Key Points
CRh correlated with TI and delayed time to severe bleeding or infection with nonmyelosuppressive targeted therapies.
CRh may denote palliative benefits for nonmyelosuppressive treatments of AML.
Visual Abstract
Complete remission with partial hematological recovery (CRh) has been used as an efficacy endpoint in clinical trials of nonmyelosuppressive drugs for acute myeloid leukemia (AML). We conducted a pooled analysis to characterize the clinical outcomes for patients with AML who achieved CRh after treatment with ivosidenib, olutasidenib, enasidenib, or gilteritinib monotherapy in clinical trials used to support marketing applications. The study cohort included 841 adult patients treated at the recommended drug dosage; 64.6% were red blood cell or platelet transfusion dependent at study baseline. Correlations between disease response and outcomes were assessed by logistic regression modeling for categorical variables and by Cox proportional hazards modeling for time-to-event variables. Patients with CRh had a higher proportion with transfusion independence (TI) for at least 56 days (TI-56; 92.3% vs 22.3%; P < .0001) or TI for at least 112 days (TI-112; 63.5% vs 8.7%; P < .0001), a reduced risk over time for severe infection (hazard ratio [HR], 0.43; P = .0007) or severe bleeding (HR, 0.17; P = .01), and a longer overall survival (OS; HR, 0.31; P < .0001) than patients with no response. The effects were consistent across drugs. In comparison with patients with CR, the effect sizes for CRh were similar for TI-56 and for risk over time of infection or bleeding but less for TI-112 and OS. CRh is associated with clinical benefits consistent with clinically meaningful palliative effects for the treatment of AML with nonmyelosuppressive drugs, although less robustly than for CR.
Introduction
Intensive chemotherapy administered on an episodic schedule is the current standard of care for the treatment of acute myeloid leukemia (AML). Although such therapies are associated with serious organ toxicities and treatment-related mortality, the improvement in overall survival (OS) outweighs these risks, especially in the curative setting. Hence, OS has long been an accepted measure of the efficacy of new intensive therapeutics for the treatment of AML with curative intent.1 Durable complete remission (CR) has also been used to support the approval of drugs for the treatment of AML, especially for patients with relapsed or refractory disease in which curative therapies are not available, because the associated prolonged resolution of cytopenias and cytopenia-related morbidities during remission after completion of the treatment course are considered to be of benefit to the patient.2
More recently, molecularly targeted drugs, such as inhibitors of isocitrate dehydrogenase 1, isocitrate dehydrogenase 2, and fms-like tyrosine kinase 3, have been developed with the potential to reduce leukemia cell burden without substantial myelosuppression even when administered on a continuous schedule. Although improvement in OS is always a goal for the treatment of AML, the moderated safety profile of molecularly targeted drugs confers a favorable benefit-risk assessment for treatment in the palliative setting as well. In this setting, durable CR and CR with partial hematological recovery (CRh) have been used in part for regulatory decision-making with an expectation of maintenance of blood counts adequate to protect against infection and avoid transfusions while on continuous treatment.3
Herein, we provide a pooled analysis to characterize the clinical outcomes of patients with AML who achieved CRh after treatment with the targeted therapies ivosidenib, olutasidenib, enasidenib, and gilteritinib. The objectives of the study were to assess the correlation of response with clinical outcomes and to determine whether CRh provides a clinical benefit better than no response (NR) and comparable with that of CR in this treatment setting.
Methods
Data source and study population
This analysis included data from study AG120-C-001 for ivosidenib, study 2102-HEM-101 for olutasidenib, study AG221-C-001 for enasidenib, and studies 2215-CL-0101 and 2215-CL-0301 for gilteritinib submitted to the US Food and Drug Administration (FDA) in support of marketing applications. Eligibility criteria and treatment details have been described elsewhere.4-9 This analysis included only study participants who had AML having the target gene variant detected by the companion diagnostic assay and who had been treated with at least 1 dose of the relevant drug at the approved recommended dosage. All patients in the included trials were required to provide written informed consent, and the trials had institutional review board or ethics committee approval.
Response categorizations
The schedule of disease assessments varied by protocol, being generally on day 1 of every or every other 28-day cycle. For this analysis, patients were categorized by the best response (CR, CRh, CR with lesser hematological recovery [CRL], and NR) achieved within the on-treatment period based on marrow blasts, complete blood counts and differential, extramedullary disease, and transfusion history. Complete blood counts performed within 7 days of the marrow sampling were used for the disease assessment. Table 1 shows the main criteria used for the response categorizations. Note that patients could be assigned the response of CRL based on either lesser recovery of blood counts or with adequate counts but when transfusions were given within the prior 7 days; additionally, the NR category included patients with clinically active disease who died or withdrew before completing a posttreatment marrow assessment. Some exploratory analyses used alternative criteria, specifically based on either blasts, blood counts, and extramedullary disease but not transfusion history (alternative criteria 1) or blasts, blood counts, extramedullary disease, transfusion history, and hematopoietic growth factor use (alternative criteria 2). The on-treatment period is defined as the time from day 1 of therapy to the last day of therapy plus 28 days, the day of the start of new systemic therapy, the day of withdrawal from study, the day of death, or the data cutoff date, whichever came first.
Criteria used for the main assessment of response type
| Type . | Marrow . | Counts . | Clinical . | Transfusions . |
|---|---|---|---|---|
| CR | Blasts <5% | ANC >1 × 109/L and PLT >100 × 109/L | and Absence of circulating blasts; absence of extramedullary disease | and No transfusions in the prior 7 d |
| CRh | Blasts <5% | ANC >0.5 × 109/L and PLT >50 × 109/L, but not fulfilling count recovery for CR | and Absence of circulating blasts; absence of extramedullary disease | and No transfusions in the prior 7 d |
| CRL | Blasts <5% | and ANC ≤0.5 × 109/L and PLT ≤50 × 109/L | and Absence of circulating blasts; absence of extramedullary disease | or Any transfusions in the prior 7 d |
| NR | Blasts ≥5% | NA | or Circulating blasts or extramedullary disease | NA |
| Type . | Marrow . | Counts . | Clinical . | Transfusions . |
|---|---|---|---|---|
| CR | Blasts <5% | ANC >1 × 109/L and PLT >100 × 109/L | and Absence of circulating blasts; absence of extramedullary disease | and No transfusions in the prior 7 d |
| CRh | Blasts <5% | ANC >0.5 × 109/L and PLT >50 × 109/L, but not fulfilling count recovery for CR | and Absence of circulating blasts; absence of extramedullary disease | and No transfusions in the prior 7 d |
| CRL | Blasts <5% | and ANC ≤0.5 × 109/L and PLT ≤50 × 109/L | and Absence of circulating blasts; absence of extramedullary disease | or Any transfusions in the prior 7 d |
| NR | Blasts ≥5% | NA | or Circulating blasts or extramedullary disease | NA |
ANC, absolute neutrophil count; NA, not applicable; PLT, platelet count.
Clinical outcomes categorizations
Transfusions, safety events, and survival were captured continuously during the on-treatment period in all protocols. Transfusion independence (TI) was defined as having had no red blood cell or platelet transfusions for at least 56 (TI-56) or 112 (TI-112) consecutive days on treatment. Adverse events were coded according to Medical Dictionary for Regulatory Activities (MedDRA) version 24.0 and graded according to Common Terminology Criteria for Adverse Events (CTCAE) version 4.03. Severe infections included grades 3 to 5 events in the System Organ Class Infections and infestations, febrile neutropenia events were ascertained by the Preferred Term Febrile neutropenia, and severe bleeding events included grades 3 to 5 events in the narrow Standardised MedDRA Query Haemorrhage terms (excluding laboratory terms). Survival was defined as the time from the first day of treatment to the day of death from any cause; for those alive by the end of study follow-up, survival was censored at the last date known alive, that is, the last contact date or last visit date that the patient was known to be alive.
PRO categorizations
Patient-reported outcome (PRO) data were available from study 2215-CL-0301 for gilteritinib and study 2102-HEM-101 for olutasidenib (see supplemental Methods, available on the Blood website). Longitudinal analyses of the gilteritinib data at the study level were published previously.10 This analysis used data for the Brief Fatigue Inventory Global Fatigue (BFI-GF) score, the EQ-5D 5-level (EQ-5D-5L) visual analogue scale (EQ-VAS) score, and the Functional Assessment of Cancer Therapy (FACT)–Leukemia Functional Well-Being (FACT-FWB), –Physical Well-Being (FACT-PWB), and –Leukemia Subscale (FACT-LEUS) scores, focusing on the best PRO score while on treatment. Supplemental Table 1 provides the criteria for the categorization of the scores.
VAF evaluation
Variant allele frequencies (VAFs) in marrow or peripheral blood were measured at varying time points by molecular methods; the assays used for these exploratory analyses were investigational and not previously cleared by the FDA for assessing minimal residual disease, disease burden, or remission quality (see supplemental Methods). Longitudinal analyses of these data at the study level were published previously.9,11-13 The stated sensitivities of the methods ranged from 0.01% to 1%. Taking into consideration this range of sensitivities, the pooled analysis focused on the proportion of patients with observed VAF <1% by any assay method while on treatment. The analyses included VAFs for only the target gene variant identified at baseline by central testing.
Statistical methods
Patients’ characteristics, disease responses, and data for clinical, PRO, and VAF outcomes were reported descriptively. Time-to-event variables were displayed graphically using Kaplan-Meier estimates. Correlations between disease response and each of the outcomes were assessed by logistic regression modeling for categorical variables and by Cox proportional hazards modeling for time-to-event variables adjusting for a selected set of potential prognostic factors. Because disease response was found to correlate with each outcome by the likelihood ratio test (supplemental Tables 2 and 3), pair-wise analyses were then performed to assess for correlations between an outcome and individual disease response (CR, CRh, and CRL) relative to NR. The effect size relative to NR was quantitated using risk difference for categorical outcomes and hazard ratio (HR) for time-to-event outcomes. When relevant, comparisons were also made for outcomes with CRh relative to CR. All P values are reported without correction for multiplicity in this retrospective descriptive study. The analyses were performed using JMP version 16.0 and Statistical Analysis System (SAS) Studio 3.81 (SAS Institute, Inc, Cary, NC); MedDRA Adverse Events Diagnostic v3.0 (FDA, Silver Spring, MD) was used to assess the adverse event outcomes.
Results
Characteristics of the study population
The analysis cohort included 841 adult patients of median age 67 years (range, 18-100). Thirty-three patients (4%) were newly diagnosed, and the remainder had relapsed or refractory AML. The demographic and disease characteristics for all 841 patients are shown in Table 2. There were 207 patients treated with ivosidenib, 147 treated with olutasidenib, 193 treated with enasidenib, and 294 treated with gilteritinib. The characteristics of the population by treatment are shown in supplemental Table 4. The median on-treatment time was 4.2 months (range, 0.2-81.3); 34% were on treatment for >6 months and 14% for >1 year.
Baseline demographic and disease characteristics
| Characteristic . | N = 841 . |
|---|---|
| Median age, (range), y | 67 (18-100) |
| Age categories, n (%) | |
| <40 y | 59 (7.0) |
| ≥40 y to <65 y | 285 (33.9) |
| ≥65 y to <75 y | 308 (36.6) |
| ≥75 y | 189 (22.5) |
| Sex, n (%) | |
| Male | 416 (49.5) |
| Female | 425 (50.5) |
| Race, n (%) | |
| White | 538 (64.0) |
| Asian | 82 (9.7) |
| Black | 42 (5.0) |
| Other or unknown | 179 (21.3) |
| Ethnicity, n (%) | |
| Hispanic/Latino | 49 (5.8) |
| Not Hispanic/Latino | 577 (68.6) |
| Not reported or unknown | 215 (25.6) |
| Baseline ECOG PS 0-1, n (%) | 682 (81.2) |
| Pre-existing cardiac disorder, n (%) | 293 (34.8) |
| Pre-existing pulmonary disorder, n (%) | 395 (47.0) |
| Baseline total bilirubin >1.5 × ULN, n (%) | 10 (1.2) |
| Baseline creatinine clearance <45 mL/min, n (%) | 41 (4.9) |
| Disease status | |
| Untreated AML, n (%) | 33 (3.9) |
| Primary refractory AML, n (%) | 329 (39.1) |
| Untreated relapse AML, n (%) | 369 (43.9) |
| Refractory relapse AML, n (%) | 110 (13.1) |
| Median number of relapses (range) | 1 (0-3) |
| Prior stem cell transplantation, n (%) | 144 (17.1) |
| Transfusion dependent at baseline, n (%)∗ | 543 (64.6) |
| Characteristic . | N = 841 . |
|---|---|
| Median age, (range), y | 67 (18-100) |
| Age categories, n (%) | |
| <40 y | 59 (7.0) |
| ≥40 y to <65 y | 285 (33.9) |
| ≥65 y to <75 y | 308 (36.6) |
| ≥75 y | 189 (22.5) |
| Sex, n (%) | |
| Male | 416 (49.5) |
| Female | 425 (50.5) |
| Race, n (%) | |
| White | 538 (64.0) |
| Asian | 82 (9.7) |
| Black | 42 (5.0) |
| Other or unknown | 179 (21.3) |
| Ethnicity, n (%) | |
| Hispanic/Latino | 49 (5.8) |
| Not Hispanic/Latino | 577 (68.6) |
| Not reported or unknown | 215 (25.6) |
| Baseline ECOG PS 0-1, n (%) | 682 (81.2) |
| Pre-existing cardiac disorder, n (%) | 293 (34.8) |
| Pre-existing pulmonary disorder, n (%) | 395 (47.0) |
| Baseline total bilirubin >1.5 × ULN, n (%) | 10 (1.2) |
| Baseline creatinine clearance <45 mL/min, n (%) | 41 (4.9) |
| Disease status | |
| Untreated AML, n (%) | 33 (3.9) |
| Primary refractory AML, n (%) | 329 (39.1) |
| Untreated relapse AML, n (%) | 369 (43.9) |
| Refractory relapse AML, n (%) | 110 (13.1) |
| Median number of relapses (range) | 1 (0-3) |
| Prior stem cell transplantation, n (%) | 144 (17.1) |
| Transfusion dependent at baseline, n (%)∗ | 543 (64.6) |
ECOG PS, Eastern Cooperative Oncology Group performance status; ULN, upper limit of normal.
Patients were defined as transfusion dependent at baseline if they received any red blood cell or platelet transfusion within 56 days before the first dose of study drug.
Disease responses
The best on-treatment disease response was CR for 163 patients (19.4%), CRh for 52 (6.2%), CRL for 142 (16.9%), and NR for 484 (57.6%). The proportion of patients by best disease response differed only slightly using the alternative disease response criteria (supplemental Figure 1).
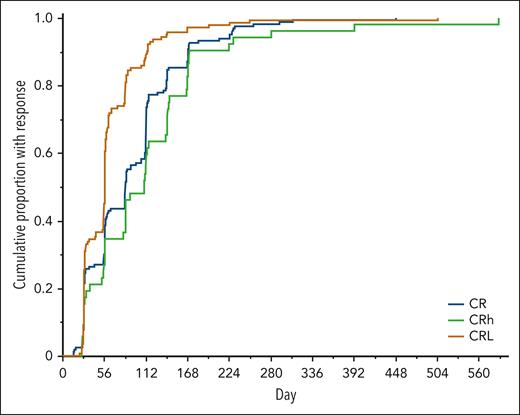
The cumulative proportion of responders over time is shown in Figure 1, grouped by best disease response achieved on treatment. The median time to a first response of CR or CRh was 2.8 months (range, 0.5-19.3), with generally overlapping time to first response when assessed by drug (supplemental Figure 2). Of the patients who achieved CR or CRh as best response, 92.1% had the first response observed by 6 months on treatment, and only 1.4% were observed after 1 year on treatment (Figure 1; supplemental Figure 3). For the analyses of correlations of response with outcomes below, patients were categorized by best response at any time on treatment to ensure that all responders are reflected as such, including late responders.
Cumulative proportion with response over time. The analysis included the 163 patients with a best response of CR (blue line), 52 patients with a best response of CRh (green line), and 142 patients with a best response of CRL (brown line). The x-axis shows the day on treatment when a first response of CR or CRh was observed for those with a best response of CR or CRh and a first response of CRL for those with a best response of CRL.
Cumulative proportion with response over time. The analysis included the 163 patients with a best response of CR (blue line), 52 patients with a best response of CRh (green line), and 142 patients with a best response of CRL (brown line). The x-axis shows the day on treatment when a first response of CR or CRh was observed for those with a best response of CR or CRh and a first response of CRL for those with a best response of CRL.
TI by response
TI-56 was observed for 352 patients (41.9%), and TI-112 was observed for 221 (26.3%). Of the patients with TI-56 or TI-112, >98% had their period of TI within the first 6 months on treatment. Table 3 shows the proportions of patients with TI-56 or TI-112 by best disease response on treatment and the risk differences relative to NR. TI-56 was observed for >90% of patients with CR or CRh but for only 27.5% of patients with CRL, and the risk differences were significant for patients with CR or CRh compared with those with NR. The pattern of outcomes by disease response was similar for TI-112 (Table 3), when assessed by drug (supplemental Table 5) and when assessed by baseline transfusion status (supplemental Table 6). When comparing between patients with CR vs CRh, the proportions were not different for TI-56 (difference, –4.0%; 95% confidence interval [CI], –11.8 to 3.8), but a smaller proportion of patients with CRh achieved TI-112 (CRh vs CR risk difference, –17.5%; 95% CI, –31.9 to –3.1). We conclude that TI was conferred consistently by CR or CRh, although the effect was slightly less for CRh than for CR.
Clinical outcomes by best response on treatment
Outcome . | Observed outcome by best response on treatment . | RD compared with patients with best response of NR . | |||||
|---|---|---|---|---|---|---|---|
| CR (n = 163) . | CRh (n = 52) . | CRL (n = 142) . | NR (n = 484) . | CR vs NR . | CRh vs NR . | CRL vs NR . | |
| TI | |||||||
| TI-56, % (95% CI) | 96.3%∗ (92.2-98.6) | 92.3%∗ (81.5-97.9) | 27.5%† (20.3-35.6) | 22.3% (18.7-26.3) | 74.0 (69.3-78.7) | 70.0 (61.9-78.1) | 5.2 (–3.1 to 13.4) |
| TI-112, % (95% CI) | 81.0%∗ (74.1-86.7) | 63.5%∗ (49.0-76.4) | 9.9% (5.5-16.0) | 8.7% (6.3-11.5) | 72.3 (65.8-78.8) | 54.8 (41.5-68.1) | 1.2 (–4.3 to 6.7) |
| Median longest TI period (IQR), d | 287 (133-596) | 146 (84.5-227.5) | 28.5 (14-59) | 26 (11-50) | |||
| Other clinical outcomes | |||||||
| Febrile neutropenia, % (95% CI) | 20.3%∗ (14.4-27.2) | 30.8% (18.7-45.1) | 34.5% (26.7-42.9) | 36.2% (31.9-40.6) | –15.9 (–23.4 to –8.4) | –5.4 (–18.6 to 7.9) | –1.6 (–10.6 to 7.3) |
| Severe infection, % (95% CI) | 23.9%∗ (17.6-31.2) | 34.6% (22.0-49.1) | 50.0% (41.5-58.5) | 46.9% (42.4-51.5) | –23.0 (–30.9 to –15.1) | –12.3 (–26 to 1.4) | 3.1 (–6.2 to 12.4) |
| Severe bleeding, % (95% CI) | 5.5%† (2.6-10.2) | 3.8% (0.5-13.2) | 10.6% (6.0-16.8) | 13.0% (10.1-16.3) | –7.5 (–12.1 to –2.9) | –9.1 (–15.2 to –3.1) | –2.5 (–8.3 to 3.4) |
Outcome . | Observed outcome by best response on treatment . | RD compared with patients with best response of NR . | |||||
|---|---|---|---|---|---|---|---|
| CR (n = 163) . | CRh (n = 52) . | CRL (n = 142) . | NR (n = 484) . | CR vs NR . | CRh vs NR . | CRL vs NR . | |
| TI | |||||||
| TI-56, % (95% CI) | 96.3%∗ (92.2-98.6) | 92.3%∗ (81.5-97.9) | 27.5%† (20.3-35.6) | 22.3% (18.7-26.3) | 74.0 (69.3-78.7) | 70.0 (61.9-78.1) | 5.2 (–3.1 to 13.4) |
| TI-112, % (95% CI) | 81.0%∗ (74.1-86.7) | 63.5%∗ (49.0-76.4) | 9.9% (5.5-16.0) | 8.7% (6.3-11.5) | 72.3 (65.8-78.8) | 54.8 (41.5-68.1) | 1.2 (–4.3 to 6.7) |
| Median longest TI period (IQR), d | 287 (133-596) | 146 (84.5-227.5) | 28.5 (14-59) | 26 (11-50) | |||
| Other clinical outcomes | |||||||
| Febrile neutropenia, % (95% CI) | 20.3%∗ (14.4-27.2) | 30.8% (18.7-45.1) | 34.5% (26.7-42.9) | 36.2% (31.9-40.6) | –15.9 (–23.4 to –8.4) | –5.4 (–18.6 to 7.9) | –1.6 (–10.6 to 7.3) |
| Severe infection, % (95% CI) | 23.9%∗ (17.6-31.2) | 34.6% (22.0-49.1) | 50.0% (41.5-58.5) | 46.9% (42.4-51.5) | –23.0 (–30.9 to –15.1) | –12.3 (–26 to 1.4) | 3.1 (–6.2 to 12.4) |
| Severe bleeding, % (95% CI) | 5.5%† (2.6-10.2) | 3.8% (0.5-13.2) | 10.6% (6.0-16.8) | 13.0% (10.1-16.3) | –7.5 (–12.1 to –2.9) | –9.1 (–15.2 to –3.1) | –2.5 (–8.3 to 3.4) |
%, percentage of patients with the listed outcome on treatment; IQR, interquartile range; n, number of patients with the best response in the column header on treatment; RD, risk difference.
P < .01 for comparison with NR by logistic regression adjusting for drug and baseline transfusion dependence status.
P < .05 for comparison with NR by logistic regression adjusting for drug and baseline transfusion dependence status. See supplemental Table 2 for details.
Other clinical outcomes by response
The leukemia-related adverse events assessed included febrile neutropenia, severe infection, and severe bleeding. Table 3 shows the proportions of patients reported to have these leukemia-related adverse events by best disease response on treatment. There was a lower incidence of all 3 leukemia-related adverse events in patients who achieved CR and a lower incidence of severe bleeding in patients who achieved CRh than in those with NR; but for the patients who achieved CRL, the incidences of these leukemia-related adverse events did not differ from those of patients with NR. The incidence rates were somewhat heterogenous when assessed by the drug used for treatment (supplemental Table 7), although some subgroups were very small.
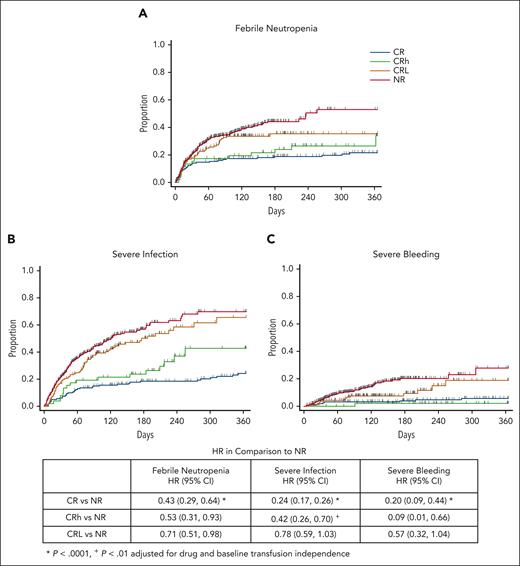
It should be noted, however, that the analysis of incidence while on therapy does not differentiate between early events that reflect immediate treatment failure and later events after a period of clinical benefit. Therefore, we also conducted a time-to-event analysis. As shown in Figure 2, with 1 year of follow-up, there was a lower HR for all 3 leukemia-related adverse events in patients who achieved CR or CRh than in patients with NR, and there was a modest decrease in the HR for febrile neutropenia in those who achieved CRL. There was a trend for an increased risk over time of febrile neutropenia (HR, 1.24; 95% CI, 0.66-2.32) and severe infection (HR, 1.73; 95% CI, 0.97-3.11) but not for severe bleeding (HR, 0.44; 95% CI, 0.06-3.57) when comparing patients in CRh with those in CR. We conclude that the achievement of CR or CRh conferred a meaningful reduction in time to febrile neutropenia, severe infection, and severe bleeding compared with those with NR, although the effect of CRh was somewhat less than that of CR.
Time to adverse event outcomes on treatment by best response. Cumulative incidence of febrile neutropenia (A), severe infection (B), and severe bleeding (C) up to 1 year during the on-treatment period. Table shows the HRs and 95% CI in comparison with NR. See supplemental Table 3 for statistical details and supplemental Figure 4 for long-term follow-up.
Time to adverse event outcomes on treatment by best response. Cumulative incidence of febrile neutropenia (A), severe infection (B), and severe bleeding (C) up to 1 year during the on-treatment period. Table shows the HRs and 95% CI in comparison with NR. See supplemental Table 3 for statistical details and supplemental Figure 4 for long-term follow-up.
Figure 3 shows the OS by best response on treatment. The OS was higher for patients with CR, CRh, or CRL than for those with NR, and this did not change in analyses using 30- or 60-day landmarks (supplemental Table 3). Survival was lower for patients with CRh (CRh vs CR HR, 2.80; 95% CI, 1.86-4.22) or with CRL (CRL vs CR HR, 4.00; 95% CI, 2.90-5.52) than for those with CR. CR had a strong impact on survival across drugs (HRs 0.07-0.15), but the magnitude of the impact of CRh and CRL on survival was less consistent across drugs (supplemental Figure 5). We conclude that CR is the only response reliably associated with long-term survival.
OS by best response on treatment. Kaplan-Meier estimates of survival by best response observed on treatment. The x-axis shows the time from start of treatment. The legend shows the median OS (mOS) and HRs in comparison with NR. ∗P < .0001 for comparison with NR adjusting for drug, baseline TI status, baseline ECOG performance status, age group, and prior HSCT. See supplemental Table 3 for details. ECOG, Eastern Cooperative Oncology Group; HSCT, hematopoietic stem cell transplantation.
