Atypical acute promyelocytic leukemias (aAPLs) are usually clinically resistant to arsenic trioxide and retinoic acid (ATRA) therapy. In this issue of Blood, Zhou et al1 have demonstrated, through careful genomic exploration, the existence of tripartite RARG or RARA fusions deleted from their ATRA-responsive transactivation domain, molecularly explaining these resistances.
“Classic” APLs are driven by the PML/RARA fusion, explaining their exquisite sensitivity to ATRA, which respectively binds the PML and RARA parts of the fusion to initiate its degradation.2 Rare APL variants are driven by PLZF/RARA fusions and are resistant to arsenic, as expected, but also to ATRA. In aAPLs, a constellation of extremely rare other fusions were described, always involving 1 of the 3 retinoic acid receptors (RARs),3 pointing to the central role of deregulated ATRA signaling in APL initiation. RARs are composed of separated protein modules including a DNA-binding domain, which ensures sequence-specific recognition of target genes regulatory sequences and an allosterically regulated hormone-binding domain, where the ligands shift the conformation from a repressive form to an activated one. Molecularly, this is achieved by exposing a short α-helix (H12) that recruits the transcriptional machinery. In the setting of patients with aAPL treated with ATRA, it may be difficult to unambiguously assess immediate differentiation and long-term leukemia clearance, particularly in the context of ATRA given with chemotherapy. An unresolved issue is the general lack of ATRA response of most aAPLs associated with X-RARA fusions and of all aAPLs driven by X-RARG fusions. Unexpectedly, mouse models where RARA, RARB, or RARG were fused to PML induced APLs that were all exquisitely sensitive to ATRA-induced differentiation.4
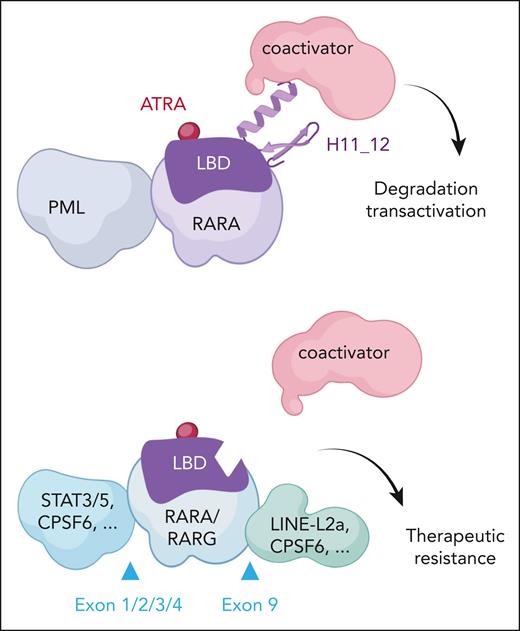
Zhou et al show definitive evidence that provides a new explanation for the pathogenesis of aAPL and for the reason aAPLs are ATRA resistant. Generalizing from sporadically reported cases, the authors demonstrate that all aAPLs involving RARG actually exhibit 2, rather than 1, genetic changes, so that the coding sequence of RARG is not only placed under the transcriptional control of another gene (sometimes acquiring novel N-terminal sequences) but also critically loses H12 (see figure). Occurrence of these 2 independent hits targeting the same RARG allele is more complicated than a single rearrangement, likely explaining the extreme rarity of these aAPLs. It is fascinating that many of the H12 deletions could be traced to endogenous retroviruses that perturbed splicing. In the emerging field of retroelement implication in homeostasis or pathogenesis, this constitutes a striking example of their involvement in oncogenesis.5 The encoded RARG proteins are devoid of transactivation capacity, likely behaving as dominant negatives. Therapeutically, absence of H12 clearly precludes ATRA-induced transcriptional activation (as demonstrated by the authors), and resistance to ATRA-induced degradation is also expected6 but was not assessed.
RAR fusions in classical and atypical APL response to ATRA therapy. (Top) Classic PML::RARA responds to ATRA therapy. ATRA binds the ligand binding domain (LBD), inducing a conformational change, coactivator recruitment, transactivation, and fusion protein degradation. (Bottom) The various tripartite RARA or RARG fusions with LBD-H11_12 truncations fail to bind coactivators, leading to ATRA resistance. Examples of N- or C-terminal sequences, as well as positions of the junctions, are shown.
RAR fusions in classical and atypical APL response to ATRA therapy. (Top) Classic PML::RARA responds to ATRA therapy. ATRA binds the ligand binding domain (LBD), inducing a conformational change, coactivator recruitment, transactivation, and fusion protein degradation. (Bottom) The various tripartite RARA or RARG fusions with LBD-H11_12 truncations fail to bind coactivators, leading to ATRA resistance. Examples of N- or C-terminal sequences, as well as positions of the junctions, are shown.
A set of very similar observations were made in many, but not all, variant X-RARA fusions associated with aAPLs, pointing to a broader implication of this novel mechanism of tripartite oncogenic retinoic acid receptor fusions (see figure). For example, 73% of RARA tripartite fusions were detected in those APLs involving STAT3/5 as a 5′ fusion partner. The tripartite RARA fusions again deleted H12, creating a dominant-negative RARA, functionally similar to those with established oncogenic properties.7 Here again, the resulting fusion protein was unresponsive to ATRA for transcriptional control.
These observations are important, clarify some long-standing issues, but also raise intriguing questions. Active repression by RAR is important in development,8 and deregulated retinoic acid signaling is clearly at the root of APL initiation. It is generally assumed that this is through repression. Yet PML/RARA was shown to be an activator for some genes.9 The tripartite fusions identified here highlight the key role of fusion-enforced repression in APL pathogenesis, since their transcriptional activation domain was lost. How basal repression is related to the preferential involvement of PML and RARA in classic APLs remains unknown. In several models, RARA overexpression on its own can promote immortalization ex vivo and even initiate an RA-responsive APL-like disease in patients as a result of insertional mutagenesis by torque teno virus.10 Here, all 5′ fusion partners displayed medium to high expression in hematopoietic cells, suggestive for a significant level of protein expression of the tripartite RAR fusions.
Overall, these exciting new developments help explain the basis for ATRA resistance of many aAPLs, highlighting a novel facet of APL as a model of targeted therapies, and provide an unambiguous implication of endogenous transposable elements in leukemogenesis.
Conflict-of-interest disclosure: The authors declare no competing financial interests.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal