Key Points
A novel form of X::RAR::X– or X::RAR::Y–type tripartite fusion was identified in certain RARA- and all RARG-related atypical APL.
Tripartite fusion leads to LBD-H11_12/H12 truncation of the chimeric RAR protein, rendering ATRA unresponsiveness in atypical APL.
Visual Abstract
Atypical acute promyelocytic leukemia (aAPL) presents a complex landscape of retinoic acid receptor (RAR) fusion genes beyond the well-known PML::RARA fusion. Among these, 31 individually rare RARA and RARG fusion genes have been documented, often reported in the canonical X::RAR bipartite fusion form. Intriguingly, some artificially mimicked bipartite X::RAR fusions respond well to all-trans retinoic acid (ATRA) in vitro, contrasting with the ATRA resistance observed in patients. To unravel the underlying mechanisms, we conducted a comprehensive molecular investigation into the fusion transcripts in 27 RARA fusion gene–positive aAPL (RARA-aAPL) and 21 RARG-aAPL cases. Our analysis revealed an unexpected novel form of X::RAR::X– or X::RAR::Y–type tripartite fusions in certain RARA-aAPL and all RARG-aAPL cases, with shared features and notable differences between these 2 disease subgroups. In RARA-aAPL cases, the occurrence of RARA 3′ splices was associated with their 5′ fusion partner genes, mapping across the coding region of helix 11_12 (H11_12) within the ligand-binding domain (LBD), resulting in LBD-H12 or H11_12 truncation. In RARG-aAPL cases, RARG 3′ splices were consistently localized to the terminus of exon 9, leading to LBD-H11_12 truncation. Significant differences were also observed between RARA and RARG 5′ splice patterns. Our analysis also revealed extensive involvement of transposable elements in constructing RARA and RARG 3′ fusions, suggesting transposition mechanisms for fusion gene ontogeny. Both protein structural analysis and experimental results highlighted the pivotal role of LBD-H11_12/H12 truncation in driving ATRA unresponsiveness and leukemogenesis in tripartite fusion–positive aAPL, through a protein allosteric dysfunction mechanism.
Introduction
All-trans retinoic acid (ATRA) combined with arsenic has proven to be a highly effective treatment for acute promyelocytic leukemia (APL) characterized by the canonical PML::RARA fusion gene.1,2 However, a subset of atypical APL (aAPL) cases lack the PML::RARA fusion and instead presents with diverse and individually rare fusion genes involving retinoic acid receptor (RAR) genes, including RARA, RARB, and RARG.3-5 Literature has documented 23 fusion partners beyond PML in >100 RARA fusion gene–positive aAPL (RARA-aAPL) cases (supplemental Table 1, available on the Blood website), including the recently identified open reading frame 2 (ORF2) of torque teno mini virus (TTMV).3-29 Similarly, studies have also reported 8 fusion partners in >30 RARG-aAPL cases (supplemental Table 2).30-54
Recent increases in the reporting of RARA- and RARG-aAPL cases are attributed to the widespread use of whole-transcriptome sequencing (WTS) and whole-genome sequencing (WGS). Previous studies have primarily focused on identifying the 5′ partner gene of RARs, describing these fusions in the canonical X::RAR (X represents the partner gene) bipartite form. Although expanding the list of RARs 5′ fusion partners advances our understanding of aAPL pathogenesis, significant questions remain. For instance, the ATRA sensitivity of RARA fusion genes varies with its 5′ partner gene, but all evaluable patients with RARG fusion genes are resistant to ATRA, and the mechanism remains undefined. Additionally, why are these fusions individually rare? More intriguingly, cells and mouse models with predicted NUP98::RARG, PML::RARG, STAT3::RARA, and STAT5B::RARA bipartite fusions respond well to ATRA,14,55-57 contrasting with the patient resistance, suggesting intricate yet undiscovered mechanisms. This study aims to elucidate the shared and pivotal etiologies driven by a novel form of tripartite fusion and ligand-binding domain (LBD) truncation in all RARG-aAPL and certain RARA-aAPL cases, providing explanations for these puzzles.
Methods
Patients
This study enrolled 27 RARA-aAPL and 21 RARG-aAPL cases (Table 1) with accessible WTS data, WGS data, or archived samples. Although 17 RARA-aAPL and 14 RARG-aAPL cases were previously reported individually, their genomic data or archived samples were retrieved and reanalyzed. WTS data of 42 PML::RARA-APL cases and 50 healthy donors from a previously reported acute leukemia cohort58 were also included for comparison. This study was approved by the ethics committees of Hebei Yanda Lu Daopei Hospital and conducted in accordance with the Declaration of Helsinki.
Demographic characteristics, genomic data, and gene fusion information of RARA-aAPL cases
| UPN . | Sex . | Age (y) . | Methods . | 5′ fusion partner . | RARA . | 3′ fusion partner . | Reference . |
|---|---|---|---|---|---|---|---|
| A1∗ | Female | 35 | WTS; WGS; OGM | STAT3 exon 1_21 | RARA exon 3_9@c.1225 | LTR40a | |
| A2 | Female | 45 | WGS | STAT3 exon 1_21 | RARA exon 3_9@c.1215 | L1MC4 | |
| A3 | Male | 24 | WGS | STAT3 exon 1_21 | RARA exon 3_9@c.1205 | AluSp | 14 |
| A4 | Male | 26 | WTS; WGS | STAT3 exon 1_23 | RARA exon 3_9@c.1203 | MIRb | 14 |
| A5 | Male | 50 | WTS | STAT5B exon 1_15 | RARA exon 3_9@c.1239 | AluJo | |
| A6 | Male | 46 | WTS; WGS | STAT5B exon 1_15 | RARA exon 3_9@c.1227 | HUMUT5218 | |
| A7 | Male | 47 | NGS-targeted | STAT5B exon 1_15 | RARA exon 3_9@c.1224 | MIRb-AluSc | 16 |
| A8 | Male | 32 | WGS | STAT5B exon 1_15 | RARA exon 3_9@c.1176 | AluSx4 | |
| A9 | Male | 34 | WTS | THRAP3 exon 1_2 | RARA exon 3_9@c.1238 | THRAP3 exon 6_12 | 25 |
| A10 | Female | 29 | WTS | HNRNPC exon 1_3 | RARA 3_8 | HNRNPCP2 | 20 |
| A11 | Male | 41 | WTS | HNRNPC exon 1_3 | RARA 3_8 | HNRNPC exon 4_9 | 21 |
| A12 | Female | 4 | WTS | FIP1L1 exon 1_13 | RARA exon 3_9 | None | 19 |
| A13 | Male | 31 | WTS | GTF2I exon 1_19 | RARA exon 3_9 | None | |
| A14 | Female | 51 | WTS | IRF2BP1 exon 1@c.322 | RARA exon 3_9 | None | 7 |
| A15 | Male | 66 | WGS | IRF2BP2 exon 1@c.1000 | RARA exon 3_9 | None | 23 |
| A16 | Male | 60 | WTS | IRF2BP2 exon 1@c.1000 | RARA exon 3_9 | None | 24 |
| A17 | Male | 60 | WTS | TNRC18 exon 1_5 | RARA exon 3_9 | None | |
| A18 | Male | 50 | WTS | TNRC18 exon 1_5 | RARA exon 3_9 | None | 26 |
| A19 | Female | 6 | WTS | TTMV-ORF2 c.1_209 | RARA exon 3_9 | None | 9 |
| A20 | Male | 3 | WTS | TTMV-ORF2 c.1_328 | RARA exon 3_9 | None | 9 |
| A21 | Male | 3 | WTS; WGS | TTMV-ORF2 c.1_215 | RARA exon 3_9 | None | 10 |
| A22 | Female | 11 | WTS; WGS | TTMV-ORF2 c.1_413 | RARA exon 3_9 | None | 11 |
| A23 | Male | 7 | WTS; WGS | TTMV-ORF2 c.1_217 | RARA exon 3_9 | None | 13 |
| A24 | Male | 35 | WTS | TTMV-ORF2 c.1_189 | RARA exon 3_9 | None | |
| A25 | Male | 9 | WTS; WGS | TTMV-ORF2 c.1_213 | RARA exon 3_9 | None | 12 |
| A26 | Male | 15 | WTS; WGS | TTMV-ORF2 c.1_226 | RARA exon 3_9 | None | |
| A27 | Female | 5 | WTS; WGS | TTMV-ORF2 c.1_175 | RARA exon 3_9 | None |
| UPN . | Sex . | Age (y) . | Methods . | 5′ fusion partner . | RARA . | 3′ fusion partner . | Reference . |
|---|---|---|---|---|---|---|---|
| A1∗ | Female | 35 | WTS; WGS; OGM | STAT3 exon 1_21 | RARA exon 3_9@c.1225 | LTR40a | |
| A2 | Female | 45 | WGS | STAT3 exon 1_21 | RARA exon 3_9@c.1215 | L1MC4 | |
| A3 | Male | 24 | WGS | STAT3 exon 1_21 | RARA exon 3_9@c.1205 | AluSp | 14 |
| A4 | Male | 26 | WTS; WGS | STAT3 exon 1_23 | RARA exon 3_9@c.1203 | MIRb | 14 |
| A5 | Male | 50 | WTS | STAT5B exon 1_15 | RARA exon 3_9@c.1239 | AluJo | |
| A6 | Male | 46 | WTS; WGS | STAT5B exon 1_15 | RARA exon 3_9@c.1227 | HUMUT5218 | |
| A7 | Male | 47 | NGS-targeted | STAT5B exon 1_15 | RARA exon 3_9@c.1224 | MIRb-AluSc | 16 |
| A8 | Male | 32 | WGS | STAT5B exon 1_15 | RARA exon 3_9@c.1176 | AluSx4 | |
| A9 | Male | 34 | WTS | THRAP3 exon 1_2 | RARA exon 3_9@c.1238 | THRAP3 exon 6_12 | 25 |
| A10 | Female | 29 | WTS | HNRNPC exon 1_3 | RARA 3_8 | HNRNPCP2 | 20 |
| A11 | Male | 41 | WTS | HNRNPC exon 1_3 | RARA 3_8 | HNRNPC exon 4_9 | 21 |
| A12 | Female | 4 | WTS | FIP1L1 exon 1_13 | RARA exon 3_9 | None | 19 |
| A13 | Male | 31 | WTS | GTF2I exon 1_19 | RARA exon 3_9 | None | |
| A14 | Female | 51 | WTS | IRF2BP1 exon 1@c.322 | RARA exon 3_9 | None | 7 |
| A15 | Male | 66 | WGS | IRF2BP2 exon 1@c.1000 | RARA exon 3_9 | None | 23 |
| A16 | Male | 60 | WTS | IRF2BP2 exon 1@c.1000 | RARA exon 3_9 | None | 24 |
| A17 | Male | 60 | WTS | TNRC18 exon 1_5 | RARA exon 3_9 | None | |
| A18 | Male | 50 | WTS | TNRC18 exon 1_5 | RARA exon 3_9 | None | 26 |
| A19 | Female | 6 | WTS | TTMV-ORF2 c.1_209 | RARA exon 3_9 | None | 9 |
| A20 | Male | 3 | WTS | TTMV-ORF2 c.1_328 | RARA exon 3_9 | None | 9 |
| A21 | Male | 3 | WTS; WGS | TTMV-ORF2 c.1_215 | RARA exon 3_9 | None | 10 |
| A22 | Female | 11 | WTS; WGS | TTMV-ORF2 c.1_413 | RARA exon 3_9 | None | 11 |
| A23 | Male | 7 | WTS; WGS | TTMV-ORF2 c.1_217 | RARA exon 3_9 | None | 13 |
| A24 | Male | 35 | WTS | TTMV-ORF2 c.1_189 | RARA exon 3_9 | None | |
| A25 | Male | 9 | WTS; WGS | TTMV-ORF2 c.1_213 | RARA exon 3_9 | None | 12 |
| A26 | Male | 15 | WTS; WGS | TTMV-ORF2 c.1_226 | RARA exon 3_9 | None | |
| A27 | Female | 5 | WTS; WGS | TTMV-ORF2 c.1_175 | RARA exon 3_9 | None |
OGM, optical genome mapping; UPN, unique patient number.
The index case.
NGS
Next-generation sequencing (NGS)-based WTS and WGS were conducted using the Illumina platform in paired-end 150–base pair mode. Raw sequencing reads in compressed FASTQ format were processed by fastp59 for adapter removal and quality control.
Long-read sequencing
Long-read sequencing was conducted on the Oxford Nanopore Technology (ONT) platform. Libraries were prepared with the Ligation Sequencing Kit SQK-LSK109 and sequenced on the PromethION platform using the R9.4.1 chemistry flow cell.
Optical genome mapping
Optical genome mapping analysis was used to explore genomic structural variation.60 High molecular weight DNA was extracted from bone marrow (BM) sample using the Bionano Prep SP BMA DNA isolation kit. The CTTAAG motif of genomic DNA was labeled, and the DNA backbone was stained using the Bionano Prep DLS Kit. Labeled DNA was loaded onto chips to collect 1600 gigabase coverage data. Qualified data were processed using the Rare Variant Analysis pipeline in Bionano Solve version 3.7 software and visualized using Bionano Access version 1.7.
Fusion gene calling and PCR confirmation
For WTS, routine fusion gene calling was performed using Arriba version 2.1.0.58,61 For WGS, LUMPY62 and CNVnator63 were used to call structural variants, including translocations and rearrangements. Both programs started from processed FASTQ format reads as input and selected GRCh37 as the human reference genome. All RARs 5′ and 3′ splices were manually investigated using the Integrative Genomics Viewer (IGV) version 2.3.92.64 Reverse transcription polymerase chain reaction (RT-PCR) and Sanger sequencing were used to validate the fusion transcripts and assess the cis alignment of RARs 5′ and 3′ splices, as described in detail in supplemental Methods and Results. Accession numbers of reference sequences and genomic loci of genes and transposable elements (TEs) analyzed are provided in supplemental Tables 3 and 4, respectively.
Gene expression and clustering analysis
Gene expression analysis was performed using the vst method in DESeq2,65 followed by uniform manifold approximation and projection66 for dimension reduction and clustering. TE expression was manually investigated using IGV version 2.3.92 with WTS reads concerning the coverage and depth of each TE locus.
In vitro experiment
The responsiveness of chimeric proteins to ATRA was assessed using a GAL4-UAS reporter system.37,67 Bipartite and tripartite chimeric sequences were cloned in the pBIND vector with Renilla luciferase, as described in detail in the supplemental Methods. Inducible Firefly luciferase reporter plasmid pGL4.15 containing 5 copies of the GAL4 response element (5 × GAL4UAS) was constructed. Recombinant pBIND vectors and pGL4.15 containing 5 × GAL4UAS were cotransfected into 293T cells, respectively. Responses to ATRA management were evaluated by a dual-luciferase reporter assay system (Promega).68 ATRA was purchased from Sigma-Aldrich. For statistical analysis, analysis of variance with Turkey correction for multiple comparisons was used. The experiments were independently repeated.
Results
Patient characteristics
The 27 RARA-aAPL cases (A1-A27; Table 1) included 8 females and 19 males, aged 3 to 66 years (median, 32 years). The 21 RARG-aAPL cases (G1-G21; Table 2) included 6 females and 15 males, aged 0.9 to 69 years (median, 38 years). All cases morphologically resembled APL, and their karyotypes are provided in supplemental Table 5. Uniform manifold approximation and projection analysis of gene expression profiles for 19 RARA-aAPL and 18 RARG-aAPL cases with available WTS data showed similarity to those of the 42 PML::RARA-APL cases (supplemental Figure 1).
