We report on the antileukemic activity of homoharringtonine (HHT) in T-cell acute lymphoblastic leukemia (T-ALL). We showed that HHT inhibited the NOTCH/MYC pathway and induced significantly longer survival in mouse and patient-derived T-ALL xenograft models, supporting HHT as a promising agent for T-ALL.
TO THE EDITOR:
T-cell acute lymphoblastic leukemia (T-ALL) accounts for 15% of pediatric and 25% of adult ALL cases.1,2 Although allogeneic hematopoietic stem cell transplantation may be curative for patients with T-ALL, disease relapses often occur. Once relapsed, the disease has a very dismal prognosis. Therefore, novel agents are urgently needed.
Herein, we report on the activity of homoharringtonine (HHT), a known potent inhibitor of messenger RNA (mRNA) translation, on T-ALL. HHT is an alkaloid derived from trees of the genus Cephalotaxus3 and has been approved by the US Food and Drug Administration for the treatment of chronic myeloid leukemia (CML).4 The antileukemic activity of HHT has been reported in patients with acute myeloid leukemia (AML) or CML5-7 but not in T-ALL. Mechanistic studies showed that HHT binds to the A-site cleft of the large ribosomal subunit and interferes with the elongation of protein biosynthesis, thereby blocking the transcription of short–half-life oncoproteins, for example, myelocytomatosis oncogene (MYC), catenin beta 1 (CTNNB1), and myeloid leukemia 1 (MCL1).3,8 Additional studies showed that HHT could downregulate MYC transcriptional expression by directly binding to the NF-κB repressing factor in AML.9
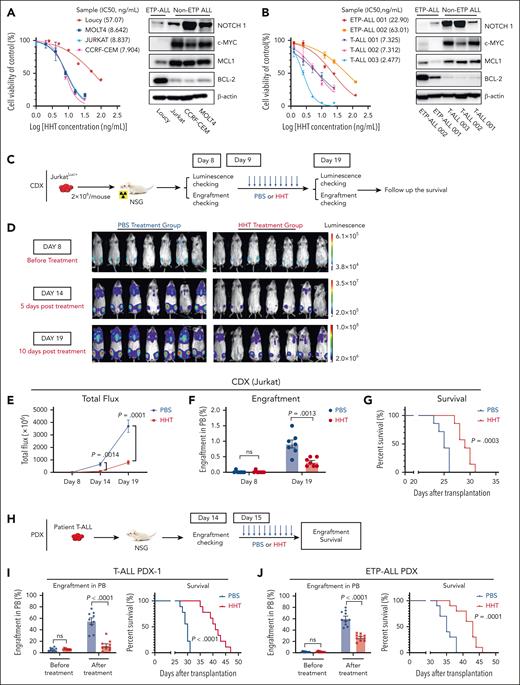
It has been reported that aberrant MYC expression is responsible for initiating and maintaining T-ALL in zebrafish10 and murine models,11-13 and high MYC levels are frequently detected in patients with T-ALL,12,14 suggesting that targeting MYC may be a therapeutic strategy. Therefore, although several new agents including nelarabine,15 bortezomib,16 and CD38-specific daratumumab17 have been reported to be effective in patients with T-ALL, novel agents that inhibit MYC pathway are worth pursuing. To study the activity of HHT in T-ALL, we first treated 4 T-ALL cell lines, including 1 early T-cell precursor (ETP)-ALL (Loucy) and 3 differentiated T-ALL (ie, Jurkat, MOLT4, and CCRF-CEM) cell lines. All 3 differentiated T-ALL cell lines showed a significantly higher sensitivity to HHT (50% inhibitory concentration [IC50], 5-10 ng/mL) than Loucy cells (IC50: 57.07 ng/mL) after 24 hours’ treatment (Figure 1A, left) with a dose-dependent reduction in viability and an increase in apoptosis and cell cycle arrest in G0 and a reduction in colony formation cells (supplemental Figure 1A-C, available on the Blood website). Interestingly, we observed higher levels of neurogenic locus notch homolog protein 1 (NOTCH1), c-MYC, and MCL1 in differentiated T-ALL than in ETP-ALL cells, which seem to negatively correlate with their IC50 values for HHT (Figure 1A; supplemental Figure 2A-B). Consistent with a previous report,18 B-cell lymphoma 2 (BCL-2) levels were higher in ETP-ALL cells. We also observed a higher sensitivity to HHT, higher levels of NOTCH1, c-MYC, and MCL1, and lower levels of BCL-2 in cells of patients with T-ALL (IC50, 2-10 ng/mL) than in blasts of patients with ETP-ALL (IC50, 22.90-63.01 ng/mL) after 24 hours’ treatment (correlation of IC50 and NOTCH1 level, Spearman r = −1.000; P = .0167; Figure 1B; supplemental Figure 2C-D; supplemental Table 1). Of note, peripheral blood mononuclear cells from healthy donors were not sensitive to HHT (IC50: 45-70 ng/mL; supplemental Figure 1D). HHT induced a dose-dependent increase in apoptosis in primary T-ALL cells (supplemental Table 2 contains demographic data, mutations, and cytogenetics; supplemental Figure 3). Of note, the ETP-ALL patient sample (ETP-ALL-001) with Notch1 mutation and in turn higher protein level of NOTCH1 also has lower IC50 value for HHT (Figure 1B). These results suggest that the higher sensitivity of T-ALL to HHT is likely associated with their higher levels of NOTCH1, c-MYC, and MCL1.
HHT showed in vitro and in vivo antileukemic activity in T-ALL. (A) T-ALL cell lines, including the ETP-ALL cell line Loucy, were treated with HHT for 24 hours. Cell viability was assessed using the CellTiter-Glo, and IC50 at 24 hours was calculated (left). The values were normalized to the average of the untreated samples for each cell line. Western blot analysis was conducted to assess NOTCH1, c-MYC, MCL1, and BCL-2 levels in T-ALL cell lines with β-actin as the loading control (right). (B) Cell viability of primary blasts of patients with T-ALL treated with HHT for 24 hours was assessed using CellTiter-Glo. The IC50 at 24 hours was calculated (left). Western blot analysis of NOTCH1, c-MYC, MCL1, and BCL-2 levels was performed in samples of patients with T-ALL with β-actin as the loading control (right). (C-G) Experimental design and results of the cell line-derived xenograft (CDX) model. (C) Schematic experimental design. JurkatLuc+ T-ALL cells (2 × 106 cells per mouse) were IV injected into NSG mice. Eight days later, these mice (n = 7 for each group) were treated with vehicle (PBS) or HHT (1 mg/kg body weight) for 10 days. (D-E) Leukemic burden was determined by luminescence imaging on day 8 (before treatment), day 14 (5 days after treatment), and day 19 (10 days after treatment). (F-G) Percentages of blasts in peripheral blood (PB) (F) and survival (G) of the above 2 groups of mice are shown. (H-J) Experimental design and results of the PDX models. (H) Schematic experimental design of the PDX models. Two PDXs were generated by transplanting blasts from 1 patient with T-ALL or from 1 patient with ETP-ALL (1 × 106 cells per mouse) IV into NSG mice. Two weeks later, these mice were treated with vehicle (PBS) or HHT (1 mg/kg body weight) for 10 to 12 days. (I-J) The percentage of blasts in the PB and survival of the T-ALL PDX (I; n = 9 mice for each group) and ETP-ALL PDX (J; n = 10 mice for each group) models are shown. For the western blot (WB) analysis in panels A-B, 1 of 3 independent experiments with similar results are shown. Statistical analysis was conducted using the 2-tailed, unpaired Student t test. Survival was analyzed using the log-rank test. Results shown represent the mean ± standard error of mean (SEM). Significance values: ns, not significant.
HHT showed in vitro and in vivo antileukemic activity in T-ALL. (A) T-ALL cell lines, including the ETP-ALL cell line Loucy, were treated with HHT for 24 hours. Cell viability was assessed using the CellTiter-Glo, and IC50 at 24 hours was calculated (left). The values were normalized to the average of the untreated samples for each cell line. Western blot analysis was conducted to assess NOTCH1, c-MYC, MCL1, and BCL-2 levels in T-ALL cell lines with β-actin as the loading control (right). (B) Cell viability of primary blasts of patients with T-ALL treated with HHT for 24 hours was assessed using CellTiter-Glo. The IC50 at 24 hours was calculated (left). Western blot analysis of NOTCH1, c-MYC, MCL1, and BCL-2 levels was performed in samples of patients with T-ALL with β-actin as the loading control (right). (C-G) Experimental design and results of the cell line-derived xenograft (CDX) model. (C) Schematic experimental design. JurkatLuc+ T-ALL cells (2 × 106 cells per mouse) were IV injected into NSG mice. Eight days later, these mice (n = 7 for each group) were treated with vehicle (PBS) or HHT (1 mg/kg body weight) for 10 days. (D-E) Leukemic burden was determined by luminescence imaging on day 8 (before treatment), day 14 (5 days after treatment), and day 19 (10 days after treatment). (F-G) Percentages of blasts in peripheral blood (PB) (F) and survival (G) of the above 2 groups of mice are shown. (H-J) Experimental design and results of the PDX models. (H) Schematic experimental design of the PDX models. Two PDXs were generated by transplanting blasts from 1 patient with T-ALL or from 1 patient with ETP-ALL (1 × 106 cells per mouse) IV into NSG mice. Two weeks later, these mice were treated with vehicle (PBS) or HHT (1 mg/kg body weight) for 10 to 12 days. (I-J) The percentage of blasts in the PB and survival of the T-ALL PDX (I; n = 9 mice for each group) and ETP-ALL PDX (J; n = 10 mice for each group) models are shown. For the western blot (WB) analysis in panels A-B, 1 of 3 independent experiments with similar results are shown. Statistical analysis was conducted using the 2-tailed, unpaired Student t test. Survival was analyzed using the log-rank test. Results shown represent the mean ± standard error of mean (SEM). Significance values: ns, not significant.
Next, we established a xenograft model by transplanting firefly luciferase and green fluorescent protein (GFP)-expressing Jurkat cells (JurkatLuc+, 2 × 106 per mouse) IV into NSG mice (Figure 1C). On day 9 after engraftment, these mice were randomly divided into 2 groups and treated with either vehicle (phosphate-buffered saline [PBS]) or HHT (1 mg/kg per day) for 10 days (Figure 1C). We observed a significantly decreased leukemic burden measured by bioluminescence imaging on day 14 and 19 (Figure 1D-E), decreased GFP+CD3+ T-ALL engraftment rates (P = .0013; Figure 1F), and a significantly longer survival (P = .0003; Figure 1G) in HHT-treated vs PBS-treated mice.
Furthermore, we generated 4 patient-derived xenograft (PDX) models by transplanting blasts from 3 patients with T-ALL and 1 patient with ETP-ALL into NSG mice. Two to 3 weeks after transplantation, all 4 models were treated with PBS or HHT (1 mg/kg per day) for 10 to 12 days (Figure 1H). HHT significantly reduced circulating T-ALL (human CD45+) engraftment and prolonged the survival of all 4 PDX models when compared with the PBS treatment (median survival: T-ALL PDX-1 [41 vs 30 days; P < .0001]; T-ALL PDX-2 [54 vs 44.50 days; P = .0098]; T-ALL PDX-3 [80 vs 63 days; P < .0001]; ETP-ALL PDX [43 vs 35 days, P = .0001]; Figure 1I-J; supplemental Figure 4A-D). For all 4 models, no significant changes in behavior, activity, and weight were observed in the HHT-treated vs PBS-treated mice (supplemental Figure 4E).
To gain further insight into the antileukemic mechanisms of HHT in T-ALL cells, we performed RNA sequencing on Jurkat cells treated with PBS or HHT (10 or 15 ng/mL for 24 hours). We identified 566 differentially expressed genes (DEGs), 418 upregulated and 148 downregulated (supplemental Figure 5A). Gene set enrichment analysis identified 6 gene sets significantly downregulated in HHT-treated cells (Figure 2A; supplemental Figure 5B). Compared with the control cells, HHT-treated T-ALL cells exhibited decreased expression of several genes linked to the NOTCH (NOTCH1, NOTCH3, FZD1, and FZD5), MYC (MYC, MYCL, EIF4A1, and CDK4), mTORC1 (MCM2, G6PD, and PRA1), or PI3K-AKT (AKT1, AKT2, and GSK3A) pathways (supplemental Figure 5C).
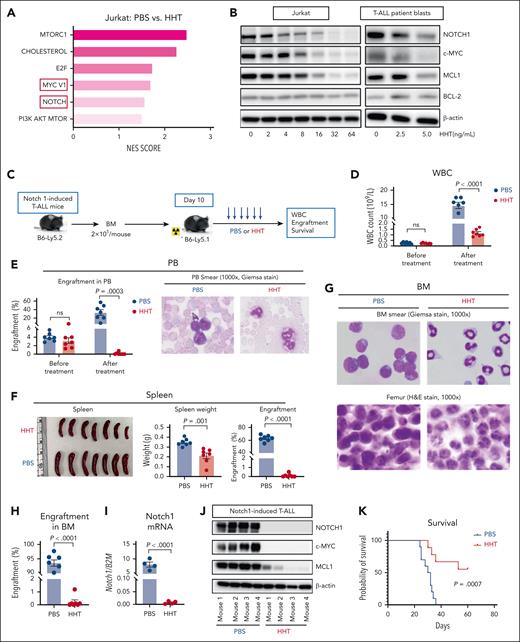
HHT exhibits antileukemic activity by inhibiting the NOTCH1/MYC pathway. (A) RNA sequencing analysis was performed on Jurkat cells treated with PBS or HHT (10 or 15 ng/mL) for 24 hours. Normalized enrichment scores (NES) of the top 6 gene sets downregulated by HHT treatment were identified through integrated analysis. (B) Jurkat cells (left) and blasts from patient with T-ALL (right, T-ALL 003) were treated with increasing concentrations of HHT for 24 hours, followed by western blot analysis for NOTCH1, c-MYC, MCL1, and BCL-2 levels with β-actin as the loading control. (C-K) Experimental design and results of the Notch1–induced T-ALL model. (C) Schematic experimental design. BM cells (2 × 105 cells per mouse) from the Notch1–induced T-ALL mice (B6-Ly5.2) were transplanted into congenic recipient mice (B6-Ly5.1, n = 7 mice per group), and 10 days after transplantation these mice were treated with vehicle (PBS) or HHT (1 mg/kg body weight). (D) White blood cell (WBC) counts; (E) percentages of leukemic blasts in PB and representative PB smears; (F) spleen size, weight, and percentages of leukemic blasts; (G) representative BM smears and femur hematoxylin and eosin (H&E) staining; (H) percentages of blasts in BM; (I) mRNA expression levels of Notch1 in BM cells; (J) western blot analysis of NOTCH1; c-MYC, and MCL1 in BM cells, with β-actin as the loading control; and (K) survival of the HHT-treated (n = 9) vs PBS-treated (n = 10) mice are shown. Statistical analysis was conducted using the 2-tailed, unpaired Student t test. Survival was analyzed using the log-rank test. Results shown represent the mean ± SEM. Significance values: ns, not significant.
HHT exhibits antileukemic activity by inhibiting the NOTCH1/MYC pathway. (A) RNA sequencing analysis was performed on Jurkat cells treated with PBS or HHT (10 or 15 ng/mL) for 24 hours. Normalized enrichment scores (NES) of the top 6 gene sets downregulated by HHT treatment were identified through integrated analysis. (B) Jurkat cells (left) and blasts from patient with T-ALL (right, T-ALL 003) were treated with increasing concentrations of HHT for 24 hours, followed by western blot analysis for NOTCH1, c-MYC, MCL1, and BCL-2 levels with β-actin as the loading control. (C-K) Experimental design and results of the Notch1–induced T-ALL model. (C) Schematic experimental design. BM cells (2 × 105 cells per mouse) from the Notch1–induced T-ALL mice (B6-Ly5.2) were transplanted into congenic recipient mice (B6-Ly5.1, n = 7 mice per group), and 10 days after transplantation these mice were treated with vehicle (PBS) or HHT (1 mg/kg body weight). (D) White blood cell (WBC) counts; (E) percentages of leukemic blasts in PB and representative PB smears; (F) spleen size, weight, and percentages of leukemic blasts; (G) representative BM smears and femur hematoxylin and eosin (H&E) staining; (H) percentages of blasts in BM; (I) mRNA expression levels of Notch1 in BM cells; (J) western blot analysis of NOTCH1; c-MYC, and MCL1 in BM cells, with β-actin as the loading control; and (K) survival of the HHT-treated (n = 9) vs PBS-treated (n = 10) mice are shown. Statistical analysis was conducted using the 2-tailed, unpaired Student t test. Survival was analyzed using the log-rank test. Results shown represent the mean ± SEM. Significance values: ns, not significant.
Interestingly, both the MYC target V1 and NOTCH signaling pathways were among the top 6 gene sets altered by HHT treatment with NOTCH1 and MYC being downregulated (Figure 2A; supplemental Figure 5B-C). These results were validated by western blot analyses, which showed an HHT dose-dependent downregulation of NOTCH1 and MYC both in the T-ALL cell line (ie, Jurkat) and in patient blasts (Figure 2B; supplemental Figure 6A). Of note, NOTCH1 is one of the most prevalently mutated oncogenes in T-ALL with >70% of T-ALL cases presenting with aberrant activation of NOTCH1 signaling,19,20 and the NOTCH1/MYC axis feeds a forward-loop transcriptional regulatory network, thereby promoting T-cell growth and transformation.14,21 Our data showed that, in addition to MYC, HHT also effectively inhibited NOTCH1, leading to eradication of T-ALL cells, a finding that has not been reported.
MCL1, a reported target of HHT in AML and CML,3,4,6 was also downregulated in HHT-treated Jurkat cells and blasts of patients with T-ALL, whereas no change in BCL-2 was observed (Figure 2B; supplemental Figure 6A). Consistent with the increased apoptosis in HHT-treated T-ALL cells (supplemental Figure 1A), we also observed increased mRNA expression of proapoptotic genes, for example, caspases-3, -6, -9 and -10, harakiri (HRK), and the BCL2 associated agonist of cell death (BAD) (supplemental Figure 6B).
Finally, we used a Notch1–induced T-ALL mouse model22,23 to confirm the HHT activity on NOTCH/MYC signaling and T-ALL in vivo. Bone marrow (BM) cells from the Notch1-induced T-ALL mice (CD45.2/B6) were transplanted into congenic normal wild-type recipients (CD45.1/B6, 2 × 105 per mouse; Figure 2C). Ten days after transplantation, the recipient mice were randomly divided into 2 groups and treated with either PBS or HHT (1 mg/kg per day; Figure 2C). After 6 day’s treatment, HHT-treated mice had significantly lower white blood cell counts and circulating leukemic blasts than the PBS-treated control mice (Figure 2D-E). HHT-treated mice also had smaller spleens (mean weight, 0.211 g vs 0.348 g; P = .001), decreased splenic or BM engraftment rates, and no or few detectable blasts (Figure 2F-H). We also observed a significant reduction in the mRNA levels of Notch1 and protein levels of NOTCH1, c-MYC, and MCL1 in the BM cells from HHT-treated vs PBS-treated mice (Figure 2I-J). Another cohort of Notch1-induced T-ALL mice was treated with HHT or PBS for 6 days and followed for survival. HHT-treated mice had significantly lower engraftment rates (supplemental Figure 7A) and longer survival than vehicle-treated mice (median survival, unreached vs 31 days; P = .0007; Figure 2K). Of the 9 mice treated with HHT, 5 had long-term survival with no evidence of blasts in the BM at day 60 after transplantation (supplemental Figure 7B), suggesting a likely cure with HHT monotherapy.
In summary, our results showed that HHT exhibited antileukemic activity in T-ALL mouse and PDX models, potentially through the inhibition of the NOTCH1/MYC pathway. Our study strongly supports HHT as a new promising agent that may optimize the current combination of chemotherapy for T-ALL.
ALL samples were obtained from donors and patients at the City of Hope National Medical Center (COHNMC) through the City Of Hope institutional review board (number 18067). Sample acquisition and analysis was approved by the institutional review board of the COHNMC and met all the requirements of the Declaration of Helsinki. Mouse care and experimental procedures were performed in accordance with the protocols approved by the COHNMC Institutional Animal Care and Use Committee.
Acknowledgments
The authors thank OE Biotech (Shanghai, China) for performing RNA sequencing and Jinyan Huang for the assistance with bioinformatics analysis. The authors acknowledge the support of the Animal Resources Center, Analytical Cytometry, and Pathology (Liquid Tumor) at the City of Hope Comprehensive Cancer Center. The authors are grateful to the City of Hope Comprehensive Cancer Center, the patients, and their physicians for providing primary patient material for this study.
This work was supported, in part, by the National Institutes of Health, National Cancer Institute (grants CA258981 and CA248475 [G.M., B.Z.]), the Gehr Family Foundation (G.M.), the National Natural Science Foundation of China (grants 82100160 [S.S.] and 82100159 [Y.Z.]), and the Zhejiang Provincial Natural Science Foundation of China (grant LQ22H080003 [S.S.]).
Authorship
Contribution: S.S. designed and conducted experiments, analyzed data, and wrote the manuscript; D.Z., F.L., Y.Z., S.R., and L.T.N. conducted experiments and analyzed data; L.G. provided samples of patients with T-cell acute lymphoblastic leukemia (T-ALL); N.C. provided Notch1–induced T-ALL mouse model and reviewed the manuscript; G.M., B.Z., and J.J. designed the experiments, analyzed data, wrote the manuscript, and provided administrative support; and all authors approved the final version of the manuscript.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Jie Jin, Department of Hematology, The First Affiliated Hospital, Zhejiang University School of Medicine, 79 Qingchun Rd, Hangzhou, 310003, China; email: jiej0503@zju.edu.cn; Bin Zhang, City of Hope Medical Center, 1500 E Duarte Rd, Duarte, CA 91010; email: bzhang@coh.org; and Guido Marcucci, City of Hope Medical Center, 1500 E Duarte Rd, Duarte, CA 91010; email: gmarcucci@coh.org.
References
Author notes
S.S. and D.Z. contributed equally to this study.
The RNA sequencing data were deposited in the National Center for Biotechnology Information Sequence Read Archive (BioProject accession number PRJNA1014743).
Supplemental information, including supplemental Figures 1-7 and supplemental Tables 1-3, are provided with the online version of this manuscript. All other data sets generated during this study are available on reasonable request from the corresponding author, Jie Jin (jiej0503@zju.edu.cn).
The online version of this article contains a data supplement.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal