Although it has been known that platelets have a role beyond hemostasis in orchestrating malignancy, the extent of their control of the tumor microenvironment has remained elusive. In this issue of Blood, Schaubaecher et al have identified a subpopulation of platelets supporting tumor progression by delivering immune checkpoint (IC) molecules and redirecting immune responses in triple negative breast cancer (TNBC).1 Utilizing mouse models of breast cancer, the authors have defined an elaborate system of communication between tumor cells, immune cells, and platelets (see figure).
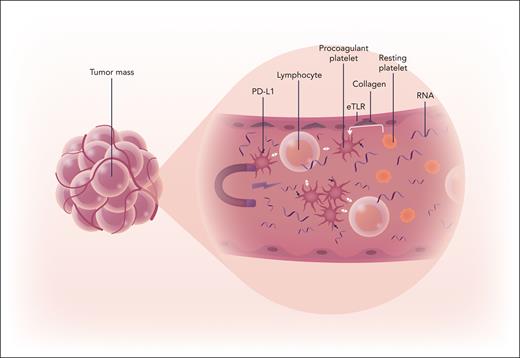
Platelets are attracted into the tumor vasculature by nucleic acids (RNA), where they become activated to procoagulant platelets, which deliver IC molecules and recruit immune cells to promote tumor growth. Professional illustration by Somersault18:24.
Platelets are attracted into the tumor vasculature by nucleic acids (RNA), where they become activated to procoagulant platelets, which deliver IC molecules and recruit immune cells to promote tumor growth. Professional illustration by Somersault18:24.
Nucleic acids released from the tumor interact with macrophage in conjunction with endosomal toll-like receptors (eTLRs) to induce adhesion molecules on the endothelial surface, which attract the immune cells and platelets. Upon recruitment the platelets are converted to a subpopulation of procoagulant platelets with a protumorigenic effect. Importantly, these procoagulant platelets are enriched with inhibitory IC molecules such as programmed death ligand-1 not only directing immune modulation but also regulating neutrophil responses to ultimately fuel tumor progression. In an effort to translate their findings to the clinic, the authors demonstrated that the platelet receptor glycosylphosphatidylinositol bα (GPIbα) orchestrated the binding of the procoagulant platelets to surrounding lymphocytes and that blockade of GPIbα conferred effective tumor suppression comparable to combined programmed death receptor 1 (PD-1) and cytotoxic T lymphocyte-associated protein-4 (CTLA-4) inhibition in their mouse model of TNBC. Their findings shed a new light on the role of platelets in malignancy, demonstrating that not all platelet populations contribute equally to the tumor immune environment.
Recent advances in platelet biology have identified the diversity in platelet subtypes and demonstrated their role in disease.2 A variety of different types of platelets have been classified based on platelet size, RNA content, and receptor surface expression. Procoagulant platelets arise when negatively charged phospholipids including phosphatidylserine become exposed on the platelet surface in response to subendothelial collagen. A recent consensus committee of the International Society of Thrombosis and Hemostasis established that procoagulant platelets require the presence of 3 surface markers including annexin V, P-selectin, and either GPIX or GPIIb.3 Previously this subpopulation of platelets has been demonstrated to have a role in clot stability via generation of thrombin and fibrin. In their article, the authors identify that the tumor vasculature is highly enriched for procoagulant platelets.
Demonstrating their distinct physiological role, procoagulant platelets have a correlative function in thrombotic disease, with increased levels of this subspecialized platelet associated with transient ischemic attack, stroke, and heparin-induced thrombocytopenia.4-6 Given that malignancy leads to hypercoagulability, understanding if cancer leads to increased numbers of procoagulant platelets locally or if there is also a systemic increase in this subpopulation of platelets could be of prognostic value in patients with malignancy.
Although some studies have suggested antiplatelet agents can disrupt platelet and tumor cell interactions in breast cancer, not all studies have shown benefit of platelet blockade in cancer patients.7 Given that it has been shown that patients utilizing aspirin and P2Y12 blockade still have full procoagulant platelet function, understanding heterogeneity of platelet subtypes may lead to novel approaches to platelet blockade.8 What remains to be determined, however, is if targeting specific platelet subpopulations can improve outcomes in malignancy. In their article, the authors described utilizing platelet receptor inhibition to deter procoagulant platelets within the tumor microenvironment. They demonstrated that blockade of the surface receptor GPIbα was as effective as combined PD-1 and CTLA-4 inhibition. Given its essential role in primary hemostasis, pharmacological blockade of GPIbα is not without hemorrhagic risk. Initial studies of this class of antiplatelet agents, however, have shown efficacy in blocking platelet function without increased risk of bleeding.9 What remains to be defined is if there is value of augmenting current cancer treatments with GPIbα blockade or if there is a subgroup of patients with systemically more highly activated or increased number of platelets that would benefit from antiplatelet agents alone or in combination with known IC modulators.
Beyond platelet receptor inhibition, other therapies targeting procoagulant platelets have shown promise as antithrombotic agents. Carbonic anhydrase inhibitors, which are used clinically as a mild diuretic, also have been shown to suppress the procoagulant platelet by attenuating cell membrane electrolyte transport while still maintaining platelet secretion properties.10 Incorporating these novel mechanisms of platelet blockade into the cancer armamentarium to target the procoagulant platelet subpopulation may represent an area of drug discovery for malignancy.
Although much remains to be learned about the value of subpopulation of platelets, the article by Schaubaecher and colleagues defines a novel role for procoagulant platelets as central to tumor immune modulation. What remains to be understood is the applicability of these findings to other types of malignancy, the prognostic value of identifying platelet subpopulations in cancer patients, and whether targeting this platelet subpopulation has clinical utility to improve outcomes in patients with malignancy.
Conflict-of-interest disclosure: The author declares no competing financial interests.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal