerase chain reaction occurred within the droplets. Droplets were then disrupted, and barcoded DNA was extracted for library amplification. Final libraries were purified and sequenced on an Illumina Novaseq 6000 sequencer to reach ∼80×/reads/cell.
This study has been approved by the Ethics Committee of Institut Universitaire du Cancer Toulouse.
Results and discussion
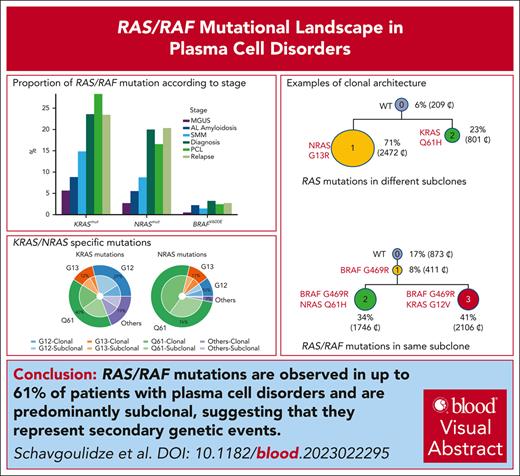
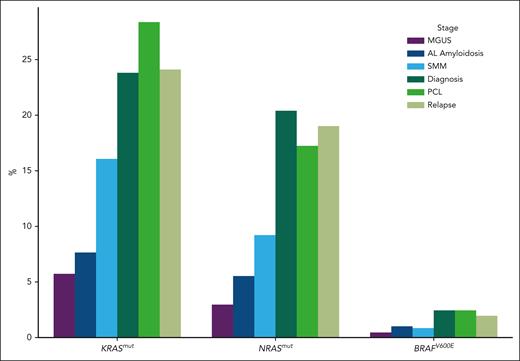
Among the 10 189 patients analyzed, we found a KRAS mutation in 18.7%, and an NRAS mutation in 14.8%. As expected, codons 12, 13, and 61 were the most frequently mutated (Table 1). However, in contrast to solid tumors, codon 61 was the most frequently mutated for KRAS and NRAS (most predominantly). Also different from solid tumors, a high incidence of subclonality was observed. Only 41% of the KRAS mutations, and 57% of the NRAS ones, were clonal. BRAF mutations were observed in 617 cases (6% of the cohort). However, 41.5% were V600E, activating the gene function. Among V600E, only 29% were clonal. We also found other BRAF mutations, supposed to activate or inactivate the kinase domain, such as D594 (inactivating), G466 (inactivating), G469 (activating), or K601 (activating), representing 16.7%, 6.1%, 9%, and 3.4%, respectively, of the BRAF-mutated cases. Comparison between the disease stages showed that RAS mutations were significantly less frequent in presymptomatic individuals (Figure 1), but stable between diagnoses and relapses. We also investigated the association between RAS/RAF mutations and major cytogenetic abnormalities in patients. We observed significantly less frequent KRAS/NRAS mutated cases (15.3% and 5.9%, respectively; P < .009 and P < .0001) in t(4;14) MM, whereas this subgroup had more frequent BRAF V600E cases compared with the general population (6.6% vs 3.2%, respectively; P = .02) (Table 2). In contrast, we did not find any difference in the incidence of t(11;14) for all KRAS/NRAS/BRAF V600E mutated cases. Finally, we did observe significant change for del(17p) in the RAS-mutated population (NRAS mutations in 8.6% vs 20% [P < .001] and KRAS mutations in 12.6% vs 23.6% [P = .0002]). We also looked at the co-occurrence of RAS/RAF mutations with other frequent mutations (namely, TP53, DIS3, and FAM46C). Significant associations were observed only for NRAS, and less frequently associated with TP53 or FAM46C mutations (P < .0001 and P = .0008, respectively). Because KRAS mutations have been described to predict SMM evolution, we did explore these data in patients with SMM and at least 2 years of follow-up. We did observe a trend (P = .08) to early progression in case of KRAS mutations.
KRAS/NRAS-specific mutations
| Codon . | KRAS . | NRAS . | Clonal KRAS/NRAS . | Subclonal KRAS/NRAS . | KRAS low frequency . | NRAS low frequency . |
|---|---|---|---|---|---|---|
| G12 | 29 | 10 | 31/52 | 69/48 | 28 | 9 |
| G13 | 12 | 11 | 34/51 | 66/49 | 14 | 15 |
| Q61 | 40 | 76 | 47/58 | 53/42 | 38 | 72 |
| K117 | 4 | 43 | 57 | 5 | ||
| A146 | 3 | 67 | 33 | 3 | ||
| A59 | 1 | 52 | 48 | 0 | ||
| Q22 | 2 | 40 | 60 | 3 | ||
| Y64 | 2 | 3 | 53 | 47 | 3 | 4 |
| Others | 5 | 6 |
| Codon . | KRAS . | NRAS . | Clonal KRAS/NRAS . | Subclonal KRAS/NRAS . | KRAS low frequency . | NRAS low frequency . |
|---|---|---|---|---|---|---|
| G12 | 29 | 10 | 31/52 | 69/48 | 28 | 9 |
| G13 | 12 | 11 | 34/51 | 66/49 | 14 | 15 |
| Q61 | 40 | 76 | 47/58 | 53/42 | 38 | 72 |
| K117 | 4 | 43 | 57 | 5 | ||
| A146 | 3 | 67 | 33 | 3 | ||
| A59 | 1 | 52 | 48 | 0 | ||
| Q22 | 2 | 40 | 60 | 3 | ||
| Y64 | 2 | 3 | 53 | 47 | 3 | 4 |
| Others | 5 | 6 |
Data are given as percentages.
Proportion of RAS/RAF mutations according to stage. MGUS, monoclonal gammopathy of undetermined significance; SMM, smoldering multiple myeloma.
Proportion of RAS/RAF mutations according to stage. MGUS, monoclonal gammopathy of undetermined significance; SMM, smoldering multiple myeloma.
KRAS/NRAS/BRAF V600E incidences in the different subpopulations
| Variable . | t(4;14) (n = 575) . | del(17p) (n = 478) . | t(11;14) (n = 1260) . | Whole diagnostic cohort (n = 5934) . | Mut TP53 (n = 344) . | Mut DIS3 (n = 617) . | Mut FAM46C (n = 280) . | AL amyloidosis (n = 489) . | PCL (n = 247) . | Relapses (n = 1429) . | SMM (n = 922) . | MGUS (n = 1168) . |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| NRAS | 34 (5.9) | 41 (8.6) | 292 (23.2) | 1189 (20) | 26 (7.6) | 137 (22.2) | 21 (7.5) | 27 (5) | 41 (16) | 290 (20) | 80 (8.7) | 32 (2.7) |
| KRAS | 88 (15.3) | 60 (12.6) | 339 (26.9) | 1401 (23.6) | 65 (18.9) | 131 (21.2) | 62 (22) | 43 (9) | 70 (28) | 334 (23) | 136 (14.8) | 65 (5.6) |
| BRAF V600E | 38 (6.6) | 3 (0.6) | 40 (3.2) | 190 (3.2) | 4 (1.2) | 17 (2.8) | 7 (2.5) | 11 (2) | 6 (2.4) | 38 (2.7) | 13 (1.4) | 6 (0.5) |
| Variable . | t(4;14) (n = 575) . | del(17p) (n = 478) . | t(11;14) (n = 1260) . | Whole diagnostic cohort (n = 5934) . | Mut TP53 (n = 344) . | Mut DIS3 (n = 617) . | Mut FAM46C (n = 280) . | AL amyloidosis (n = 489) . | PCL (n = 247) . | Relapses (n = 1429) . | SMM (n = 922) . | MGUS (n = 1168) . |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| NRAS | 34 (5.9) | 41 (8.6) | 292 (23.2) | 1189 (20) | 26 (7.6) | 137 (22.2) | 21 (7.5) | 27 (5) | 41 (16) | 290 (20) | 80 (8.7) | 32 (2.7) |
| KRAS | 88 (15.3) | 60 (12.6) | 339 (26.9) | 1401 (23.6) | 65 (18.9) | 131 (21.2) | 62 (22) | 43 (9) | 70 (28) | 334 (23) | 136 (14.8) | 65 (5.6) |
| BRAF V600E | 38 (6.6) | 3 (0.6) | 40 (3.2) | 190 (3.2) | 4 (1.2) | 17 (2.8) | 7 (2.5) | 11 (2) | 6 (2.4) | 38 (2.7) | 13 (1.4) | 6 (0.5) |
Data are given as number (percentage).
MGUS, monoclonal gammopathy of undetermined significance; Mut, mutated; SMM, smoldering multiple myeloma.
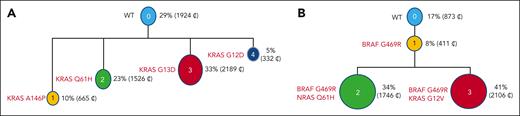
Two different RAS mutations were observed in 2.6% of the patients, and both mutations were always subclonal (KRAS/KRAS in 114 cases, NRAS/NRAS in 55 cases, and KRAS/NRAS in 94 cases). We also observed BRAF V600E comutated with KRAS or NRAS in 12 patients each. We were able to perform single-cell DNA sequencing in 29 patients with co-occurring KRAS/NRAS or BRAF V600E mutations. Single-cell DNA sequencing showed that in 22 of 29 patients, mutations occurred in different cells, indicating coevolving 2 subclones (supplemental Figure). However, in 7 cases (patients 1 to 7), we found a minor subclone harboring the 2 mutations, suggesting an ongoing mutational process (Figure 2). This finding was enhanced by the observation of tiny subclones harboring mutations not detected in the bulk analysis in 15 patients analyzed at the single-cell level. These data encouraged us to look at the bulk data with a lower VAF. With a 200× sequencing depth, we did estimate that these specific VAFs were significant until 0.03. With this cutoff, we did detect 2527 patients with further KRAS, NRAS, or BRAF V600E mutations (1 to 5 per patient). These minor subclones were significantly more frequent in patients with a mutation already detected at the bulk level (56.4%), but the repartition of the different mutation subtypes was exactly the same as with a VAF >0.1. With the addition of these cases, we found a final incidence of KRAS/NRAS/BRAF V600E mutations of 60.7%.
Single-cell sequencing showing 2 different subclones with different RAS mutations (A), and different mutations in the same subclone (B). WT, wild type.
Single-cell sequencing showing 2 different subclones with different RAS mutations (A), and different mutations in the same subclone (B). WT, wild type.
We also investigated these mutations in the AL amyloidosis and PCL subgroups. In patients with amyloidosis, all KRAS, NRAS, and BRAF mutations were significantly lower, but again, this lower incidence may reflect the subclonal pattern, with many cases keeping polyclonal plasma cells. For primary PCL, we did not observe any particularity compared with MM diagnostic cases.
Activation of the MAPK pathway by specific gain-of-function mutations targeting the KRAS/NRAS/BRAF genes is a frequent event in cancer in general. This activation has been shown to control growth, proliferation, differentiation, migration, and apoptosis. In solid tumors, such as colorectal, lung, or pancreatic, these mutations are more often observed in KRAS than in NRAS or BRAF. For KRAS, codons 12 and 13 are by far more frequently involved than codon 61. In MM, KRAS and NRAS are approximately equally mutated (20%-25%),10 whereas the BRAF V600E mutation is rarely observed (2%-3%). We observed differences in the incidence of these mutations between the different stages of the disease. For instance, KRAS and NRAS mutations were observed in ∼25% and ∼20% in symptomatic stages, respectively (MM diagnosis, relapses, and primary PCL) vs 3% to 8% in presymptomatic stages (monoclonal gammopathy of undetermined significance and AL amyloidosis), and 9% to 16% in SMM. These incidence differences can reflect an ongoing process, but can also be related to differences in plasma cell clonality (fully clonal in symptomatic stages, vs subclonal in others). The second hypothesis is highly probable because we did not observe any increase between diagnoses and relapses. The fact that the MAPK pathway increases from presymptomatic stages to MM supports that this pathway is necessary for the development of the disease; however, it does not increase at relapse because it does not make the disease particularly aggressive, explaining the absence of prognostic value for these mutations in MM.11
Another difference with solid tumors is the more frequently mutated codon 61 rather than codons 12 and 13. This preferential mutation in codon 61 has been already described in other lymphoid malignancies, suggesting a tumor effect. The most important difference with solid tumors is the frequent mutation subclonality observed in MM. This subclonality cannot be explained by the frequent hyperdiploidy observed in MM, with chromosomes 1p and 12p being almost never gained. In a small but significant number of patients, we observed 2 different RAS mutations with a subclonal distribution. This observation was confirmed in the single-cell sequencing experiments. The 2 mutations were observed in different cells in general, but few cells with 2 mutations were present in some patients. To our knowledge, this process has not been published before, probably because single-cell analyses were not performed. Mechanistically, it is rather difficult to understand what advantage a cell will gain with 2 activating mutations in the same pathway. However, this finding represents a minority of cases, and might be just stochastic. Furthermore, more mutations not detected in the bulk analyses were found in 15 patients. This finding pushed us to go back to the bulk sequencing data with a lower threshold than the arbitrarily 0.1 chosen. We considered that a 3% VAF level was accurate for analysis. With this threshold, a much higher number of patients were detected, up to 60%. Of note, several KRAS/NRAS/BRAF V600E mutations were found in a significant number of patients (up to 5). This observation favors the hypothesis of an ongoing mutational process. Because we did not observe a higher incidence of mutations between diagnoses and relapses, this ongoing process is probably restricted to some patients more dependent to KRAS/NRAS/BRAF mutations.
Finally, because of the high frequency of RAS mutations in MM, could we expect to therapeutically target them? In solid tumors, RAS inhibitors were rather disappointing, except for KRAS G12C and sotorasib.12 However, this specific mutation represents only 2.5% of all the KRAS mutations. Furthermore, approximately half of them are subclonal. BRAF V600E mutations are also targetable. However, they are mostly subclonal and thus not an ideal target, although a recent small series showed this approach feasible, but in a low number of patients.13 Thus, it is rather unlikely that targeting RAS in MM would (will) be an attractive option. Some investigators were also investigating the possibility to use these frequent RAS mutations to follow residual disease on circulating cell-free DNA.14 Although conceptually promising, this approach might be not effective in that case because approximately half of these mutations are subclonal.
Acknowledgments
This work was supported by grants from the Riney Foundation and from the ARC Foundation.
Authorship
Contribution: A.S., J.C., and H.A.-L. designed and analyzed the data and wrote the manuscript; A.S. helped in the data analysis; and all other authors provided samples and clinical data.
Conflict-of-interest disclosure: A.S. is the CEO of Mission Bio. The remaining authors declare no competing financial interests.
Correspondence: Herve Avet-Loiseau, Myeloma Genomics Lab, IUCT-Oncopole, 1 Avenue Irene Joliot-Curie, Toulouse, France; email: avetloiseau.herve@iuct-oncopole.fr.
References
Author notes
A.S. and J.C. are joint first authors.
The online version of this article contains a data supplement.
There is a Blood Commentary on this article in this issue.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal