Key Points
Procoagulant platelets deliver large amounts of IC molecules to malignant tumors.
Procoagulant platelets use these IC molecules to misguide immune cell responses, thus promoting tumor progression.
Visual Abstract
Triple-negative breast cancer (TNBC) is an aggressive tumor entity in which immune checkpoint (IC) molecules are primarily synthesized in the tumor environment. Here, we report that procoagulant platelets bear large amounts of such immunomodulatory factors and that the presence of these cellular blood components in TNBC relates to protumorigenic immune-cell activity and impaired survival. Mechanistically, tumor-released nucleic acids attract platelets to the aberrant tumor microvasculature, where they undergo procoagulant activation, thus delivering specific stimulatory and inhibitory IC molecules. This concomitantly promotes protumorigenic myeloid leukocyte responses and compromises antitumorigenic lymphocyte activity, ultimately supporting tumor growth. Interference with platelet-leukocyte interactions prevented immune cell misguidance and suppressed tumor progression, nearly as effective as systemic IC inhibition. Hence, our data uncover a self-sustaining mechanism of TNBC by using platelets to misdirect immune-cell responses. Targeting this irregular multicellular interplay may represent a novel immunotherapeutic strategy for TNBC without the adverse effects of systemic IC inhibition.
Introduction
Breast cancer is the most prevalent oncological disorder in women worldwide and is one of the most common solid cancers overall. Thereof, triple-negative breast cancer (TNBC) accounts for up to 20% of the cases. These tumors do not express the therapeutically targetable receptors for estrogen (ER), progesterone, or human epidermal growth factor receptor 2 (HER2) and belong to the most aggressive breast cancer entities.1
The immune system protects the organism against the development of malignant tumors. In particular, cytotoxic T lymphocytes (CTLs) recognize neoplastic cells and eliminate them through the release of perforins and granzymes. Furthermore, Th1-polarized CD4+ T lymphocytes collaborate with CTLs in cytotoxic killing, increase antigen presentation in neoplastic cells, and induce antitumor activity in peritumoral macrophages. Strategies to enhance the responses of these antitumorigenic immune cells (eg, immune checkpoint inhibitors [ICI]) have already proven effective in clinical trials for different cancer entities.2 Here, primary interference with the interactions of tumor-released programmed death-ligand 1 (PD-L1) with programmed death receptor 1 (PD-1) expressed on the surface of CTLs and/or CD80 and CD86 with their receptor cytotoxic T-lymphocyte-associated protein-4 (CTLA-4) increased the antitumor activity of CTLs, ultimately compromising malignant growth.2 However, in TNBC, PD-L1 expression is largely restricted to the tumor environment, while tumor cells produce only low amounts of this IC molecule.3 Importantly, >10% of anti-PD-L1/PD-1 ICI-treated patients experience severe or life-threatening autoimmune-related side effects due to compromised homeostatic PD-L1 activity in healthy tissues, causing excessive local CTL activity. The prevalence of these side effects is further enhanced upon the addition of other ICI (eg, anti-CTLA-4 antibodies) to a single ICI treatment.4 Consequently, the benefits of ICI in TNBC are subject to controversial discussions.5 Moreover, distinct leukocyte subsets can interfere with CTL activity or even increase B-cell–mediated protumorigenic humoral responses (eg, T helper cell type 2 [Th2] T cells; regulatory T cells and Treg). Specifically, myeloid leukocytes (eg, neutrophils, and monocytes) exhibit potent protumorigenic properties by producing pro-proliferative, proangiogenic, and immunosuppressive factors, notwithstanding that these immune cells are also able to exhibit antitumorigenic properties.6,7 Although tremendous insights have been gained into the individual anti- and protumorigenic functions of different immune cell populations, it remains poorly understood how specific leukocyte subsets reach malignant lesions.
In addition to their essential role in hemostasis, platelets are increasingly being recognized to participate in tumorigenesis.8-11 To this end, platelets release growth factors and angiogenic mediators,12-14 reduce tumor cell apoptosis and anoikis,15 induce gene expression16 and epithelial-mesenchymal transition in malignant cells,17-19 and protect tumor cells from immune responses,20-23 thus promoting tumor progression and metastasis.24 Accordingly, cyclooxygenase inhibitors (eg, acetylsalicylic acid)25-27 and adenosine diphosphate (ADP) receptor antagonists (eg, clopidogrel, ticagrelor, or prasugrel) have been reported to exhibit beneficial effects in the prevention of different malignancies, including breast cancer.28 Importantly, activated platelets acquire distinct phenotypes in thrombosis: although “aggregatory” platelets tighten the platelet plug, “procoagulant” platelets expose negatively charged phospholipids (particularly phosphatidylserine), on their surface upon contact to subendothelial collagen to promote plasmatic coagulation.29-31 However, the role of activated platelet phenotypes in cancer remains unclear.
Reciprocal interactions with platelets are well-known to support the extravasation of immune cells to the perivascular tissue under inflammatory conditions.32-38 However, the functional relevance of platelets in the regulation of immune cell responses in malignant tumors is still elusive. Interestingly, platelets have recently been reported to express IC molecules, including PD-L1.39-41 With respect to the already known protumorigenic effects of platelets and their established role in immune cell trafficking in inflammation, we hypothesized that these anucleate cell particles use their specific immunomodulatory properties to promote tumor progression in TNBC.
Methods
Systemic trafficking dynamics of platelets including their interplay with immune cells in the intra-/peritumoral microvasculature were analyzed in female BALB/c mice using orthotopic (mammary fat pad: flow cytometry and confocal microscopy) and heterotopic (auricle: in vivo microscopy) syngeneic TNBC models (4T1 tumor cells) at early tumor stages. To characterize the underlying mechanisms, flow cytometry in a mouse peritoneal assay, in vivo microscopy in a mouse cremaster muscle assay, and different in vitro assays were used. Toward translational perspectives, immunohistochemical analyses of human breast cancer samples, flow cytometry in primary human platelets, and bioinformatic analyses of published transcriptomic data were performed.
Additional details on methods are provided in supplemental Data, available on the Blood website.
All animal experiments were performed according to the German law for animal protection and were approved by the local government authorities (Regierung von Oberbayern).
Results
Procoagulant platelets accumulate in the tumor microvasculature
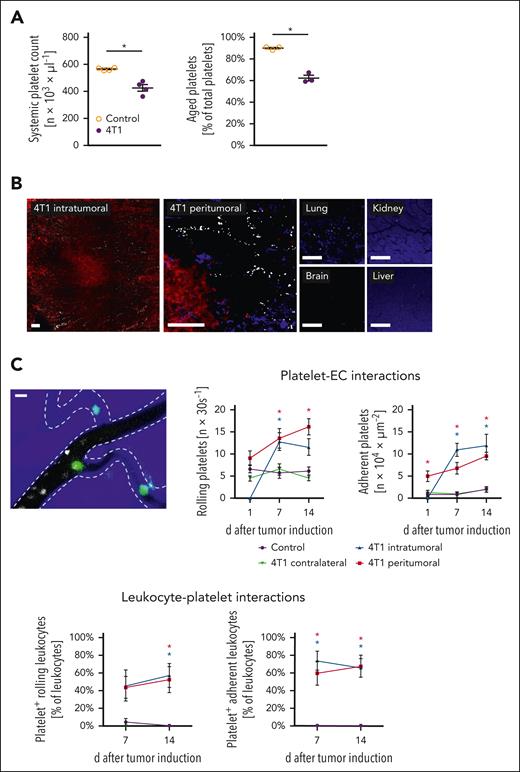
In this study, we hypothesized that platelets use their immunomodulatory properties to regulate tumor growth in TNBC. To prove this, we first sought to follow the tracks of platelets in an orthotopic syngeneic mouse model of TNBC at the early stages. Flow cytometry revealed that the total number of circulating platelets initially declined in tumor-bearing animals compared with that in healthy controls before increasing (supplemental Table 1A). A pulse-labeling approach further documented that the proportion of aged platelets of the total circulating platelets was significantly diminished in diseased animals (Figure 1A), pointing to a reduced circulatory time of these anucleate cell particles in the peripheral blood. In this context, confocal microscopy of tissue sections demonstrated that platelets primarily accumulated in tumors, but barely in the major peripheral organs of the diseased animals (Figure 1B; supplemental Table 1B). These events were associated with a (compensatory) increase in bone marrow megakaryocyte and platelet content (supplemental Table 1B-C), as well as slight splenic organ enlargement (supplemental Table 1D), and enhanced splenic accumulation of platelets (supplemental Table 1E), collectively unveiling an accelerated platelet turnover in diseased animals. In particular, in vivo microscopy in a heterotopic 4T1 breast cancer model (mouse auricle) documented that platelets (together with neutrophils, lymphocytes, and, to a lesser degree, classical monocytes (cMOs), but not non-cMOs (supplemental Figure 1A) increasingly roll and adhere to the endothelial surface of peritumoral and, later on, of the developing intratumoral aberrant microvessels with the progression of the tumors (Figure 1C). Importantly, the accumulating immune cells were preferentially bound to adherent platelets (Figure 1C; supplemental Video 1). As a result of these events, fewer aggregates of platelets and immune cells were formed in the peripheral blood of diseased animals than in healthy controls (Figure 1D). Interestingly, immunostaining and flow cytometry of tumor tissue homogenates revealed an almost 100-fold higher content of platelets exhibiting high surface levels of phosphatidylserine (supplemental Table 1F) than in the peripheral blood, which is indicative of the interactive “procoagulant” phenotype of these anucleate cell particles (exhibiting a larger cell size, more nucleic acid content, as well as higher surface levels of P-selectin, GPIIb/IIIa, and coagulation factor Xa as opposed to nonprocoagulant platelets including platelets with “aggregatory” or resting phenotypes; supplemental Figure 1B).29-31 Hence, circulating platelets “settle down” in malignant lesions, in which they interact with immune cells upon procoagulant activation (Figure 1E).
Platelet trafficking in experimental breast cancer. (A) Systemic platelet count as well as a proportion of aged platelets of total platelets in the peripheral blood of tumor-free control or orthotopic 4T1 tumor-bearing mice as assessed by flow cytometry; quantitative data are shown (mean ± standard error of the mean [SEM] for n = 3-4 mice per group; ∗P < .05 vs control). (B) Representative confocal microscopy images of GPIbβ+ platelets (white) in the intra- and peritumoral microvasculature of 4T1 tumors (tdTomato-transduced fluorescent tumor cells; red) and lungs, brain, kidneys, and liver (parenchymal structure in blue; scale bars: 100 μm) harvested from 4T1 tumor-bearing mice. (C) Interactions of platelets (white), endothelial cells (EC; broken lines), and leukocytes (green) in the peri- and intratumoral microvasculature of 4T1 tumors (blue) implanted into the auricle as assessed by in vivo microscopy, a representative image (scale bar: 10 μm) and quantitative data for the tumor microvasculature and the tumor-free auricular microvasculature are shown (mean ± SEM for n = 6 mice per group; ∗P < .05 vs control). (D) Proportion of neutrophils, monocytes, and lymphocytes bound to platelets of total neutrophils/monocytes/lymphocytes in the peripheral blood of mice 10 days after 4T1 tumor induction; quantitative data are shown (mean ± SEM for n = 10 mice per group; ∗P < .05 vs control). (E) Proportion of leukocytes bound to procoagulant platelets (phosphatidylserine+ GPIbβ+ cells) of leukocytes (CD45+ cells) bound to platelets (GPIbβ+ cells) accumulating in the intra- and peritumoral microvasculature, quantitative data and a representative in vivo microscopy image is shown (mean ± SEM for n = 3 mice per group).