OS by best response on treatment. Kaplan-Meier estimates of survival by best response observed on treatment. The x-axis shows the time from start of treatment. The legend shows the median OS (mOS) and HRs in comparison with NR. ∗P < .0001 for comparison with NR adjusting for drug, baseline TI status, baseline ECOG performance status, age group, and prior HSCT. See supplemental Table 3 for details. ECOG, Eastern Cooperative Oncology Group; HSCT, hematopoietic stem cell transplantation.
PRO by response
We also sought to determine whether disease response was reflected in the resolution of disease symptoms or normalization of well-being (PRO category 1, see supplemental Table 1). As shown in Table 4, for the patients treated with gilteritinib, a higher proportion of patients in CR reported category 1 PRO scores than patients with NR; but with the exception of the FACT-LEUS score, the proportions of patients in CRh or CRL with a category 1 PRO score were not substantially higher than for those with NR. For patients treated with olutasidenib, results were available only for EQ-VAS score; by disease response, postbaseline EQ-VAS score ≥80 was reported by 87.8% in CR, 71.4% in CRh, 58.8% in CRL, and 30.5% with NR. No data were available for patients treated with enasidenib or ivosidenib.
PROs by best response on treatment with gilteritinib in study 2215-CL-0301
PRO outcome . | Observed outcome by best response on treatment . | Risk difference in comparison to patients with best response of NR . | |||||
|---|---|---|---|---|---|---|---|
| CR (n = 32) . | CRh (n = 19) . | CRL (n = 66) . | NR (n = 124) . | CR vs NR . | CRh vs NR . | CRL vs NR . | |
| BFI-GF score <1, % (95% CI) | 87.5%∗ (71.0-96.5) | 52.6% (28.9-75.6) | 51.5% (38.9-64.0) | 39.5% (30.9-48.7) | 48.0 (33.7-62.3) | 13.1 (–10.9 to 37.2) | 12.0 (–2.8 to 26.8) |
| EQ-VAS score ≥80, % (95% CI) | 84.4%∗ (67.2-94.7) | 52.6% (28.9-75.6) | 51.5% (38.9-64.0) | 41.9% (33.1-51.1) | 42.4 (27.2-57.7) | 10.7 (–13.4 to 34.8) | 9.6 (–5.3 to 24.4) |
| FACT-FWB score >21, % (95% CI) | 62.5%∗ (43.7-78.9) | 47.4%† (24.4-71.1) | 27.3% (17.0-39.6) | 25.8% (18.4-34.4) | 36.7 (18.2-55.2) | 21.6 (–2.2 to 45.3) | 1.5 (–11.8 to 14.7) |
| FACT-PWB score >21, % (95% CI) | 96.9%∗ (83.8-99.9) | 78.9% (54.4-93.9) | 77.3% (65.3-86.7) | 62.9% (53.8-71.4) | 34.0 (23.6-44.4) | 16.0 (–4.2 to 36.3) | 14.4 (1.2-27.6) |
| FACT-LEUS score >51, % (95% CI) | 90.6%∗ (75.0-98.0) | 78.9%∗ (54.4-93.9) | 54.5% (41.8-66.9) | 49.2% (40.1-58.3) | 41.4 (28.0-54.8) | 29.8 (9.4-50.1) | 5.4 (–9.5 to 20.2) |
PRO outcome . | Observed outcome by best response on treatment . | Risk difference in comparison to patients with best response of NR . | |||||
|---|---|---|---|---|---|---|---|
| CR (n = 32) . | CRh (n = 19) . | CRL (n = 66) . | NR (n = 124) . | CR vs NR . | CRh vs NR . | CRL vs NR . | |
| BFI-GF score <1, % (95% CI) | 87.5%∗ (71.0-96.5) | 52.6% (28.9-75.6) | 51.5% (38.9-64.0) | 39.5% (30.9-48.7) | 48.0 (33.7-62.3) | 13.1 (–10.9 to 37.2) | 12.0 (–2.8 to 26.8) |
| EQ-VAS score ≥80, % (95% CI) | 84.4%∗ (67.2-94.7) | 52.6% (28.9-75.6) | 51.5% (38.9-64.0) | 41.9% (33.1-51.1) | 42.4 (27.2-57.7) | 10.7 (–13.4 to 34.8) | 9.6 (–5.3 to 24.4) |
| FACT-FWB score >21, % (95% CI) | 62.5%∗ (43.7-78.9) | 47.4%† (24.4-71.1) | 27.3% (17.0-39.6) | 25.8% (18.4-34.4) | 36.7 (18.2-55.2) | 21.6 (–2.2 to 45.3) | 1.5 (–11.8 to 14.7) |
| FACT-PWB score >21, % (95% CI) | 96.9%∗ (83.8-99.9) | 78.9% (54.4-93.9) | 77.3% (65.3-86.7) | 62.9% (53.8-71.4) | 34.0 (23.6-44.4) | 16.0 (–4.2 to 36.3) | 14.4 (1.2-27.6) |
| FACT-LEUS score >51, % (95% CI) | 90.6%∗ (75.0-98.0) | 78.9%∗ (54.4-93.9) | 54.5% (41.8-66.9) | 49.2% (40.1-58.3) | 41.4 (28.0-54.8) | 29.8 (9.4-50.1) | 5.4 (–9.5 to 20.2) |
%, percentage of patients with the listed PRO outcome on treatment.
P < .01 for comparison to NR by logistic regression adjusting for drug and baseline PRO category.
P < .05 for comparison to NR by logistic regression adjusting for drug and baseline PRO category. See supplemental Table 2 for details.
Because an improvement in PRO cannot be detected in patients with a category 1 PRO score at baseline, we also assessed for a shift from category 2 scores at baseline to category 1 on treatment. The shift analysis demonstrated a similar trend for improved PRO score category in patients with CR and an inconsistent trend in improvement for patients with CRh (supplemental Table 8). For patients treated with olutasidenib, the results for a shift to category 1 EQ-VAS score (82.4% in CR, 60.0% in CRh, 66.7% in CRL, and 27.5% with NR) were similar in trend to those for patients treated with gilteritinib. It should be noted, however, that because many patients had a category 1 PRO score on one of the tools at baseline, including 25% to 63% of those with best disease response of CR or CRh, the number of patients remaining for the shift analysis was small. In all, the data were not sufficient to demonstrate that disease responses other than CR were consistently associated with the resolution of symptoms or normalization of well-being scores.
VAF by response
Lastly, we evaluated whether VAFs differed by disease response. As shown in Table 5, the proportions of patients with reported VAF <1% in marrow or peripheral blood were higher in those with CR or CRh than those with NR but not for patients with CRL. The longitudinal graphical depiction of VAFs by disease response and by drug was largely consistent with the pooled analysis (supplemental Figures 6-8). Due to the differences in sampling times, assays used, and the levels of missing data across studies, these results should be interpreted with caution.
VAF less than 1% by best response on treatment
Matrix . | Observed outcome by best response on treatment . | Risk difference in comparison with patients with best response of NR . | |||||
|---|---|---|---|---|---|---|---|
| CR . | CRh . | CRL . | NR . | CR vs NR . | CRh vs NR . | CRL vs NR . | |
| Marrow subgroup, n | 90 | 26 | 58 | 272 | |||
| Marrow VAF <1%, % (95% CI) | 34.4%∗ (24.7-45.2) | 26.9%∗ (11.6-47.8) | 8.6% (2.9-19.0) | 3.3% (1.5-6.2) | 31.1 (21.1-41.2) | 23.6 (6.4-40.8) | 5.3 (–2.2 to 12.8) |
| Peripheral blood subgroup, n | 127 | 32 | 57 | 331 | |||
| Peripheral blood VAF <1%, % (95% CI) | 40.2%∗ (31.6-49.2) | 21.9%∗ (9.3-40.0) | 7.0% (1.9-17.0) | 4.5% (2.6-7.4) | 35.6 (26.8-44.4) | 17.3 (2.8-31.8) | 2.5 (–4.5 to 9.5) |
Matrix . | Observed outcome by best response on treatment . | Risk difference in comparison with patients with best response of NR . | |||||
|---|---|---|---|---|---|---|---|
| CR . | CRh . | CRL . | NR . | CR vs NR . | CRh vs NR . | CRL vs NR . | |
| Marrow subgroup, n | 90 | 26 | 58 | 272 | |||
| Marrow VAF <1%, % (95% CI) | 34.4%∗ (24.7-45.2) | 26.9%∗ (11.6-47.8) | 8.6% (2.9-19.0) | 3.3% (1.5-6.2) | 31.1 (21.1-41.2) | 23.6 (6.4-40.8) | 5.3 (–2.2 to 12.8) |
| Peripheral blood subgroup, n | 127 | 32 | 57 | 331 | |||
| Peripheral blood VAF <1%, % (95% CI) | 40.2%∗ (31.6-49.2) | 21.9%∗ (9.3-40.0) | 7.0% (1.9-17.0) | 4.5% (2.6-7.4) | 35.6 (26.8-44.4) | 17.3 (2.8-31.8) | 2.5 (–4.5 to 9.5) |
See supplemental Table 2 for details.
%, percentage of patients with the VAF <1% on treatment; n, number of patients with the disease response listed in the column header in the marrow or peripheral blood cohort tested for VAF; VAF, variant allele frequency for the allele identified by the companion diagnostic assay at study baseline.
P < .01 for comparison with NR by logistic regression adjusting for drug and baseline transfusion dependence status.
Discussion
Reductions in the need for transfusions and the occurrence of severe infection or bleeding would seem appropriate salutary effects of a palliative therapy for AML. In this pooled analysis of clinical outcomes of patients with AML treated with targeted therapy, we showed that achieving CRh was associated with a higher rate of TI and a decreased risk over time of severe infection or severe bleeding compared to those with NR, and the impact of CRh on these outcomes was comparable with or slightly less than that in patients achieving CR. These results suggest that CRh would be an appropriate efficacy endpoint in addition to CR for such palliative therapies. It should be noted, however, that this analysis is not a validation of CRh as a surrogate for TI, infection, or bleeding; formal validation would require a study-level assessment of the association between the treatment effect of the drugs on the response and the treatment effect on the associated clinical outcomes across randomized trials, and no such randomized trials have been performed. Hence, supporting data on transfusions, infections, and bleeding would still be needed if CRh is used in a marketing application to establish the treatment effect of a palliative therapy for AML.
de Botton et al9 reported previously that patients treated with olutasidenib who achieved CRh had very high rates of TI-56. We confirm that here and extend that observation to all 4 targeted therapies in our pooled analysis (TI-56 in 92%) and for each drug (TI-56 in 88%-100%; supplemental Table 5). Moreover, a substantial proportion of patients with CRh (64%) were independent of transfusions for an even longer period of time, 112 days, comparable to the rate seen in patients with CR (TI-112 in 81%). These findings suggest that ≥112 days may be a feasible benchmark for clinically meaningful duration of TI for new drugs for the palliative treatment of AML.
Despite the recent emphasis on patient-centered outcomes in clinical trial design, very little has been written on the association of AML disease response and PROs. In a preliminary report, Bennet et al14 indicated that morphological leukemia-free state but not CRh or CR with incomplete hematologic recovery differed from CR in symptom burden or physical function for patients with AML. Herein, we evaluated for differences in measures of fatigue and well-being in patients with response compared with those with NR, expecting that if CRh was associated with TI and a delay in time to severe cytopenia-related adverse events, it may also correlate with the resolution of symptoms and normalization of well-being. Using the available data for the patients treated with gilteritinib, we found a strong correlation with CR for all 5 PRO measures but only modest or less correlations with CRh that were limited to the FACT-FWB score and the FACT-LEUS score. Using the available data for patients treated with gilteritinib or olutasidenib, the results were inconsistent across drugs for the EQ-VAS score. Interestingly, Ritchie et al10 reported from their longitudinal analysis that patients who converted from transfusion dependent to TI on treatment with gilteritinib had an 8-point increase in the EQ-VAS score but only small mean changes in the other PRO measures, suggesting that the achievement of TI itself is not sufficient to confer a meaningful change in PROs. Both we and Ritchie et al noted that a rather high proportion of patients in this data set had high PRO scores at study baseline, making improvements difficult to discern; as shown in our shift analysis (supplemental Table 8), the number of patients in whom resolution of symptoms and normalization of well-being could be assessed was too small for a meaningful analysis. These findings highlight the need for more research into how well AML disease responses reflect the effect of these palliative therapies on how the patients feel and function.
In randomized trials, a small (3.7 months) but statistically significant survival advantage was reported for gilteritinib monotherapy but none for enasidenib.7,15 In responder analyses,5,9,16 OS was higher in patients who achieved CR or CRh when grouped together vs those with NR. Acknowledging that the survival analysis may be confounded by varying use of effective poststudy salvage therapies, including hematopoietic stem cell transplantation, favoring responders, we show here that the survival for patients with a best response of CRh was superior to that for patients with NR but quite inferior to that for patients with CR (Figure 3). The relative survival rates are consistent with the mutation clearance data showing lower VAF levels in patients with CR than in those with CRh, although deep molecular remissions that would confer long-term disease control occurred in only a minority of patients.5,11,12,16 This would not detract from the value of CRh as an efficacy endpoint for a palliative therapy, because the associated TI and delay in infection or bleeding may be viewed as beneficial even in the absence of a survival improvement when the safety profile was favorable.
For the purposes of this analysis, we categorized disease response into 4 mutually exclusive groups: CR, CRh, CRL, and NR. The category CRL, which includes those with marrow and extramedullary remission but impaired recovery of blood counts, was used solely to describe the data set and not to suggest it as a measure of efficacy. In fact, we show here that despite the modest difference in survival compared with that of those with NR (median OS, 9.9 vs 5.1 months), CRL did not consistently correlate strongly with the other clinical outcome measures assessed. As such, just clearing blasts without adequate count recovery, as occurs in the patients categorized as CRL, does not appropriately reflect a palliative benefit for these targeted therapies.
Although the schedule of assessments for disease response, transfusions, and adverse events were similar among the clinical trials used in this pooled analysis, differences in the duration of follow-up for survival and differences in the schedule of testing and sensitivity of the assay platforms used for monitoring VAF levels present limitations of the pooled data. These limitations were mitigated to some extent by using drug as a covariate in the multivariable analyses and by presenting the subgroup analyses by drug; nonetheless, there remains some uncertainty in the quantitative outcomes for survival and VAF levels as reported.
We conclude that CRh provides for meaningful palliative outcomes compared with patients with no disease response, and the effects are similar to those in patients with CR during continuous treatment with nonmyelosuppressive targeted therapies. We caution, however, that these findings cannot be extrapolated to episodic myelosuppressive treatments, which have treatment-related risks that may outweigh modest palliative benefits.17-19
Acknowledgments
The authors thank Sheila Ryan, Esther Park, and Saumya Nathan for expert project management.
Authorship
Contribution: R.Q.L., D.P., H.C., Y.L.S., E.D.P., K.N., and R.A.D.C. participated in designing the study; R.Q.L. and D.P. collected and assembled the study data; R.Q.L, D.P., H.C., and Y.L.S. analyzed and interpreted the study data and wrote the manuscript; and all authors reviewed the manuscript and provided final approval.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Robert Q. Le, US Food and Drug Administration, Center for Drug Evaluation and Research, Office of Oncologic Diseases, WO22, 10903 New Hampshire Ave, Silver Spring, MD 20993; email: robert.le@fda.hhs.gov.
References
Author notes
For patient-level data, please refer to the instructions in the original publications of the clinical trial results.4-9
The online version of this article contains a data supplement.
There is a Blood Commentary on this article in this issue.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.



This feature is available to Subscribers Only
Sign In or Create an Account Close Modal