Demographic characteristics, genomic data, and gene fusion information of RARG-aAPL cases
| UPN . | Sex . | Age (y) . | Methods . | 5′ fusion partner . | RARG . | 3′ fusion partner . | Reference . |
|---|---|---|---|---|---|---|---|
| G1∗ | Male | 69 | WGS | NPM1 exon 1_4 | RARG exon 4_9 | NPM1 exon 11 | 31 |
| G2∗ | Male | 26 | WGS | PRPF19 exon 1_4 | RARG exon 4_9 | PRPF19 exon 5_16 | 32 |
| G3∗ | Female | 0.9 | WGS | HNRNPC exon 1_2 | RARG exon 3a_9 | HNRNPC exon 4_9 | 33 |
| G4 | Male | 43 | WTS | HNRNPC exon 1_3 | RARG exon 4_9 | HNRNPC exon 4_9 | 34 |
| G5 | Male | 30 | WTS | HNRNPC exon 1_3 | RARG exon 4_9 | HNRNPC exon 4_9 | 35 |
| G6 | Male | 49 | WTS | HNRNPC exon 1_3 | RARG exon 4_9 | HNRNPC exon 4_9 | |
| G7 | Female | 1 | WTS | HNRNPC exon 1_2 | RARG exon 4_9 | HNRNPC exon 4_9 | |
| G8 | Female | 25 | WTS | HNRNPM exon 1_12 | RARG exon 1_9 | HNRNPM exon 3_16 | 36 |
| G9 | Female | 51 | WTS | CPSF6 exon 1_4 | RARG exon 1_9 | LINE-L2a | 54 |
| G10 | Male | 38 | WTS | CSPF6 exon 1_4 | RARG exon 1_9 | CSPF6 exon 5_10 | |
| G11 | Male | 38 | WTS | CPSF6 exon 1_4 | RARG exon 2_9 | LINE-L2a | 39 |
| G12 | Female | 65 | WTS | CPSF6 exon 1_4 | RARG exon 4_9 | CPSF6 exon 5_10 | |
| G13 | Female | 48 | WTS | CPSF6 exon 1_4 | RARG exon 4_9 | LTR17 | 54 |
| G14 | Male | 41 | WTS | CPSF6 exon 1_4 | RARG exon 4_9 | Tigger2 | |
| G15 | Male | 55 | WTS | CPSF6 exon 1_5 | RARG exon 1_9 | LINE-L2a | 40 |
| G16 | Male | 22 | WGS | CPSF6 exon 1_5 | RARG exon 4_9 | LINE-L2a | 41 |
| G17 | Male | 53 | WTS | NUP98 exon 1_11 | RARG exon 4_9 | LINE-L2a | 43 |
| G18 | Male | 32 | WTS | NUP98 exon 1_12 | RARG exon 4_9 | LINE-L2a | 49 |
| G19 | Male | 32 | WTS | NUP98 exon 1_12 | RARG exon 4_9 | LINE-L2a | |
| G20 | Male | 33 | WTS | NUP98 exon 1_12 | RARG exon 4_9 | AluSx | |
| G21 | Male | 46 | WTS | SART3 exon 1_18 | RARG exon 3-9 | LINE-L2a | 53 |
| UPN . | Sex . | Age (y) . | Methods . | 5′ fusion partner . | RARG . | 3′ fusion partner . | Reference . |
|---|---|---|---|---|---|---|---|
| G1∗ | Male | 69 | WGS | NPM1 exon 1_4 | RARG exon 4_9 | NPM1 exon 11 | 31 |
| G2∗ | Male | 26 | WGS | PRPF19 exon 1_4 | RARG exon 4_9 | PRPF19 exon 5_16 | 32 |
| G3∗ | Female | 0.9 | WGS | HNRNPC exon 1_2 | RARG exon 3a_9 | HNRNPC exon 4_9 | 33 |
| G4 | Male | 43 | WTS | HNRNPC exon 1_3 | RARG exon 4_9 | HNRNPC exon 4_9 | 34 |
| G5 | Male | 30 | WTS | HNRNPC exon 1_3 | RARG exon 4_9 | HNRNPC exon 4_9 | 35 |
| G6 | Male | 49 | WTS | HNRNPC exon 1_3 | RARG exon 4_9 | HNRNPC exon 4_9 | |
| G7 | Female | 1 | WTS | HNRNPC exon 1_2 | RARG exon 4_9 | HNRNPC exon 4_9 | |
| G8 | Female | 25 | WTS | HNRNPM exon 1_12 | RARG exon 1_9 | HNRNPM exon 3_16 | 36 |
| G9 | Female | 51 | WTS | CPSF6 exon 1_4 | RARG exon 1_9 | LINE-L2a | 54 |
| G10 | Male | 38 | WTS | CSPF6 exon 1_4 | RARG exon 1_9 | CSPF6 exon 5_10 | |
| G11 | Male | 38 | WTS | CPSF6 exon 1_4 | RARG exon 2_9 | LINE-L2a | 39 |
| G12 | Female | 65 | WTS | CPSF6 exon 1_4 | RARG exon 4_9 | CPSF6 exon 5_10 | |
| G13 | Female | 48 | WTS | CPSF6 exon 1_4 | RARG exon 4_9 | LTR17 | 54 |
| G14 | Male | 41 | WTS | CPSF6 exon 1_4 | RARG exon 4_9 | Tigger2 | |
| G15 | Male | 55 | WTS | CPSF6 exon 1_5 | RARG exon 1_9 | LINE-L2a | 40 |
| G16 | Male | 22 | WGS | CPSF6 exon 1_5 | RARG exon 4_9 | LINE-L2a | 41 |
| G17 | Male | 53 | WTS | NUP98 exon 1_11 | RARG exon 4_9 | LINE-L2a | 43 |
| G18 | Male | 32 | WTS | NUP98 exon 1_12 | RARG exon 4_9 | LINE-L2a | 49 |
| G19 | Male | 32 | WTS | NUP98 exon 1_12 | RARG exon 4_9 | LINE-L2a | |
| G20 | Male | 33 | WTS | NUP98 exon 1_12 | RARG exon 4_9 | AluSx | |
| G21 | Male | 46 | WTS | SART3 exon 1_18 | RARG exon 3-9 | LINE-L2a | 53 |
exon 3a, alternative exon 3; UPN, unique patient number.
The index cases.
The index RARA-aAPL case
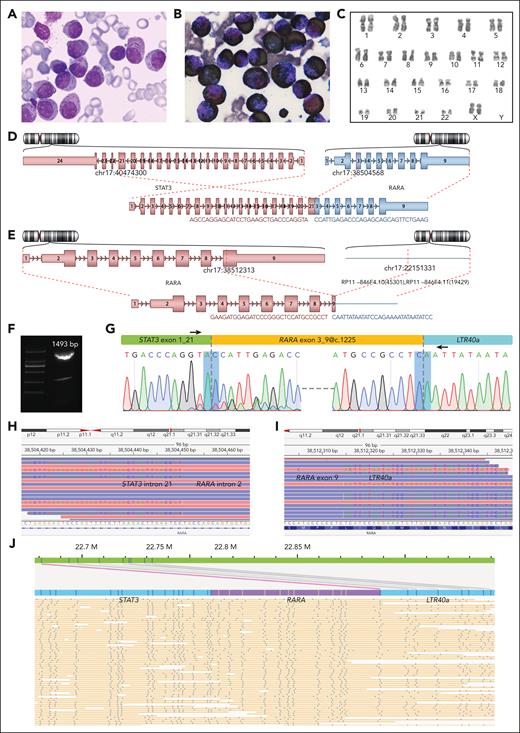
A 35-year-old female (A1) presented with 2 months of skin ecchymosis and fatigue, followed by 1 week of fever, diarrhea, and lower abdominal pain. Laboratory tests revealed anemia, thrombocytopenia, and coagulation abnormalities. BM examination showed 79.0% aberrant promyelocytes with occasional Auer rods (Figure 1A) and diffuse myeloperoxidase reactivity (Figure 1B). Immunophenotyping showed an APL phenotype. Karyotype analysis identified 46,XX,inv(17)(p12q21) (Figure 1C). However, neither RT-PCR nor fluorescence in situ hybridization detected PML::RARA fusion. She received ATRA and arsenic trioxide treatment because of the APL-like manifestations, but the BM still displayed 85.2% aberrant promyelocytes on day 6. Subsequent treatment was modified to dexamethasone and daunorubicin. However, the patient discontinued treatment on day 24 for nonmedical reasons.
Morphological and genetic investigation of the index RARA-aAPL case. (A) Wright staining of BM smear. (B) Myeloperoxidase chemical staining of BM smear. (C) Karyotype 46,XX,inv(17)(p12q21) of BM sample. (D-E) Schematic diagram of the STAT3 exon 21::RARA exon 3 (D) and RARA exon 9@c.1225::LTR40a (E) splices, reported by Arriba version 2.0.1 software with NGS WTS data. (F-G) Electrophoresis result of the RT-PCR product (F), a diagram indicating primer locations (G, upper), and the Sanger sequencing results (G, lower) of the STAT3::RARA::LTR40a tripartite fusion transcript. The bottom peaks in forward Sanger sequencing are due to the minor STAT3 exon 20::RARA exon 3 splice. (H-I) STAT3 intron 21::RARA intron 2 (H) and RARA exon 9::LTR40a (I) splices at the genomic level, as revealed by NGS WGS visualized using the IGV version 2.3.92. (J) Abundant STAT3::RARA::LTR40a tandem genomic splicing fragments captured through optical genome mapping analysis. The human GRCh37 annotation reference was used to annotate gene coordinates on chromosome 17.
Morphological and genetic investigation of the index RARA-aAPL case. (A) Wright staining of BM smear. (B) Myeloperoxidase chemical staining of BM smear. (C) Karyotype 46,XX,inv(17)(p12q21) of BM sample. (D-E) Schematic diagram of the STAT3 exon 21::RARA exon 3 (D) and RARA exon 9@c.1225::LTR40a (E) splices, reported by Arriba version 2.0.1 software with NGS WTS data. (F-G) Electrophoresis result of the RT-PCR product (F), a diagram indicating primer locations (G, upper), and the Sanger sequencing results (G, lower) of the STAT3::RARA::LTR40a tripartite fusion transcript. The bottom peaks in forward Sanger sequencing are due to the minor STAT3 exon 20::RARA exon 3 splice. (H-I) STAT3 intron 21::RARA intron 2 (H) and RARA exon 9::LTR40a (I) splices at the genomic level, as revealed by NGS WGS visualized using the IGV version 2.3.92. (J) Abundant STAT3::RARA::LTR40a tandem genomic splicing fragments captured through optical genome mapping analysis. The human GRCh37 annotation reference was used to annotate gene coordinates on chromosome 17.
NGS-based WTS was conducted on the patient's BM sample at diagnosis, with the gene expression profile clustering with PML::RARA-APL cases (supplemental Figure 1). Arriba fusion gene calling reported STAT3 exon 21::RARA exon 3 splice (Figure 1D) and another splice of RARA exon 9 fused with a 3′ sequence mapped to a TE of LTR40a (Figure 1E). Through RT-PCR and Sanger sequencing with primers spanning both splices, we confirmed that the 2 RARA 5′ and 3′ splices were cis aligned on the same transcript (Figure 1F-G). NGS-based WGS was conducted and confirmed the presence of STAT3 intron 21::RARA intron 2 and RARA exon 9@c.1225::LTR40a splices (Figure 1H-I). Optical genome mapping analysis exhibited abundant cis-aligned fragments composed of STAT3, RARA, and LTR40a in tandem (Figure 1J). ONT WGS and ONT WTS detected tandem splicing reads of STAT3::RARA::LTR40a at genomic and transcript levels, respectively (supplemental Figures 2 and 3; supplemental Result 1). Therefore, multiple approaches confirmed that the fusion is STAT3::RARA::LTR40a that splices in cis, at both genomic and transcriptomic levels, rather than 2 individually bipartite fusion transcripts of STAT3::RARA and RARA::LTR40a. We introduce the term “tripartite fusion” to characterize this novel form of gene fusion.
The index RARG-aAPL cases
The 3 index RARG-aAPL cases (G1-G3; Table 1) have been individually reported by Chen et al from Lu Daopei Hospital and collaborative hospitals.31-33 Previous reports indicated that they were all clinically resistant to ATRA and featured both RARG 5′ and 3′ splices, resulting in RARG exon 10 truncations. However, we did not explore the pathogenic relevance of RARG 3′ splice and exon 10 truncations. Furthermore, this phenomenon has not been addressed in other aAPL studies or reviews to date.4,5,30 In this study, we retrieved archived samples and confirmed that the RARG 3′ and 5′ splices are cis aligned in all 3 cases by RT-PCR and Sanger sequencing with primers that spanned both splices (supplemental Result 2). Therefore, the fusion transcripts in these 3 cases are all X::RARG::X–type tripartite fusion with both RARG 5′ and 3′ splices in tandem, not 2 separate bipartite fusions of X::RARG and RARG::X, nor a pair of reciprocal fusions.
Meta-analysis and reanalysis of validation cases
The finding of tripartite fusion motivated us to investigate additional cases. We conducted a comprehensive meta-analysis to reexamine the overlooked characteristics of RARA and RARG splices in documented aAPL cases. Additionally, we reinvestigated WTS data of 42 PML::RARA-APL cases in our previously reported cohort,58 in which no RARA 3′ splicing was detected. Furthermore, we searched for reported tripartite-type fusions in all other leukemias and tumors but found no results.
Upon thorough investigation of the 111 RARA-aAPL cases documented with detailed genomic aberrations (supplemental Table 1), we noted a few instances of RARA exon 9 abnormalities. Notably, both reported STAT3::RARA-aAPL cases refractory to ATRA-containing therapy were described with RARA exon 9 breakpoints.14 Intriguingly, in vitro experiments showed that the predicted bipartite STAT3::RARA fusion without RARA 3′ aberration responded well to ATRA, contrary to clinical resistance. When we retrieved and reanalyzed WGS and WTS data of both cases (A3 and A4; supplemental Results 3 and 4), we identified STAT3::RARA::AluSp and STAT3::RARA::MIRb tripartite fusions, respectively. Similarly, 22 STAT5B::RARA-aAPL cases have been reported, with all evaluable cases displaying resistance to ATRA,3,17 and RARA exon 9 breakpoints were described in 2 cases.15,16 Additionally, it was reported that artificial bipartite STAT5B::RARA fusion without RARA 3′ aberration responded to ATRA similarly to PML::RARA, contrasting clinical resistance.55,69 Reanalysis of NGS data of 1 reported case16 (A7) identified a STAT5B::RARA::MIRb-AluSc tripartite fusion. Two aAPL cases have been reported with coexisting RARA 5′ and 3′ splicing events.20,21 Through reanalysis of their WTS data, we identified tripartite fusions of HNRNPC::RARA::HNRNPCP2 (A10) and HNRNPC::RARA::HNRNPC (A11), respectively.
We also investigated RARG fusions in 23 documented cases (supplemental Table 2; supplemental Figure 4) with detailed genomic aberrations other than the 3 index RARG-aAPL cases.34-54 Twenty-two of them received an ATRA-based regimen, with all 20 evaluable cases showing ATRA resistance. All cases were reported to possess bipartite RARG fusion, including 19 with X::RARG (R5-R23)38-54 and 2 with RARG::X fusions (R3 and R4).36,37 Coexistence of HNRNPC exon 3::RARG exon 4 and RARG exon 9::HNRNPC exon 4 splices were reported as reciprocal fusions in 2 cases (R1 and R2).34,35 However, the paired fusion isoforms do not conform to the characteristics that reciprocal fusions should have. Breakpoints within RARG intron 9 were reported in 4 cases (R4, R6, R8, and R20) but without further investigation.37,40,54
Intriguingly, in vitro experimental outcomes for speculated bipartite X::RARG fusions were diametrically opposed to those observed in vivo and patient-derived leukemia cells. The first reported RARG-aAPL case was identified as NUP98::RARG positive, and the leukemia cells displayed ATRA resistance both in vivo and in vitro.44,70 However, cells transfected with speculated bipartite NUP98::RARG fusion exhibited extreme ATRA sensitivity.55 Similarly, cells transfected with speculated bipartite CPSF6::RARG fusion responded well to ATRA, contrasting observed clinical resistance.54 Moreover, in vitro studies preceding the first reports of RARG-aAPL cases had documented that the proposed bipartite PML::RARG fusion was ATRA sensitive.56,57
These combined indications of RARs 3′ abnormalities suggest unexplained molecular etiologies beyond 5′ splices in aAPL cases. We further enrolled WTS and WGS data of 15 previously reported and 11 unreported RARA-aAPL cases (A2-A27; Table 1), and 11 previously reported and 7 unreported RARG-aAPL cases (G4-G21; Table 2) as a validation cohort for detailed bioinformatic scrutiny. We confirmed that RARG 5′ and 3′ splices were cis aligned in 3 more cases with archived samples available (G8, G16, and G21; supplemental Result 2). Thus, we validated that the 5′ and 3′ splices of RARA or RARG were cis aligned in all 7 cases with available specimens. Therefore, although limited specimen availability hindered direct verification in other cases, we inferred that their RARA or RARG 5′ and 3′ splices should be cis aligned if both are present.
The landscape of RARA and RARG fusions
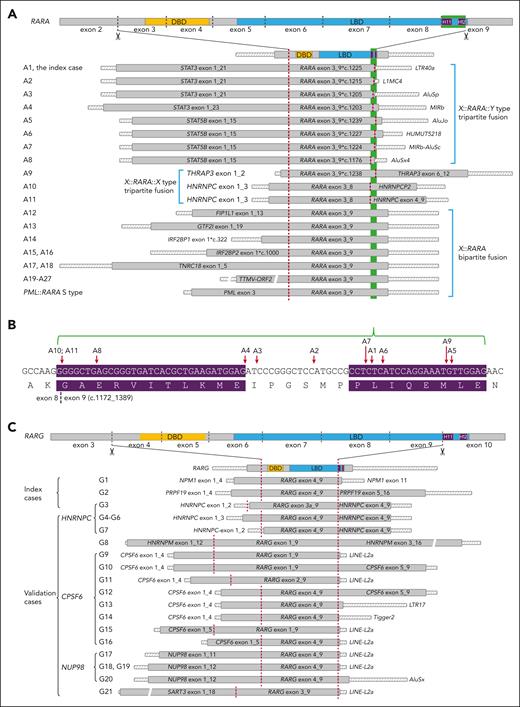
A hitherto unpredicted landscape of bipartite and tripartite RARA and RARG fusion patterns was delineated (Figure 2; supplemental Results 3-6). Altogether, RARA 3′ splices were observed in 11 (40.7%) RARA-aAPL cases with a 5′ partner gene–associated pattern. Among the 8 RARA-aAPL cases in which STAT3/5B is the 5′ fusion partner, TE-derived sequences comprised the 3′ fusion partners, forming X::RARA::Y-type fusions. In the 2 RARA-aAPL cases with HNRNPC and the 1 case with THRAP3 as the 5′ fusion partners, the 3′ fusion partner gene corresponded to the 5′ partner or its pseudogene, constructing X::RARA::X-type fusions. RARA 3′ splicing was not observed in 9 TTMV-ORF2::RARA cases, 3 IRF2BP1/2::RARA cases, 2 TNRC18::RARA cases, 1 FIP1L1::RARA, and 1 GTF2I::RARA case. In contrast to RARA-aAPL, all 21 patients with RARG-aAPL exhibited tripartite RARG fusion genes, with X::RARG::X–type fusions in 10 cases and X::RARG::Y–type fusions in 11 cases.
Structural diagram of RARA and RARG fusions. (A) Structural diagram of tripartite and bipartite RARA fusions in each RARA-aAPL case. A∗, unique patient number. (B) Red arrows indicate RARA 3′ splicing sites of each case. The purple background highlights the H11 (left) and H12 (right) coding regions. (C) Structure diagram of tripartite RARG fusion transcripts in each RARG-aAPL case. G∗, unique patient number. The 3′ end of each transcript extends to the first poly(A) signal. Crosshatched bars indicate UTRs; gray-filled bars indicate coding regions; and color-filled bars mark the DBD, LBD, and helices 11 (H11) and 12 (H12). The green background highlights the H11_12 coding region. Dark red dashed vertical lines indicate the splice sites. Bars are displayed proportionally according to their length. Bars with breaks indicate that the length of these segments is not to scale.
Structural diagram of RARA and RARG fusions. (A) Structural diagram of tripartite and bipartite RARA fusions in each RARA-aAPL case. A∗, unique patient number. (B) Red arrows indicate RARA 3′ splicing sites of each case. The purple background highlights the H11 (left) and H12 (right) coding regions. (C) Structure diagram of tripartite RARG fusion transcripts in each RARG-aAPL case. G∗, unique patient number. The 3′ end of each transcript extends to the first poly(A) signal. Crosshatched bars indicate UTRs; gray-filled bars indicate coding regions; and color-filled bars mark the DBD, LBD, and helices 11 (H11) and 12 (H12). The green background highlights the H11_12 coding region. Dark red dashed vertical lines indicate the splice sites. Bars are displayed proportionally according to their length. Bars with breaks indicate that the length of these segments is not to scale.
RARA and RARG 5′ splices
RARA 5′ splices were consistently located at the beginning of exon 3, with all 5′ fusion partners retaining partial protein-coding region, as observed in PML::RARA and all other documented RARA fusions (Figure 2A; supplemental Results 3 and 4),3,4 In contrast, RARG 5′ splice sites exhibited remarkable variability (Figure 2C; supplemental Results 5 and 6). The most common RARG 5′ splicing sites were located at the beginning of its exon 4 (15 cases, 68.2%). In 6 cases (G8-G11, G15, and G22), varying lengths of the RARG 5′ untranslated region (UTR) were preserved, contributing to derived coding sequences in the inframe fusion. An alternative RARG exon 3a was used in case G3. In case G3 and G7, HNRNPC 5′ UTR spliced to RARG, forming an inframe fusion. Thus, we presumed that the obligation of the RARG 5′ splice was to adopt its partner gene’s promoter without requiring the partner gene to retain a protein-coding region.
The expression of fusion genes is typically driven by promoter elements of the 5′ partner gene. We analyzed the expression levels of all 27 reported RARA and RARG human protein-coding partner genes in bulk WTS of 50 control healthy BM samples (supplemental Figure 5). Except for ZBTB16, all other genes showed medium to high overall expression levels. According to the Atlas of Human Blood Cells,71 ZBTB16 is primarily highly expressed in hematopoietic stem cells and early stage myeloid cells.
RARA and RARG 3′ splices
RARA 3′ splices exhibited variability within the coding region of helices 11_12 (H11_12) of its LBD in exon 9 (Figure 2A-B; supplemental Results 3 and 4). In 7 of 8 STAT3/5B-implicated cases, 3′ splices truncated RARA LBD-H12 while preserving H11. In both HNRNPC-implicated cases, the entire RARA exon 9 and LBD-H11_12 were truncated. In contrast, RARG 3′ splices consistently resided at the terminus of its exon 9, resulting in entire exon 10 and LBD-H11_12 truncation (Figure 2C; supplemental Results 5 and 6).
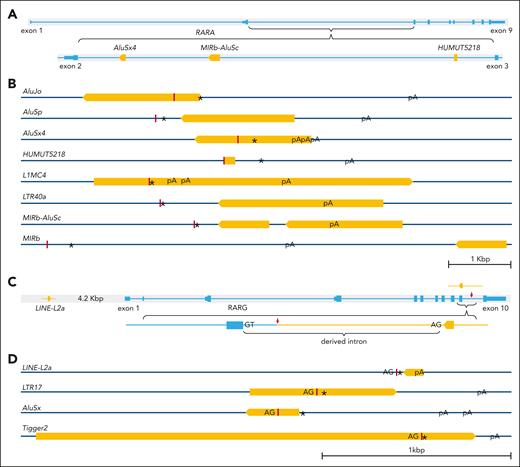
Eight distinct TE-derived 3′ splicing sequences were identified in X::RARA::Y-type fusions (Figures 2A and 3A-B; supplemental Results 3-4,7). The committed genomic loci in 4 of them were in RARA intron 2. All TE-derived sequences were spliced directly to the truncated RARA exon 9 at both genomic and transcriptomic levels. Four distinct TE-derived 3′ splicing sequences were identified in 12 cases with X::RARG::Y-type fusions (Figures 2C and 3C-D; supplemental Results 5-7), with the most implicated being a LINE-L2a in 9 cases, whose committed genomic locus is 4.2 kilobase pairs upstream of RARG. All TE-derived fusion transcript fragments exhibited 5′ contiguous intron splicing acceptor signals at the genomic level (Figure 3D), indicating exonization in conjunction with RARG exon 9. Although the splicing sites of LINE-L2a on RARG intron 9 varied, they conferred an identical derived 3′ fusion transcript in all 8 involved cases. These TE-derived 3′ fusion sequences conferred derived rapid termination coding sequences followed by ≥1 poly(A) signals, which are essential for functional messenger RNA. Consequently, the 3′ fusion partners in RARA and RARG trinary fusions may either be a protein-coding gene or a TE-derived sequence, consistently conferring at least 1 poly(A) signal.
Structural diagram of the genomic location, splicing site, derived stop codon, derived poly(A) signal, and derived intron splicing acceptor of each implicated TE. (A) Structural diagram of the 4 TEs (orange bars) located within RARA (light blue) intron 2. (B) Structure diagram of the relative positions of the splicing site (dark red vertical lines), derived stop codon (∗), and derived poly(A) signal (pA) of each implicated TE in RARA-aAPL cases. (C) Structure diagram of the relative genomic locus of LINE-L2a (orange) and RARG (light blue), the insertion of LINE-L2a in RARG intron 9 (upper), and the derived intron splicing acceptor (AG) signal that pairing with the splicing donor signal (GT) of RARG intron 9 (lower). (D) Structure diagram of the relative positions of the derived intron splicing acceptor (AG), splicing site (dark red vertical lines), stop codon (∗), and poly(A) signal (pA) of each involved TE. Orange bars are displayed proportionally according to their length, and the pointed end shows their orientation in the genome, with a scale bar marking 1 kilobase.
Structural diagram of the genomic location, splicing site, derived stop codon, derived poly(A) signal, and derived intron splicing acceptor of each implicated TE. (A) Structural diagram of the 4 TEs (orange bars) located within RARA (light blue) intron 2. (B) Structure diagram of the relative positions of the splicing site (dark red vertical lines), derived stop codon (∗), and derived poly(A) signal (pA) of each implicated TE in RARA-aAPL cases. (C) Structure diagram of the relative genomic locus of LINE-L2a (orange) and RARG (light blue), the insertion of LINE-L2a in RARG intron 9 (upper), and the derived intron splicing acceptor (AG) signal that pairing with the splicing donor signal (GT) of RARG intron 9 (lower). (D) Structure diagram of the relative positions of the derived intron splicing acceptor (AG), splicing site (dark red vertical lines), stop codon (∗), and poly(A) signal (pA) of each involved TE. Orange bars are displayed proportionally according to their length, and the pointed end shows their orientation in the genome, with a scale bar marking 1 kilobase.
Aberrant expression of TEs
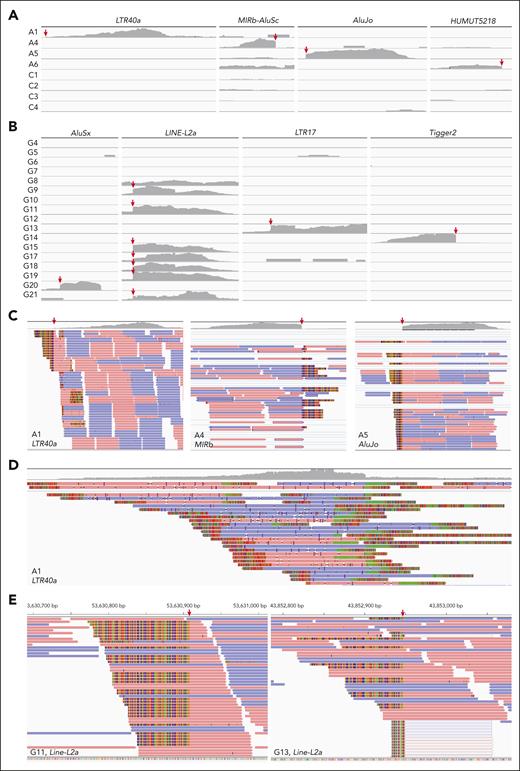
WTS sequencing reads coverage and depth of each TE locus were manually investigated using IGV. The implicated TEs were almost exclusively expressed in each related case (Figure 4A-B). Apart from the fusion sequences, no continuous transcript was aligned spanning the splice sites of TEs in RARA-aAPL cases (Figure 4C), suggesting the lack of native expression of these TEs. ONT WTS captured numerous long-read bipartite X::LTR40a fusion transcripts with various 5′ partners in the index case (Figure 4D), indicating multiple insertion events of this LTR40a in the genome. There were both fusion and native transcripts aligned spanning the splice sites of TEs in RARG-aAPL cases (Figure 4E), indicating the autonomy of aberrant TEs activation from 3′ fusion events.
Expression analysis of TEs. (A) Alignment coverage tracks of short-read WTS reads in RARA-aAPL cases with available data according to each implicated TE, visualized using the IGV version 2.3.92. Red arrows indicate the splicing sites. C1-4 were PML::RARA positive APL cases serving as controls. (B) Alignment coverage tracks of short-read WTS reads in RARG-aAPL cases according to each implicated TE. (C) Reads alignment tracks (lower) and alignment coverage tracks (upper) of short-reads WTS reads in RARA-aAPL cases at each implicated TE locus. (D) Reads alignment tracks (lower) and alignment coverage tracks (upper) of long-read WTS reads at the implicated LTR40a locus in the index case A1. (E) Reads alignment tracks of short-read WTS reads in example cases G11 and G13 at the LINE-L2a loci.
Expression analysis of TEs. (A) Alignment coverage tracks of short-read WTS reads in RARA-aAPL cases with available data according to each implicated TE, visualized using the IGV version 2.3.92. Red arrows indicate the splicing sites. C1-4 were PML::RARA positive APL cases serving as controls. (B) Alignment coverage tracks of short-read WTS reads in RARG-aAPL cases according to each implicated TE. (C) Reads alignment tracks (lower) and alignment coverage tracks (upper) of short-reads WTS reads in RARA-aAPL cases at each implicated TE locus. (D) Reads alignment tracks (lower) and alignment coverage tracks (upper) of long-read WTS reads at the implicated LTR40a locus in the index case A1. (E) Reads alignment tracks of short-read WTS reads in example cases G11 and G13 at the LINE-L2a loci.
LBD truncation in tripartite chimeric proteins
RARA exon 9 and RARG exon 10 encode H11_12 of their LBD, respectively. Therefore, all tripartite RARA chimeric proteins maintain their intact DNA binding domain (DBD) and H12 or H11_12 truncated LBD, depending on the 3′ splicing sites (Figure 2A-B). In contrast, all bipartite RARA chimeric proteins preserve both the intact DBD and LBD (Figure 2A), whereas all tripartite RARG chimeric proteins maintain their intact DBD and fully H11_12 truncated LBD (Figure 2C).
In 2010, structural biologists revealed the critical role of the allosteric transition of LBD-H11_12 in responding to ATRA ligation in RAR proteins.72 In ATRA-unliganded RAR proteins, their LBD-H11 region exhibits as an extended β-strand, and LBD-H12 lies in a more extended and flexible conformation. In this conformation, this β-strand forms an antiparallel β-sheet with corepressor motifs, rendering basal repressive activity of RARs through the recruiting of corepressor proteins. Upon ATRA binding, this β-strand conformation transitions into an α-helix, leading to the formation of LBD-H11 and the subsequent retraction and stabilization of LBD-H12, composing a coactivator docking surface. This transition provokes corepressor release and facilitates coactivator recruitment. Thus, LBD-H11_12/H12 truncation in tripartite RARA or RARG chimeric proteins structurally impairs their coactivator docking surface, preventing them from activating target genes in response to ATRA.
ATRA unresponsiveness of LBD-H11_12/H12 truncated chimeric proteins
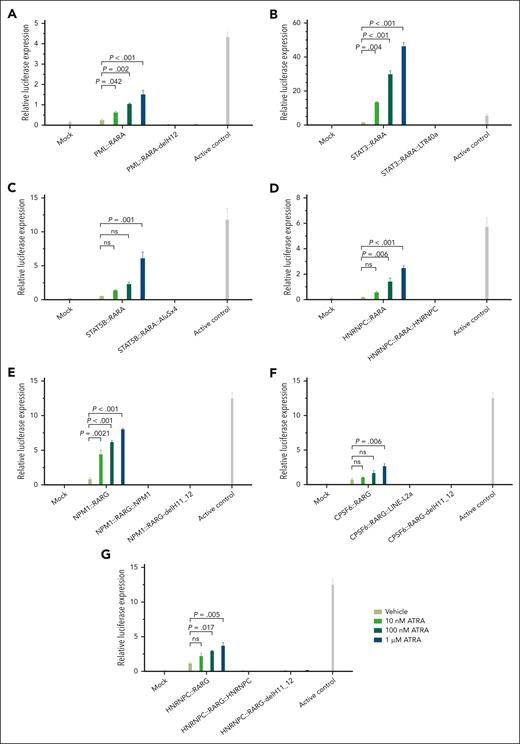
Plasmid vectors were constructed with PML, STAT3, STAT5B, and HNRNPC as RARA 5′ fusion partners and NPM1, CPSF6, and HNRNPC as RARG 5′ fusion partners to simulate the following: (1) bipartite fusions with intact LBD, (2) tripartite fusions with LBD-H11_12/H12 truncation as observed in cases, and (3) bipartite 5′ fusions with direct artificial LBD-H11_12/H12 truncation without 3′ partner sequences. Experimental results demonstrated that all chimeric proteins with intact LBD robustly responded to ATRA, whereas those with LBD-H11_12/H12 truncation exhibited minimal to no response, even at high ATRA concentrations (Figure 5).
The response of chimeric proteins to ATRA. (A) PML::RARA, bipartite PML::RARA fusion with intact LBD. PML::RARA-delH12, simulated PML::RARA fusion with artificial truncation of LBD helices 12 (LBD-H12) without a 3′ fusion partner. (B) STAT3::RARA, simulated bipartite STAT3::RARA fusion with intact LBD. STAT3::RARA::LTR40a, tripartite STAT3::RARA::LTR40a fusion as identified in index case A1. (C) STAT5B::RARA, simulated bipartite STAT5B::RARA fusion with intact LBD. STAT5B::RARA::AluSx4, tripartite STAT5B::RARA::AluSx4 fusion as identified in case A8. (D) HNRNPC::RARA, simulated bipartite HNRNPC::RARA fusion with intact LBD. HNRNPC::RARA::HNRNPC, tripartite HNRNPC::RARA::HNRNPC fusion as identified in case A11. (E) NPM1::RARG, simulated bipartite NPM1::RARG fusion with intact LBD. NPM1::RARG::NPM1, tripartite NPM1::RARG::NPM1 fusion as identified in index case G1. NPM1::RARG-delH11_12, simulated NPM1::RARG fusion with artificial truncation of LBD-H11_12 without a 3′ fusion partner. (F) CPSF6::RARG, simulated bipartite CPSF6::RARG fusion with intact LBD. CPSF6::RARG::LINE-L2a, tripartite CPSF6::RARG::LINE-L2a fusion as identified in case G9. CPSF6::RARG-delH11_12, simulated CPSF6::RARG fusion with artificial truncation of LBD-H11_H12 without a 3′ fusion partner. (G) HNRNPC::RARG, simulated bipartite HNRNPC::RARG fusion with intact LBD. HNRNPC::RARG::HNRNPC, tripartite HNRNPC::RARG::HNRNPC fusion as in case G3. HNRNPC::RARG-delH11_12, simulated HNRNPC::RARG fusion with artificial truncation of LBD-H11_H12 without a 3′ fusion partner. These results demonstrate that all bipartite chimeric proteins with intact LBD exhibit a dose-dependent response to ATRA, whereas those with LBD-H11_12/H12 truncation displayed minimal to no response. The experiments were performed using a GAL4-UAS reporter system and 293T cell line. Results are presented as the mean ± standard deviation of 3 biological replicates. Statistical analysis was performed using analysis of variance with Tukey correction for multiple comparisons. A representative experiment out of 3 with similar results is displayed.
The response of chimeric proteins to ATRA. (A) PML::RARA, bipartite PML::RARA fusion with intact LBD. PML::RARA-delH12, simulated PML::RARA fusion with artificial truncation of LBD helices 12 (LBD-H12) without a 3′ fusion partner. (B) STAT3::RARA, simulated bipartite STAT3::RARA fusion with intact LBD. STAT3::RARA::LTR40a, tripartite STAT3::RARA::LTR40a fusion as identified in index case A1. (C) STAT5B::RARA, simulated bipartite STAT5B::RARA fusion with intact LBD. STAT5B::RARA::AluSx4, tripartite STAT5B::RARA::AluSx4 fusion as identified in case A8. (D) HNRNPC::RARA, simulated bipartite HNRNPC::RARA fusion with intact LBD. HNRNPC::RARA::HNRNPC, tripartite HNRNPC::RARA::HNRNPC fusion as identified in case A11. (E) NPM1::RARG, simulated bipartite NPM1::RARG fusion with intact LBD. NPM1::RARG::NPM1, tripartite NPM1::RARG::NPM1 fusion as identified in index case G1. NPM1::RARG-delH11_12, simulated NPM1::RARG fusion with artificial truncation of LBD-H11_12 without a 3′ fusion partner. (F) CPSF6::RARG, simulated bipartite CPSF6::RARG fusion with intact LBD. CPSF6::RARG::LINE-L2a, tripartite CPSF6::RARG::LINE-L2a fusion as identified in case G9. CPSF6::RARG-delH11_12, simulated CPSF6::RARG fusion with artificial truncation of LBD-H11_H12 without a 3′ fusion partner. (G) HNRNPC::RARG, simulated bipartite HNRNPC::RARG fusion with intact LBD. HNRNPC::RARG::HNRNPC, tripartite HNRNPC::RARG::HNRNPC fusion as in case G3. HNRNPC::RARG-delH11_12, simulated HNRNPC::RARG fusion with artificial truncation of LBD-H11_H12 without a 3′ fusion partner. These results demonstrate that all bipartite chimeric proteins with intact LBD exhibit a dose-dependent response to ATRA, whereas those with LBD-H11_12/H12 truncation displayed minimal to no response. The experiments were performed using a GAL4-UAS reporter system and 293T cell line. Results are presented as the mean ± standard deviation of 3 biological replicates. Statistical analysis was performed using analysis of variance with Tukey correction for multiple comparisons. A representative experiment out of 3 with similar results is displayed.
Discussion
Pathological fusion genes play a significant role in leukemogenesis, with all historically reported as bipartite fusions.3-58 The primary mechanism responsible for fusion gene formation is chromosomal translocation, often resulting in reciprocal bipartite fusions like PML::RARA and RARA::PML. Previous studies on the canonical bipartite PML::RARA fusion in classical APL have provided comprehensive insights into its leukemogenesis and response to ATRA.1 However, diagnostic and therapeutic landscapes for PML::RARA–negative aAPL cases remain challenging.3-5
This study reveals that RAR genes undergo both 5′ and 3′ splicing, generating X::RAR::X– or X::RAR::Y–type tripartite fusions in aAPL cases. This finding expands the current understanding of leukemia fusion genes, suggesting that pathogenic fusion genes may possess a more complex structure beyond the conventional bipartite paradigm. Analyzing cis-aligned tripartite fusion remains challenging, especially with short-read NGS. The involvement of UTRs and TEs further complicates the identification and evaluation of the fusion transcripts, often leading to misreporting as inframe fusion by routine bioinformatic pipelines. Developing an optimized bioinformatic pipeline is needed to ensure the accurate reporting of tripartite fusions.
RAR proteins are highly homologous and function as ATRA receptors through their LBD.73 The canonical bipartite PML::RARA chimeric protein demonstrates dysregulated ATRA responsivity, maintaining transcriptional repression at physiological ATRA concentrations and contributing to differentiation blockade.1 Pharmacological concentrations of ATRA can rescue the hyporesponsiveness and elicit therapeutic effects in PML::RARA-APL. However, this efficacy is not observed in some aAPL cases with other RAR fusion genes, and the underlying mechanisms are not well understood.
This study presents evidence for shared etiologies of tripartite fusion in all RARG-aAPL and certain RARA-aAPL cases. Although the 3′ splicing patterns of RARA and RARG tripartite fusions differ, both lead to LBD-H11_12/H12 truncation. The pivotal role of the allosteric effect of LBD-H11_12 in RAR protein response to ATRA has been revealed for more than a decade,72 yet linking this structural property to disease was previously unrecognized. The discovery of tripartite RAR fusions illuminates the critical role of LBD-H11_12/H12 truncation in mediating ATRA unresponsiveness in some aAPL cases. Theoretically, LBD-H11_12 truncation directly prevents RAR proteins from binding with coactivator proteins in responding to ATRA, hindering the expression of their target genes. However, ATRA may also affect cell apoptosis and exert long-term response effects through other cellular pathways,74 and its multifaceted effects are complex, with potential interconnections between them. Thus, further study is necessitated to investigate the overall impact of LBD-H11_12 truncation.
ATRA responses in RARA-aAPL cases with different 5′ fusion partners vary widely, ranging from complete resistance to excellent sensitivity.4,5 This study found tripartite RARA fusions in aAPL with a 5′ partner gene–associated pattern. Specifically, all cases with STAT3/5B and HNRNPC as the 5′ partner showed tripartite RARA fusions, whereas all cases with PML, TTMV-ORF2, IRF2BP1/2, and TNRC18 as the 5′ partner showed bipartite RARA fusions. Because of the rarity and diversity of RARA-aAPL, this study covered only a limited number of RARA fusion partners and cases. Further investigations are warranted to explore tripartite fusion among the full spectrum of RARA fusion partners. The underlying mechanisms of why some RARA fusion genes require both 5′ and 3′ fusions whereas others only need 5′ fusions merit further investigation.
In contrast, RARG-aAPL cases with diverse fusion partners exhibit uniform clinical resistance to ATRA.30 In this study, all 21 RARG-aAPL cases with 8 different 5′ partner genes exhibited tripartite RARG fusion genes. These findings highlight the mechanistic differences between RARA-aAPL and RARG-aAPL. Although RARG is highly homology to RARA, it shows distinct ATRA response kinetics with significantly higher sensitivity.73 One speculated mechanism is that when the bipartite X::RARG fusion forms, its intact LBD possesses adequate ATRA responsiveness, as observed in this study and others' experiments.55-57 Therefore, an additional 3′ splicing leading to LBD-H11_12 truncation is necessary to completely abolish its high sensitivity to ATRA. This suggests that different ATRA response kinetics of RAR proteins might contribute to the disparate ontogeny of aAPL cases, warranting further study.
There were noteworthy differences in both 5′ and 3′ splicing patterns between RARA-aAPL and RARG-aAPL. RARA 5′ splices consistently reside at the beginning of exon 3, with fusion partners conferring a functional coding sequence in all X::RARA fusions.1,3,4 In contrast, RARG 5′ splice sites are variable, and the fusion partners may not retain their coding regions. In contrast, the RARG 3′ splices site is fixed, whereas the RARA 3′ splices site shows variability within the LBD-H11_12 coding region. These findings also suggest subtle differences in the properties of RAR proteins and their association with leukemia ontogeny.
We observed extensive implications of TEs in constructing tripartite fusions. Although TEs are notoriously difficult to analyze because of sequence variability, recent studies have highlighted their roles in carcinogenesis.75 TEs can impart mobile genetic fragments through transposon or retrotransposon mechanisms and, therefore, have the potential to introduce ectopic poly(A) signals. This study is, to our knowledge, the first to document TEs in constructing leukemia fusion genes, potentially through aberrant activation and transposition mechanisms. This finding expands the understanding of fusion gene ontogeny and unveils new research avenues.
This study elucidates the pivotal role of tripartite fusion and LBD-H11_12/H12 truncation in the molecular etiology of RARG-aAPL and certain RARA-aAPL cases. The tripartite fusion orchestrates the aberrant activation of RARs through 5′ splicing and renders LBD-H11_12/H12 allosteric dysfunction and ATRA unresponsiveness through 3′ splicing. The construction of tripartite fusions necessitates multiple splicing or transposition events, explaining the rarity of related aAPL. It also highlights substantial molecular etiological differences among RARG-aAPL, RARA-aAPL, and the archetypal PML::RARA-APL, inspiring several refined research directions. Clarifying the molecular etiology of ATRA unresponsiveness provides a substantial basis to avoid the ineffective use of ATRA in tripartite fusion-related aAPL cases and indicates the correct research targets for rescuing ATRA responsiveness.
Acknowledgments
The authors are grateful to Siyuan Liu and Qihui Chen for their assistance in bioinformatic analysis and valuable discussions. The authors also thank Tyler Lu for his valuable suggestions in revising the manuscript.
This work was supported, in part, by grants from the training plan for academic and technical leaders of major disciplines in Jiangxi Province (20213BCJ22015) and the Natural Science Foundation of Hunan Province (2024JJ6566).
Authorship
Contribution: H.L. and. X.C. wrote the manuscript; H.L. conceptualized and designed the study; D.L. and P.L. provided valuable discussion and direction; X. Zhou, X.C., J.C., P.C., F.W., Yang Zhang, Xiaoli Ma, J.F., P.W., T.W., Xiujuan Ma, Q.W., G.F., and D.J. were responsible for laboratory experiments, bioinformatic analysis, and interpretation; and S.C, L. Wen, Zhanglin Zhang, Y.-Z.Q., H.X., Y.M., W.W., G.Z., J.L., H.W., Zhifen Zhang, J.Z., Z.S., Yang Zhao, X. Zhang, Zhihua Zhang, L. Wang, F.M., X.X., C.W., K.S., R.T., Yun Zhang., S.W., R.G., L.Z., H.Z., Yanli Zhao, and H.-H.Z. provided study materials or recruited patient.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Hongxing Liu, 22 Tongji South Road, Beijing 100176, China; email: starliu@pku.edu.cn; Suning Chen, 899 Pinghai Road, Suzhou 215006, China; email: chensuning@suda.edu.cn; and Peihua Lu, 22 Tongji South Road, Beijing 100176, China; email: peihua_lu@126.com.
References
Author notes
X. Zhou, X.C., J.C., L. Wen, Zhanglin Zhang, Y.-Z.Q., and P.C. contributed equally to this study.
Presented, in abstract form, at the 65th annual meeting of the American Society of Hematology, San Diego, CA, 9 December 2023.
For original data, please contact corresponding author Hongxing Liu (starliu@pku.edu.cn); related genomic data will be made available upon request.
The online version of this article contains a data supplement.
There is a Blood Commentary on this article in this issue.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.


This feature is available to Subscribers Only
Sign In or Create an Account Close Modal