Platelet trafficking in experimental breast cancer. (A) Systemic platelet count as well as a proportion of aged platelets of total platelets in the peripheral blood of tumor-free control or orthotopic 4T1 tumor-bearing mice as assessed by flow cytometry; quantitative data are shown (mean ± standard error of the mean [SEM] for n = 3-4 mice per group; ∗P < .05 vs control). (B) Representative confocal microscopy images of GPIbβ+ platelets (white) in the intra- and peritumoral microvasculature of 4T1 tumors (tdTomato-transduced fluorescent tumor cells; red) and lungs, brain, kidneys, and liver (parenchymal structure in blue; scale bars: 100 μm) harvested from 4T1 tumor-bearing mice. (C) Interactions of platelets (white), endothelial cells (EC; broken lines), and leukocytes (green) in the peri- and intratumoral microvasculature of 4T1 tumors (blue) implanted into the auricle as assessed by in vivo microscopy, a representative image (scale bar: 10 μm) and quantitative data for the tumor microvasculature and the tumor-free auricular microvasculature are shown (mean ± SEM for n = 6 mice per group; ∗P < .05 vs control). (D) Proportion of neutrophils, monocytes, and lymphocytes bound to platelets of total neutrophils/monocytes/lymphocytes in the peripheral blood of mice 10 days after 4T1 tumor induction; quantitative data are shown (mean ± SEM for n = 10 mice per group; ∗P < .05 vs control). (E) Proportion of leukocytes bound to procoagulant platelets (phosphatidylserine+ GPIbβ+ cells) of leukocytes (CD45+ cells) bound to platelets (GPIbβ+ cells) accumulating in the intra- and peritumoral microvasculature, quantitative data and a representative in vivo microscopy image is shown (mean ± SEM for n = 3 mice per group).
DAMPs promote platelet trafficking into the tumor microvasculature
Cell death occurs frequently in malignant tumors, leading to the release of damage-associated molecular patterns (DAMPs) (eg, nucleic acids, and ADP) into the extracellular space.42,43 Accordingly, in vivo propidium iodide staining in our heterotopic 4T1 breast cancer model demonstrated a broad deposition of extracellular nucleic acids in the tumor environment, as assessed by in vivo microscopy (Figure 2A). With respect to these observations, we hypothesized that nucleic acid-recognizing endosomal toll-like receptors (eTLR)-7, -8, and -9 initiate the trafficking of platelets and immune cells into malignant lesions. In line with this assumption, inhibition of these eTLRs by blocking oligonucleotides significantly decreased the recruitment of platelets (Figure 2B), neutrophils, cMOs, and CD4+ T cells (but not CTLs or B cells) into the tumor microvasculature as compared with control oligonucleotide-treated animals (Figure 2C-D). These immunomodulatory effects of the blocking oligonucleotides were associated with significantly reduced tumor size and weight (Figure 2E). Notably, exposure of TLR7, -8, or -9 agonists to 4T1 cancer cells did not alter 4T1 tumor cell proliferation in vitro (Figure 2F), collectively implying that tumor-released nucleic acids recruit platelets and immune cells into malignant lesions through eTLRs and subsequently support tumor progression. Importantly, ADP receptor blockade did not significantly alter procoagulant platelet (supplemental Figure 2A) or immune cell (supplemental Figure 2B) accumulation in the orthotopic TNBC model but decreased nonprocoagulant platelet accumulation (supplemental Figure 2A) and, partially, tumor growth (supplemental Figure 2C). Furthermore, the surface levels of sialic acid on platelets (which decrease with chronological platelet aging and modulate systemic immune responses)44 were not significantly altered upon ADP receptor blockade (supplemental Figure 2D), collectively pointing to nonimmunomodulatory protumorigenic effects of nonprocoagulant platelets.
Effects of tumor-released extracellular nucleic acids on platelet and immune cell trafficking in experimental breast cancer. (A) Extracellular nucleic acids (red) released into the environment of 4T1 tumors as assessed by propidium iodide staining and in vivo microscopy on day 10 after tumor induction, a representative image (scale bar: 100 μm) and quantitative data for the fluorescence intensity relative to the distance from the tumor core (dotted line) are shown (mean ± SEM). (B) EC interactions of platelets in the peri- and intratumoral microvasculature of 4T1 tumors implanted into the left auricle in mice treated with blocking eTLR oligonucleotides or control oligonucleotides and of the left auricle of tumor-free mice; quantitative data are shown (mean ± SEM for n = 7 mice per group; #P < .05 vs control ∗P < .05 vs control oligo). Infiltration by (C) neutrophils, cMOs, B cells, CD8+ T cells, and (D) CD4+ T-cell subsets as assessed by flow cytometry as well as (E) size and weight of orthotopic 4T1 tumors, quantitative data are shown (mean ± SEM for n = 5 mice per group; ∗P < .05 vs control oligo). (F) Proliferation of 4T1 tumor cells exposed to agonists of TLR-7, -8, or -9 as assessed by 3-(4,5-dimethyldiazol-2-yl)-2,5 diphenyl tetrazolium bromide assay and quantitative data are shown (mean ± SEM for n = 6 mice per group).
Effects of tumor-released extracellular nucleic acids on platelet and immune cell trafficking in experimental breast cancer. (A) Extracellular nucleic acids (red) released into the environment of 4T1 tumors as assessed by propidium iodide staining and in vivo microscopy on day 10 after tumor induction, a representative image (scale bar: 100 μm) and quantitative data for the fluorescence intensity relative to the distance from the tumor core (dotted line) are shown (mean ± SEM). (B) EC interactions of platelets in the peri- and intratumoral microvasculature of 4T1 tumors implanted into the left auricle in mice treated with blocking eTLR oligonucleotides or control oligonucleotides and of the left auricle of tumor-free mice; quantitative data are shown (mean ± SEM for n = 7 mice per group; #P < .05 vs control ∗P < .05 vs control oligo). Infiltration by (C) neutrophils, cMOs, B cells, CD8+ T cells, and (D) CD4+ T-cell subsets as assessed by flow cytometry as well as (E) size and weight of orthotopic 4T1 tumors, quantitative data are shown (mean ± SEM for n = 5 mice per group; ∗P < .05 vs control oligo). (F) Proliferation of 4T1 tumor cells exposed to agonists of TLR-7, -8, or -9 as assessed by 3-(4,5-dimethyldiazol-2-yl)-2,5 diphenyl tetrazolium bromide assay and quantitative data are shown (mean ± SEM for n = 6 mice per group).
Toward a more comprehensive mechanistic understanding of these eTLR-dependent processes, we used flow cytometry analyses in a mouse peritoneal assay and in vivo microscopy in the mouse cremaster muscle. Here, agonists of TLR7, -8, or -9 (but not TLR3) induced the trafficking of neutrophils, cMOs, and lymphocytes (supplemental Figure 3A) and platelets (supplemental Figure 3B) to the site of application compared with the controls. In vitro analyses further revealed that agonism of these eTLRs directly activates neutrophils and cMOs (indicated by increased surface expression of Mac-1; supplemental Figure 4A) and lymphocytes (indicated by L-selectin shedding; supplemental Figure 5) but did not directly activate platelets (indicated by unchanged surface expression of activated GPIIb/IIIa) or microvascular endothelial cells (indicated by unaltered ICAM-1, VCAM-1, E-selectin, and P-selectin surface expression). Instead, agonism of TLR7, -8, or -9 profoundly induced the production of the cytokine tumor necrosis factor in macrophages (supplemental Figure 4B), which, in turn, activated endothelial cells to induce the expression of adhesion molecules on their surface (supplemental Figure 4C-D). Accordingly, endothelial expression of these molecules in the microvasculature was more pronounced in the vicinity of perivascular macrophages (supplemental Figure 4E), collectively initiating endothelial interactions with platelets and immune cells. Thus, nucleic acids mediate platelet and immune cell responses via direct and indirect eTLR-dependent effects.
Platelets differentially regulate responses of distinct immune cell subsets
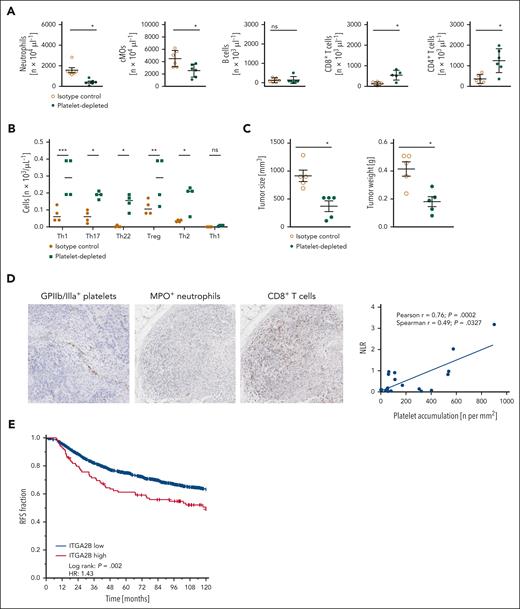
In our TNBC model, antibody-mediated depletion of neutrophils or cMOs attenuated tumor growth, whereas depletion of CTLs supported tumor progression (supplemental Figure 6). To characterize the functional relevance of platelets for responses of these immune cells in TNBC, we performed experiments in thrombocytopenic animals (supplemental Table 2A). Here, antibody-mediated depletion of platelets almost completely abolished the migration of protumorigenic neutrophils and cMOs into orthotopically grown 4T1 tumors, whereas the infiltration of antitumorigenic CTLs was significantly enhanced compared with isotype control antibody-treated tumor-bearing mice (Figure 3A). To a lesser extent, tumor infiltration by Th17 and Th22 cells, antitumorigenic Th9 cells, and protumorigenic Treg and Th2 cells, but not of B cells or antitumorigenic Th1 cells (Figure 3A-B), was intensified in thrombocytopenic animals. The resulting overall immunomodulatory effect of platelet depletion was associated with a significant reduction in tumor size and weight compared with isotype control antibody-treated controls (Figure 3C). Our findings were confirmed in the more reductionist peritoneal assay, as eTLR-dependent extravasation of neutrophils and cMOs to the peritoneal cavity was significantly attenuated upon platelet depletion, whereas responses of lymphocytes were significantly elevated (supplemental Figure 7A). Importantly, antibody-mediated depletion of neutrophils (supplemental Table 2B) significantly diminished the recruitment of cMOs into the peritoneal cavity but did not alter lymphocyte responses (supplemental Figure 7B). Thus, platelets differentially regulate innate and adaptive immune cell responses in the tumor microenvironment and (subsequent) tumor progression. Most interestingly, immunohistochemical analyses of human TNBC samples documented that intravascular accumulation of platelets positively correlated with high neutrophil-CTL ratios in the perivascular tumor immune cell infiltrate (Figure 3D), confirming our experimental data and translating them into human disease. Accordingly, the gene expression data from the METABRIC (Molecular Taxonomy of Breast Cancer International Consortium) breast cancer cohort (supplemental Figure 8A) indicated that recurrence free (Figure 3E) and overall (supplemental Figure 8B) survival of patients with breast cancer with high RNA expression of the platelet surrogate marker integrin αIIb (ITGA2B/CD41) in their tumors was significantly impaired compared to patients with low ITGA2B RNA levels. Interestingly, ITGA2Bhigh-expressing METABRIC cases were enriched with the ER+/HER2high-proliferating subtype and histological grade 3 (supplemental Figure 8A,C). At the molecular level, ITGA2B expression was positively correlated with the expression of gene sets involved in proliferation, DNA synthesis, and repair, and negatively correlated with gene sets associated with immune mechanisms and inflammation (supplemental Figure 8D). Notably, tumoral ITGA2B RNA expression did not positively correlate with the expression of the endothelial cell marker PECAM-1, whereas tumor RNA expression of PECAM-1 positively correlated with the expression of PD-L1, CD80, CD86, CD40, and CD40L (supplemental Figure 8E), collectively suggesting that higher expression levels of ITGA2B do not simply reflect higher tumor vascularization.
Effects of platelets on immune cell trafficking in breast cancer. (A) Infiltration by neutrophils, cMOs, B cells, CD8+ T cells, CD4+ T cells, and (B) CD4+ T-cell subsets as assessed by flow cytometry, as well as (C) size and weight of orthotopic 4T1 tumors in mice receiving platelet depleting or isotype control antibodies; quantitative data are shown (mean ± SEM for n = 4-6 mice per group; ∗P < .05/∗∗P < .01/∗∗∗P < .001 vs isotype control; ns, not significant). (D) Correlation between the ratio of extravascular MPO+ neutrophils and CD8+ T cells (NLR, neutrophil/lymphocyte ratio) and intravascularly accumulated integrin β3+ platelets, as assessed by immunohistochemical analyses of human TNBC samples; representative images and quantitative data are shown. (E) Recurrence-free survival of patients with breast cancer from the METABRIC breast cancer cohort exhibiting ITGA2Blow and ITGA2Bhigh expression levels (z-value ≥1.5 served as the cutoff).
Effects of platelets on immune cell trafficking in breast cancer. (A) Infiltration by neutrophils, cMOs, B cells, CD8+ T cells, CD4+ T cells, and (B) CD4+ T-cell subsets as assessed by flow cytometry, as well as (C) size and weight of orthotopic 4T1 tumors in mice receiving platelet depleting or isotype control antibodies; quantitative data are shown (mean ± SEM for n = 4-6 mice per group; ∗P < .05/∗∗P < .01/∗∗∗P < .001 vs isotype control; ns, not significant). (D) Correlation between the ratio of extravascular MPO+ neutrophils and CD8+ T cells (NLR, neutrophil/lymphocyte ratio) and intravascularly accumulated integrin β3+ platelets, as assessed by immunohistochemical analyses of human TNBC samples; representative images and quantitative data are shown. (E) Recurrence-free survival of patients with breast cancer from the METABRIC breast cancer cohort exhibiting ITGA2Blow and ITGA2Bhigh expression levels (z-value ≥1.5 served as the cutoff).
Procoagulant platelets deliver IC molecules into tumors
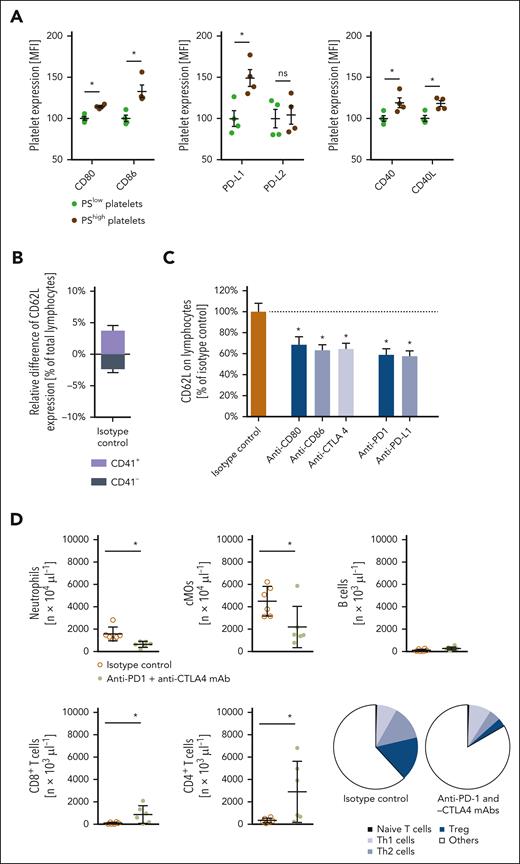
Recently, IC molecules were detected on the surface of platelets.39-41 Here, we show that particularly procoagulant platelets bear large amounts of the inhibitory IC molecules PD-L1, CD80, and CD86 (but not of PD-L2) as well as of the stimulatory IC molecules CD40 and CD40L on their surface as compared with nonprocoagulant platelets or immune cells isolated from the peripheral blood of tumor-bearing mice (Figure 4A; supplemental Figure 9). Similar results were obtained in primary human platelets (supplemental Figure 10). Confocal microscopy further documented that platelets in the aberrant microvasculature of orthotopically raised 4T1 tumors colocalized with exposed collagen (supplemental Figure 11A). Interestingly, collagen-stimulated procoagulant activation of platelets was associated in vitro, particularly with high surface expression of PD-L1, which was mediated by the platelet receptors GPVI and GPIIb/IIIa as well as through cyclophilin D- and scramblase transmembrane protein 16F-dependent pathways (supplemental Figure 11B-C). Accordingly, the blockade of GPVI and, to a lesser extent, GPIIb/IIIa significantly attenuated the accumulation of procoagulant platelets in 4T1 tumors (supplemental Figure 11D). In addition, blockade of GPVI or GPIIb/IIIa diminished intratumoral numbers of nonprocoagulant platelets and myeloid leukocytes while increasing intratumoral lymphocyte responses (supplemental Figure 11D-E). As a consequence, tumor growth was strongly impaired (supplemental Figure 11F).
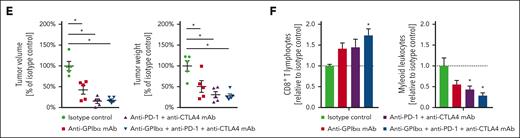
Synergistic effects of antiplatelet therapy and IC inhibition in experimental breast cancer. (A) Surface expression of CD80, CD86, PD-L1, PD-L2, CD40, and CD40L on phosphatidylserinelow or phosphatidylserinehigh platelets from the peripheral blood of mice; quantitative data are shown (mean ± SEM for n = 3 mice per group; ∗P < .05 vs phosphatidylserinelow). (B) Activation status of platelet-bound (CD41+) or -unbound (CD41−) lymphocytes isolated from orthotopically raised 4T1 tumors as assessed by L-selectin/CD62L surface expression by flow cytometry, and quantitative data are shown (mean ± SEM for n = 3 mice per group). (C) Activation status of platelet-bound (CD41+) lymphocytes, as assessed by L-selectin/CD62L surface expression in flow cytometry upon antibody blockade of CD80, CD86, CTLA4, PD-L1, or PD-1, quantitative data are shown (mean ± SEM for n = 3 mice per group). (D) Infiltration by neutrophils, cMOs, B cells, CD8+ T cells, and CD4+ T-cell subsets of orthotopic 4T1 tumors in mice receiving anti-PD-1 and -CTLA4 monoclonal antibodies (mAbs) or isotype control antibodies as assessed by flow cytometry; quantitative data are shown (mean ± SEM for n = 5 mice per group; ∗P < .05 vs isotype control). (E) Relative volume and weight of tumors as well as (F) relative infiltration by lymphocytes and myeloid leukocytes of orthotopically grown 4T1 tumors in mice receiving anti-GPIbα mAbs, and/or anti–PD-1 and -CTLA4 mAbs, or isotype control antibodies; quantitative data are shown (mean ± SEM for n = 5 mice per group; ∗P < .05 vs isotype control).
Synergistic effects of antiplatelet therapy and IC inhibition in experimental breast cancer. (A) Surface expression of CD80, CD86, PD-L1, PD-L2, CD40, and CD40L on phosphatidylserinelow or phosphatidylserinehigh platelets from the peripheral blood of mice; quantitative data are shown (mean ± SEM for n = 3 mice per group; ∗P < .05 vs phosphatidylserinelow). (B) Activation status of platelet-bound (CD41+) or -unbound (CD41−) lymphocytes isolated from orthotopically raised 4T1 tumors as assessed by L-selectin/CD62L surface expression by flow cytometry, and quantitative data are shown (mean ± SEM for n = 3 mice per group). (C) Activation status of platelet-bound (CD41+) lymphocytes, as assessed by L-selectin/CD62L surface expression in flow cytometry upon antibody blockade of CD80, CD86, CTLA4, PD-L1, or PD-1, quantitative data are shown (mean ± SEM for n = 3 mice per group). (D) Infiltration by neutrophils, cMOs, B cells, CD8+ T cells, and CD4+ T-cell subsets of orthotopic 4T1 tumors in mice receiving anti-PD-1 and -CTLA4 monoclonal antibodies (mAbs) or isotype control antibodies as assessed by flow cytometry; quantitative data are shown (mean ± SEM for n = 5 mice per group; ∗P < .05 vs isotype control). (E) Relative volume and weight of tumors as well as (F) relative infiltration by lymphocytes and myeloid leukocytes of orthotopically grown 4T1 tumors in mice receiving anti-GPIbα mAbs, and/or anti–PD-1 and -CTLA4 mAbs, or isotype control antibodies; quantitative data are shown (mean ± SEM for n = 5 mice per group; ∗P < .05 vs isotype control).
Furthermore, procoagulant platelets exhibited higher expression levels of the immunomodulatory cytokines CCL5 and transforming growth factor-β than nonprocoagulant platelets (supplemental Figure 12). The binding of platelets to T lymphocytes significantly decreased the activation status of these immune cells (indicated by elevated surface expression of the activation marker L-selectin (Figure 4B), as well as by reduced surface expression of the activation markers CD25 and CD69, cytokine interferon-gamma, promigratory integrin CD49d, and proliferation marker Ki-67 (supplemental Figure 13). Antibody blockade of the inhibitory IC molecule receptors PD-1 and CTLA-4 consequently attenuated these inhibitory effects on platelets (Figure 4C; supplemental Figure 13), suggesting that platelet-dependent, PD-1/CTLA-4 ligand-mediated inhibition of T lymphocyte activation compromises diverse T lymphocyte functions. Concomitant interference with the PD-L1 receptor PD-1 and the CD80/CD86 receptor CTLA-4 accordingly increased eTLR-dependent trafficking of CTLs to the level of platelet-depleted animals in the peritoneal assay, whereas blockade of the CD40L receptor CD40 expectedly attenuated the responses of myeloid leukocytes and lymphocytes (supplemental Figure 14). Moreover, concomitant inhibition of PD-1 and CTLA-4 significantly enhanced tumor infiltration by antitumorigenic CTLs and Th1 cells and reduced (protumorigenic) myeloid leukocyte responses as well as tumor growth in the orthotopic 4T1 breast cancer model compared with isotype control antibody-treated tumor-bearing animals (Figure 4D-E). In line with the low IC molecule expression in poorly immunogenic 4T1 tumor cells, combined PD-1 and CTLA-4 inhibition achieved comparable immunomodulatory and tumor-suppressing effects as antibody-mediated platelet depletion (Figures 4E-F and 3A-B). Importantly, upon platelet depletion, the remaining viable tumor cells exhibited a slightly enhanced proliferation status (indicated by the average Ki-67 expression; supplemental Figure 15A) as compared with control animals (which might be due to the survival of the “fittest,” most proliferative cells), but unaltered epithelial-mesenchymal transition status (measured by epithelial cellular adhesion molecule expression; supplemental Figure 15A) or, in line with the lack of IC molecule expression in human TNBC cells, tumor cell expression of IC molecules supplemental Figure 15B), as well as the vascularization of the tumors (supplemental Figure 15C), as identified in orthotopic and heterotopic 4T1 tumor models. Thus, our experimental data suggest that procoagulant platelets primarily modulate immune cell responses by delivering IC molecules to the tumor microvasculature. As a consequence, numbers of viable tumor cells were significantly decreased in platelet-depleted animals (Figure 4E; supplemental Figure 15).
GPIbα blockade and PD-1/CTLA-4 inhibition exhibit similar effects in experimental TNBC
Interestingly, because of intratumoral procoagulant platelet activation (supplemental Figure 11), the proportion of procoagulant platelets among the total platelets bound to lymphocytes was significantly higher in 4T1 tumors than in peripheral blood (supplemental Figure 16A). Toward a translational perspective, we therefore tested different platelet molecules for their ability to interfere with the formation of platelet-lymphocyte complexes in the systemic circulation before procoagulant platelet activation in the tumor. Using blocking antibodies and inhibitors, we showed that the platelet receptor GPIbα, but not GPVI, GPIX, GPIIbIIIa, ICAM-2, P-selectin, PSGL-1, P2Y12, or the enzyme cyclooxygenase, facilitates the binding of platelets to circulating lymphocytes (supplemental Figure 16B). Confocal microscopy analyses further indicate that platelet GPIbα interacts with von Willebrand factor-decorated CD3+ T cells (supplemental Figure 16C-D). Accordingly, a priori blockade of GPIbα showed similar immunomodulatory and tumor-suppressing effects as combined PD-1- and CTLA-4 inhibition in the orthotopic TNBC model (Figure 4E-F). Importantly, blocking or platelet-depleting antibodies efficiently bound to platelets but not to 4T1 tumor cells (supplemental Figure 16E). Most interestingly, triple blockade of GPIbα, PD-1, and CTLA-4 did not significantly enhance tumor infiltration by antitumorigenic CTLs or further attenuate (protumorigenic) myeloid leukocyte responses and suppression of tumor growth as compared with isolated ICI treatment. This lack of synergistic effects of ICI and GPIbα blockade in the 4T1 tumor model, in which ligands of PD-1 and CTLA-4 are predominantly expressed by platelets, suggests that platelets may promote tumor progression in this experimental model mainly via their immunomodulatory properties. From a therapeutic perspective, the treatment of existing tumors with anti-GPIbα or anti-PD-1/CTLA-4 antibodies significantly reduced tumor progression (supplemental Figure 17A) and enhanced tumoral CTL accumulation without affecting the presence of myeloid leukocytes in the tumors (supplemental Figure 17B), which might be due to the dominance of intravascular lymphocyte responses in established tumors (supplemental Figure 1A). Similar results were obtained in an orthotopic mouse model of poorly immunogenic head and neck squamous cell carcinoma (supplemental Figure 18), suggesting platelet–mediated IC molecule delivery to malignant tumors as a more general pathological mechanism. Moreover, despite the low number of platelets in the peripheral organs of orthotopically 4T1 tumor-bearing mice (Figure 1B; supplemental Table 1B), procoagulant platelet activation was also detected at sites of tumor metastases in bone marrow (7.9% ± 0.2% of total platelets), liver (11.8% ± 0.8% of total platelets), and lungs (12.5% ± 0.6% of total platelets). Platelet depletion critically modulated neutrophil (but not cMO, CD4+ T cell, CTL, and B cell) accumulation (supplemental Figure 19A) and (subsequently) compromised metastatic seeding of breast cancer cells (supplemental Figure 19B), further indicating that platelets exhibit common and distinct immunoregulatory effects in primary tumors and at sites of tumor metastasis. This might be explained by the contribution of tissue-specific properties to the control of immune cell responses (eg, the presence of DAMPs and inflammatory cytokines in tumors, as opposed to homeostatic cytokines in peripheral tissues during initial metastatic stages).
Discussion
Platelets substantially contribute to the pathogenesis of malignant tumors. Their systemic trafficking dynamics in cancer, however, remain obscure. In this study, we showed that the circulatory time of platelets in peripheral blood decreased in the early stages of experimental TNBC, despite (reactively) enhanced bone marrow megakaryocytopoiesis and stable splenic platelet clearance, because these cellular blood components massively accumulate in the aberrant tumor microvasculature. Here, platelets heavily interacted with neutrophils, cMOs, lymphocytes, and endothelial cells, resembling platelet-immune cell interactions in inflamed tissue.32-34 Most interestingly, the majority of these interactive platelets in the tumor environment exhibited a highly reactive “procoagulant” phenotype, which shares distinct phenotypic properties with the hyperreactive young, reticulated subset of platelets45 and is triggered by exposure to subendothelial collagen in defective vessels.29-31 Hence, platelets instantly traffick to neoplastic lesions in early TNBC in which they undergo procoagulant activation. At later stages of the disease, reactive thrombocytopoiesis might further intensify tumoral platelet responses and even overcompensate for the initial decline of circulating platelets, ultimately leading to clinically reported thrombocytosis and thrombotic events in advanced stages of breast cancer.46-48
DAMPs are host biomolecules released in a variety of pathologies,49 including cancer, as a consequence of inflammation, cell death, and tissue destruction.42,43 Upon recognition of these specific molecular signals by eTLRs, distinct inflammatory programs are initiated, mediating the activation of cells.35 In our experiments, we expectedly detected an enormous accumulation of extracellular nucleic acids in the tumor environment. These tumor-released DAMPs (but not ADP, which is also known to be liberated by necrotic cells) attract platelets and immune cells into the tumor environment by instructing perivascular macrophages through eTLRs to induce the expression of distinct adhesion molecules on microvascular endothelial cells capable of recruiting and activating circulating platelets and immune cells.50 Notably, previous experimental studies have suggested that the activation of endosomal TLR7 and/or TLR8 primarily stimulates antitumorigenic immune responses.51 In clinical trials, however, eTLR agonists showed beneficial effects only in precancerous skin lesions but did not improve the survival of patients with (head and neck) cancer.51 This lack of antitumorigenic effects of eTLR agonism might be due to the concomitant induction of eTLR-dependent protumorigenic platelet responses in invasive tumors.
Under inflammatory conditions, platelets are well-known to promote the migration of immune cells to their target destinations via diverse molecular interactions.32-38 The role of these anucleate cell particles in the recruitment of immune cells to malignant tumors is still unclear. Here, we unveil a previously unrecognized immunomodulatory function of platelets that concurrently promotes the trafficking of innate immune cells into neoplastic lesions while inhibiting the responses of adaptive immune cells, independent of their effects on tumor cell proliferation, epithelial-mesenchymal transition, and angiogenesis. Most importantly, analyses of human TNBC samples clearly confirmed our experimental results, as the presence of platelets in tumors was associated with a protumorigenic perivascular immune cell milieu. Accordingly, transcriptomic data from the METABRIC cohort document particularly impaired survival of individuals with high tumoral expression levels of the platelet surrogate marker ITGA2B, presumably originating from a more aggressive subtype as indicated by enrichment of histological grade 3, the ER+/HER2high-proliferating subtype, and gene sets associated with proliferation, DNA synthesis, and repair. Furthermore, the enrichment of ITGA2B across all tumor stages and different breast cancer subtypes point to a more general role of platelets in this tumor entity.
IC molecules control immune cell responses under homeostatic and pathological conditions.52 Whereas stimulatory ICs enhance immune reactions,34 inhibitory ICs dampen the immune cell activity. In cancer, particularly interactions of inhibitory PD-1 and PD-L1, as well as of CTLA-4 and its ligands CD80 and CD86, drive adaptive immune cell evasion.52 Extending previous observations,39-41 we here demonstrate that primarily procoagulant platelets expose large amounts of these IC molecules on their surface as compared with nonprocoagulant platelets or other blood cells, which is mediated via GPVI, GPIIb/IIIa, cyclophilin D, and transmembrane protein 16F. Accordingly, platelets attenuate the activity of antitumorigenic T cells and promote neutrophil responses (which are protumorigenic in experimental TNBC)53,54 in malignant lesions by using these immunomodulatory factors, ultimately fueling tumor growth. Opposite lymphocyte-supporting effects of platelets observed in viral infection36-38 might be explained by context-specific differences in the models used.
Most interestingly, we here demonstrate that platelet depletion achieved similar immunomodulatory and tumor-suppressing effects as compared with combined PD-1 and CTLA-4 inhibition in our model of TNBC. Consequently, targeting interactions between platelets and immune cells might represent a promising treatment strategy for TNBC without the side effects of systemic ICI. In line with this assumption, we showed that blockade of the platelet receptor GPIbα nearly reached the immunomodulatory effects of platelet depletion or combined PD-1 and CTLA-4 inhibition in experimental TNBC. This approach might be particularly beneficial in individual tumors lacking tumor cell expression of IC molecules,40 in which platelets primarily deliver these molecular factors to malignant lesions, and in paraneoplastic thrombocytosis55 as GPIba additionally supports thrombopoietin–dependent platelet production.56 Of note, antiplatelet strategies might enhance the risk of hemorrhage, albeit they do not exhibit the severe or life-threatening autoimmune-related adverse effects observed in ICI-treated patients.4 Although hereditary GPIba or von Willebrand factor defects associate with severe bleeding episodes,57,58 pharmacological GPIbα blockade might be safe as it does not increase the risk of intracranial hemorrhage in experimental cerebral ischemia,52,59 in contrast to conventional antiplatelet drugs and besides targeting procoagulant platelet activation via GPVI, cyclophilin D, or transmembrane protein 16F.
In conclusion, our findings uncover a previously unrecognized immunomodulatory activity of procoagulant platelets in the tumor microvasculature that concurrently promotes protumorigenic myeloid immune cell responses and impedes antitumorigenic T-cell activity, thus supporting TNBC progression. Targeting this self-sustaining mechanism of such malignant neoplasms effectively interferes with their expansion, hence providing a novel strategy to counteract immune evasion in TNBC without the side effects of systemic ICI.
Acknowledgments
The authors thank Claudia Fahney for excellent technical assistance.
This study was supported by Deutsche Forschungsgemeinschaft, Sonderforschungsbereich 914, projects B03 (C.A.R.) and B06 (K.L.), and Fritz-Bender-Stiftung (C.A.R.; B.U.).
Data presented in this article are part of the doctoral thesis of J.B.S. and B.S.
Authorship
Contribution: J.B.S., F.H., B.S., Z.W., A.U., K.S., S.B., S.M., J.L., V.S., J.H., M.C., L.A.M., C.B., G.Z., and M.M. performed the experiments and contributed to data analysis and interpretation; R.K., L.N., W.W., K.L., B.U., and C.A.R. wrote the manuscript; C.A.R. conceived and supervised the study; and all authors read and approved the manuscript.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Christoph A. Reichel, Walter Brendel Centre of Experimental Medicine, Department of Otorhinolaryngology, and Comprehensive Cancer Center, Munich Ludwig-Maximilians-Universität Hospital, Marchioninistr 15, D-81377 Munich, Germany; email: christoph.reichel@med.uni-muenchen.de.
References
Author notes
J.B.S., B.S., and F.H. are joint first authors.
B.U. and C.A.R. are joint senior authors.
Original data are available upon reasonable request from the corresponding author, Christoph A. Reichel (christoph.reichel@med.uni-muenchen.de).
The online version of this article contains a data supplement.
There is a Blood Commentary on this article in this issue.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.

![Platelet trafficking in experimental breast cancer. (A) Systemic platelet count as well as a proportion of aged platelets of total platelets in the peripheral blood of tumor-free control or orthotopic 4T1 tumor-bearing mice as assessed by flow cytometry; quantitative data are shown (mean ± standard error of the mean [SEM] for n = 3-4 mice per group; ∗P < .05 vs control). (B) Representative confocal microscopy images of GPIbβ+ platelets (white) in the intra- and peritumoral microvasculature of 4T1 tumors (tdTomato-transduced fluorescent tumor cells; red) and lungs, brain, kidneys, and liver (parenchymal structure in blue; scale bars: 100 μm) harvested from 4T1 tumor-bearing mice. (C) Interactions of platelets (white), endothelial cells (EC; broken lines), and leukocytes (green) in the peri- and intratumoral microvasculature of 4T1 tumors (blue) implanted into the auricle as assessed by in vivo microscopy, a representative image (scale bar: 10 μm) and quantitative data for the tumor microvasculature and the tumor-free auricular microvasculature are shown (mean ± SEM for n = 6 mice per group; ∗P < .05 vs control). (D) Proportion of neutrophils, monocytes, and lymphocytes bound to platelets of total neutrophils/monocytes/lymphocytes in the peripheral blood of mice 10 days after 4T1 tumor induction; quantitative data are shown (mean ± SEM for n = 10 mice per group; ∗P < .05 vs control). (E) Proportion of leukocytes bound to procoagulant platelets (phosphatidylserine+ GPIbβ+ cells) of leukocytes (CD45+ cells) bound to platelets (GPIbβ+ cells) accumulating in the intra- and peritumoral microvasculature, quantitative data and a representative in vivo microscopy image is shown (mean ± SEM for n = 3 mice per group).](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/144/2/10.1182_blood.2023022928/2/m_blood_bld-2023-022928-gr1b.jpeg?Expires=1765905041&Signature=WPaR6Do4JikWBp19n24f3Hn6JAmBEpKz7l25Pa8Q5Fbf6yErsmD1Ff5lfu9MMgvh2FeOPbFPfjtJ~nTFxaRZqy-Dgft2A0GI-85XrDNdAUfW9ehei6w1tgdKbzzaY5NM-tTBjNuhhigDsUtB1vP02ErrQYZ~Mj7AxHUGoOeeWFQxG4itHlQxFp-MAUWx9MkaNAAaT4t940c9RYnz2TBsZt4e6vWU4OgDwzWqMZFjj05ywh7ibZ~GQAGbb4eqxbaeIXoGbhGoio~ikYv79zl-ldtklr5QIubFwn3MTiqyKyZd2NOU~VIrMZ1r0c28CY3hS5XGgbCzBCCfvtFYX2UE9w__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal