Key Points
The major cause of death in patients with ataxia-telangiectasia and hematological malignancies is treatment-related toxicity.
The germ line ATM pathogenic variant functional class is a robust outcome predictor, which can be applied to therapy stratification.
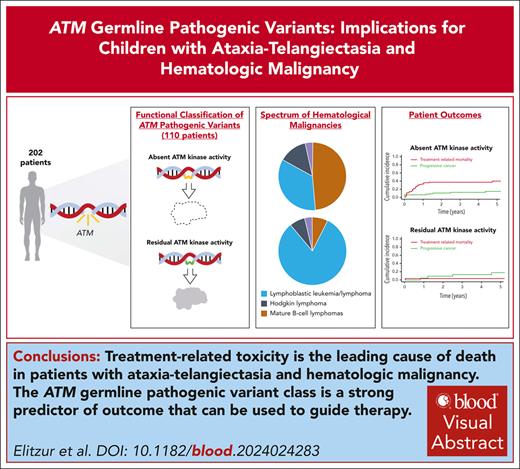
Visual Abstract
Ataxia-telangiectasia (A-T) is an autosomal-recessive disorder caused by pathogenic variants (PVs) of the ATM gene, predisposing children to hematological malignancies. We investigated their characteristics and outcomes to generate data-based treatment recommendations. In this multinational, observational study we report 202 patients aged ≤25 years with A-T and hematological malignancies from 25 countries. Ninety-one patients (45%) presented with mature B-cell lymphomas, 82 (41%) with acute lymphoblastic leukemia/lymphoma, 21 (10%) with Hodgkin lymphoma and 8 (4%) with other hematological malignancies. Four-year overall survival and event-free survival (EFS) were 50.8% (95% confidence interval [CI], 43.6-59.1) and 47.9% (95% CI 40.8-56.2), respectively. Cure rates have not significantly improved over the last four decades (P = .76). The major cause of treatment failure was treatment-related mortality (TRM) with a four-year cumulative incidence of 25.9% (95% CI, 19.5-32.4). Germ line ATM PVs were categorized as null or hypomorphic and patients with available genetic data (n = 110) were classified as having absent (n = 81) or residual (n = 29) ATM kinase activity. Four-year EFS was 39.4% (95% CI, 29-53.3) vs 78.7% (95% CI, 63.7-97.2), (P < .001), and TRM rates were 37.6% (95% CI, 26.4-48.7) vs 4.0% (95% CI, 0-11.8), (P = .017), for those with absent and residual ATM kinase activity, respectively. Absence of ATM kinase activity was independently associated with decreased EFS (HR = 0.362, 95% CI, 0.16-0.82; P = .009) and increased TRM (hazard ratio [HR] = 14.11, 95% CI, 1.36-146.31; P = .029). Patients with A-T and leukemia/lymphoma may benefit from deescalated therapy for patients with absent ATM kinase activity and near-standard therapy regimens for those with residual kinase activity.
Introduction
In recent years, it has become increasingly evident that both the spectrum and prevalence of genetic predisposition in children with cancer is higher than previously estimated.1-3 Yet, for most childhood cancer predisposition syndromes (CPS), comprehensive evidence to guide clinical practice remains limited and collaborative studies within international networks are urgently required.
Ataxia-telangiectasia (AT; Online Mendelian Inheritance in Man 208900) is an autosomal-recessive, multisystem disorder characterized by diverse manifestations,3-5 including progressive cerebellar degeneration, immunodeficiency, segmental premature aging, radiation sensitivity, and genome instability.4,6 AT arises from biallelic germ line pathogenic variants (PVs) in the ATM gene,7 resulting in complete absence or impaired function of the ATM protein. ATM, a multifunctional protein kinase, is primarily known for activating the cellular response to DNA double strand breaks and oxidative stress.8-12 The clinical phenotype of patients with AT is highly variable and ranges from individuals with an early onset, severe phenotype to patients with later-onset, milder symptoms. An important feature of AT is an increased predisposition to cancer,13 with a cumulative incidence of 23% at 20 years, mainly due to hematological malignancies.14 Cancer is a leading cause of mortality in patients with AT15,16 yet its treatment is extremely challenging. Between the risk of cancer progression, debilitating comorbidities, and a substantial susceptibility to chemotherapy-associated toxicity, the optimal therapeutic strategies have not yet been defined.17-20 Little is known regarding the treatment intensity required to provide a balance between efficacy and toxicity in this unique patient population. The lack of treatment guidelines reflects the limited data on treatment and outcomes in this patient population. Patients with AT and cancer are usually excluded from therapeutic clinical trials involving children with cancer. Limited information thus exists concerning their treatment outcomes and toxicity profiles, leaving optimal management strategies unclear and highlighting an unmet need.
In this multinational study, we investigate the characteristics and outcomes of leukemia and lymphoma in a large cohort of children with AT. Our aim is to determine risk factors associated with treatment outcomes in order to generate consensus and data-based prospective treatment recommendations.
Methods
Patients
This retrospective study, conducted from 1 February 2019 to 18 June 2023 via the International Berlin-Frankfurt-Münster Study Group with the addition of several North American centers, involved data collection through the review of medical records from institutional or national databases, overseen by the principal investigators. Eligible individuals comprised all patients aged ≤25 years diagnosed with confirmed AT and a hematological malignancy since 1985. The diagnosis of AT was based on identifying biallelic ATM PVs21; or on demonstrating absence or reduced level of activity of the ATM protein; or, in the absence of these, on international consensus clinical and laboratory criteria.22 These include ataxia and at least 2 of the following: oculocutaneous telangiectasia, elevated α-fetoprotein, chromosomal breakage/aberrations in lymphocyte metaphases,23 and/or evidence of cerebellar hypoplasia on magnetic resonance imaging.
A comprehensive review of patient medical records was conducted to collect relevant clinical data, including patient demographics, medical history, genetic testing results, hematological malignancy characteristics, treatment details, and outcomes. All patients were treated after obtaining a written informed consent from the patient, the patient’s parents, or legal guardians. This international study was conducted by the investigators at Schneider Medical Center of Israel (institutional review board approval: 0132-19-RMC), with the approval of review boards or ethics committees of all participating centers (supplemental Table 1, available on the Blood website).
Classification of germ line ATM PVs
Each reported ATM PV identified in the cohort was classified as null (resulting in complete loss of ATM activity) or hypomorphic (allowing residual ATM activity)15,24-34 according to the expected functional activity of the ATM protein and published functional studies. Patients with reported PVs or ATM protein activity testing results were then classified as those with absent ATM kinase activity (harboring biallelic null PVs) or those with residual kinase activity, harboring at least 1 hypomorphic ATM allele. Notably, monoallelic carriers of ATM PVs were not included in the study. A panel comprising scientists specializing in genetics and cancer, as well as geneticists and oncologists with expertise in ATM variant classification, conducted an independent review of the ATM PVs and their classification.
Clinical classifications
To assess the effects of comorbidities, patients were classified into 3 stages according to their baseline neurological status at cancer diagnosis: asymptomatic patients, those with an ataxic gait, and patients who were wheelchair bound.35 Patients were also classified into 3 stages according to their baseline respiratory status: asymptomatic, those with recurrent respiratory exacerbations/infections (≥3 episodes per year), and those with chronic bronchitis and/or bronchiectasis.36,37 Investigators reported whether patients had received treatment according to standard disease-based protocols or underwent significant treatment modifications, defined as administering at least 3 protocol drugs at <75% of their prescribed dosage. Patients were further classified into those treated with curative intent and those who received only supportive palliative care.
Statistical analyses
Outcome measures included event free survival (EFS), defined as time from cancer diagnosis to first event, either relapse, secondary malignancy, or death of any cause; and overall survival, calculated from cancer diagnosis to death. In patients diagnosed with >1 hematological malignancy, diagnosis of the first neoplasm was considered the time point of cancer diagnosis. To determine the causes of therapy failure in this patient population, additional outcome measures were evaluated, including the cumulative rates of progressive cancer, second malignancy, and cancer TRM. Progressive cancer was defined as relapsed (cancer recurrence after achieving complete remission, defined for leukemia as morphological remission) or refractory cancer, defined in this study as nonremission despite ≥3 months of therapy. TRM was defined as any death resulting from cancer therapy when the cancer was in remission, as well as early death (within the first 3 months of therapy) resulting from cancer therapy, regardless of remission status. The classification of death as TRM was determined by the local investigator. Only patients who were treated with curative intent were included in the outcome analyses. Time was censored at the date of last patient contact if no event had occurred. The Kaplan-Meier method was used to estimate survival rates, and curves were compared with the log-rank test. Cumulative incidence functions were constructed using the method of Kalbfleisch and Prentice and compared with the Gray test after adjusting for competing risks of other events. The Cox and Fine-Gray proportional hazards regression models38 have been used, respectively, to estimate EFS and TRM hazard ratios (HRs) in multivariate analyses of prognostic factors. Because the main focus of our study was cancer-related events, we also performed univariate and multivariate analyses at a predefined time point of 4 years from cancer diagnosis on the basis of log-log transformation.39 Focusing on the 4-year time point reduced the impact of later events, which were not associated with cancer or cancer therapy on survival estimates.
Other comparisons were performed using the Fisher exact test and the Brown-Mood median test for comparing medians. A 2-sided P value ≤.05 was considered statistically significant. All analyses were performed with the R Project for Statistical Computing, version 4.3.1.
Results
A cohort of 202 patients, diagnosed with AT and hematological malignancies between 1985 and 2023, was identified across 25 countries (supplemental Figure 1). Among these cases, 185 patients received treatment with curative intent and were included in the subsequent outcome analyses.
Patient characteristics
Of a total of 202 patients in the cohort, 79 (39%) were female. The median age at cancer diagnosis for the cohort was 8.9 years (range, 0.5-25). AT was diagnosed before cancer in 119 of 151 patients with available data (79%).
Classification of germ line ATM PV
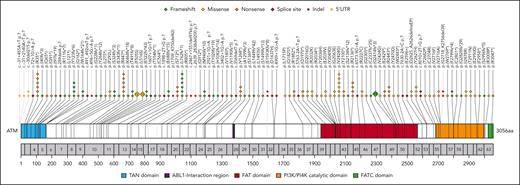
In our cohort of 202 patients, genomic sequencing confirmed AT in 102 cases (50%), revealing pathogenic ATM PVs. Of these, 58 patients were homozygous whereas 44 were compound heterozygotes. Altogether, 105 unique ATM variants were reported (Figure 1). In 14 additional cases, for which genomic sequencing was unavailable, specialized laboratories confirmed the absence or deficiency of the ATM protein or its activity. In the remaining 86 cohort patients, AT was diagnosed based on clinical and laboratory criteria (supplemental Figure 2). In total, of 116 patients with available data on germ line ATM variants or on ATM kinase activity, 81 (74%) were classified as having absent kinase activity (harboring 2 null PVs), 29 (26%) as having residual kinase activity (with at least 1 hypomorphic allele), and in 6 cases the functional consequences remained unknown (supplemental Table 2). A comparison of demographic characteristics between classifiable and nonclassifiable patients is presented in supplemental Table 3.
Deleterious germ line variants in ATM observed in the study cohort. The upper panel of the plot shows the predicted amino acid substitutions resulting from the determined germ line ATM variants identified in this study. Each rhomb/circle represents a detected pathogenic variant; for frequent variants (n>6), enlarged icons were generated, with the frequency written inside. Numbers in the grey bar denote the respective exon, and numbers below correlate to the amino acid sequence. Exonic variants are depicted by rhombs and black lines, while indels and 5' UTR variants are represented by circles and grey lines. The color codes representing the mutation types are as indicated in the upper right corner of the plot.
Deleterious germ line variants in ATM observed in the study cohort. The upper panel of the plot shows the predicted amino acid substitutions resulting from the determined germ line ATM variants identified in this study. Each rhomb/circle represents a detected pathogenic variant; for frequent variants (n>6), enlarged icons were generated, with the frequency written inside. Numbers in the grey bar denote the respective exon, and numbers below correlate to the amino acid sequence. Exonic variants are depicted by rhombs and black lines, while indels and 5' UTR variants are represented by circles and grey lines. The color codes representing the mutation types are as indicated in the upper right corner of the plot.
The spectrum of hematological malignancies in patients with AT
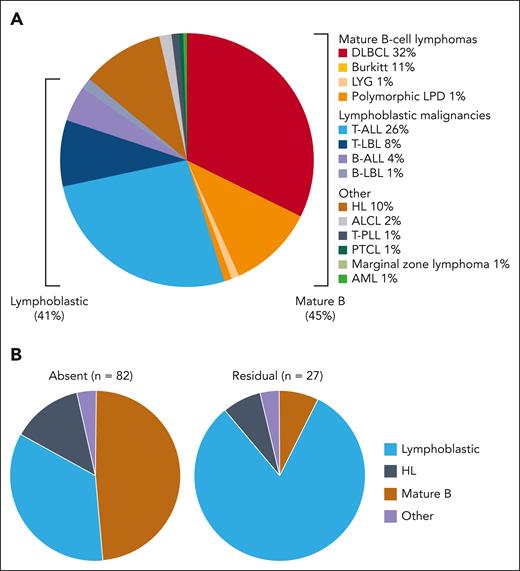
The spectrum of the hematological malignancies in our cohort is presented in Figure 2A. In total, 91 patients (45%) were diagnosed with mature B-cell lymphomas; 82 (41%) with lymphoblastic malignancies (acute lymphoblastic leukemia [ALL] or lymphoma); 21 (10%) with Hodgkin lymphoma; and 8 (4%) with other, less common, hematological malignancies. Patient characteristics according to these tumor subgroups are summarized in supplemental Table 4. Tumors of T-cell lineage were predominant in the lymphoblastic group (70 of 82 patients; 85%). Interestingly, T-cell receptor γδ expression was found positive in 10 of 16 T-cell ALL (T-ALL) cases (63%) in which it had been assessed. Importantly, the distribution of tumor types differed significantly between patients with absent and residual ATM kinase activity: 34% vs 82% presented with lymphoblastic leukemia/lymphoma, respectively (P < .001; Figure 2B).
The spectrum of hematological malignancies. (A) The spectrum of hematological malignancies in the entire cohort (202 patients). (B) The differential tumor spectrum in patients with absent ATM kinase activity and in those with residual ATM kinase activity. Patients with absent ATM kinase activity (n = 82): lymphoblastic leukemia/lymphoma (34%), mature B-cell lymphoma (49%), HL (13%), and other (4%). Patients with residual ATM kinase activity (n = 27): lymphoblastic leukemia/lymphoma (82%), mature B-cell lymphoma (7%), HL (7%), and other (4%). ALCL, anaplastic large cell lymphoma; AML, acute myeloid leukemia; DLBCL, diffuse large B-cell lymphoma; HL, Hodgkin lymphoma; LBL, lymphoblastic lymphoma; LPD, lymphoproliferative disorder; LYG, lymphomatous granulomatosis; PTCL, peripheral T-cell lymphoma; T-PLL, T-cell prolymphocytic leukemia.
The spectrum of hematological malignancies. (A) The spectrum of hematological malignancies in the entire cohort (202 patients). (B) The differential tumor spectrum in patients with absent ATM kinase activity and in those with residual ATM kinase activity. Patients with absent ATM kinase activity (n = 82): lymphoblastic leukemia/lymphoma (34%), mature B-cell lymphoma (49%), HL (13%), and other (4%). Patients with residual ATM kinase activity (n = 27): lymphoblastic leukemia/lymphoma (82%), mature B-cell lymphoma (7%), HL (7%), and other (4%). ALCL, anaplastic large cell lymphoma; AML, acute myeloid leukemia; DLBCL, diffuse large B-cell lymphoma; HL, Hodgkin lymphoma; LBL, lymphoblastic lymphoma; LPD, lymphoproliferative disorder; LYG, lymphomatous granulomatosis; PTCL, peripheral T-cell lymphoma; T-PLL, T-cell prolymphocytic leukemia.
Cancer therapy
A total of 185 patients (92% of the entire cohort) were treated with curative intent (supplemental Figure 1). The majority (135 patients) received attenuated therapy, individually modified at the discretion of the treating physician. Specifically, in 120 cases, therapy was deescalated upfront, whereas in 15 other cases, treatment was modified after therapy-associated toxicity. Overall, 32 patients received standard unmodified treatment regimens, and in 18 cases treatment data were unavailable. Of the 185 treated patients, 178 were treated with chemotherapeutic agents, 54 received immunotherapeutic agents with/without chemotherapy, 3 received cranial irradiation (supplemental Table 5), and 5 patients underwent hematopoietic stem cell transplantation. All 3 patients who underwent cranial irradiation harbored ATM PVs that allowed for residual kinase activity. None had been diagnosed with AT before cancer therapy and all subsequently developed second malignancies. Only 17 of 202 patients in our cohort (8%) were treated solely with palliative supportive care, all of whom died of progressive malignancy.
Treatment outcomes and patterns of treatment failure
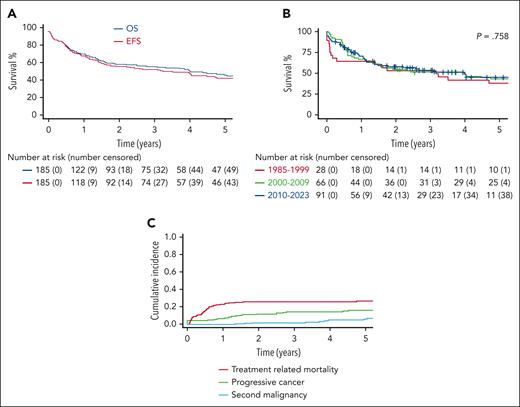
With a median follow-up of 4 years (range, 0.27-28.3), 4-year overall survival and EFS for the 185 treated patients were 50.8% (95% confidence interval [CI], 43.6-59.2) and 47.9% (95% CI, 40.8-56.2), respectively (Figure 3A). Surprisingly, cure rates of patients with AT and hematological malignancies did not improve significantly over the last 4 decades. The 4-year EFS rates were 41.6% (95% CI, 26.6-65.0), 49.6% (95% CI, 38.8-63.4), and 48.0% (95% CI, 37.4-61.7) for those treated before 2000, between 2000 and 2009, and between 2010 to 2023, respectively (P = .758; Figure 3B). The major cause of treatment failure was TRM, with a 4-year cumulative incidence of 25.9% (95% CI, 19.5-32.4), followed by progressive cancer in 14.5% (95% CI, 19.5-32.4) and second malignancy in 4.9% (95% CI, 1.3-8.4) (Figure 3C). Supplemental Figure 3 illustrates the various causes of TRM.
Treatment outcomes for all the 185 treated patients. (A) Kaplan-Meier estimates of EFS and overall survival. (B) Comparison of the EFS through different periods of time: 1985 to 1999, 2000 to 2009, and since 2010. (C) Patterns of treatment failure: TRM, progressive cancer, and second malignancy. P values are based on a comparison performed at a predefined time point of 4 years from cancer diagnosis based on log-log transformation.
Treatment outcomes for all the 185 treated patients. (A) Kaplan-Meier estimates of EFS and overall survival. (B) Comparison of the EFS through different periods of time: 1985 to 1999, 2000 to 2009, and since 2010. (C) Patterns of treatment failure: TRM, progressive cancer, and second malignancy. P values are based on a comparison performed at a predefined time point of 4 years from cancer diagnosis based on log-log transformation.
Prognostic factors
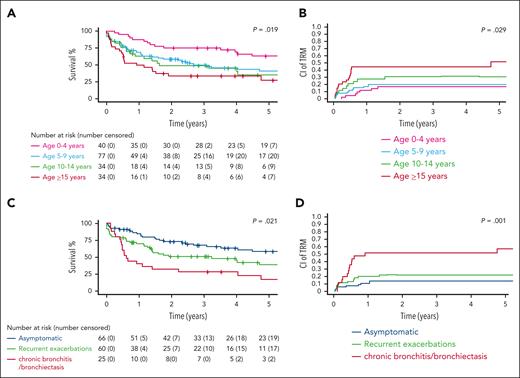
We identified factors that were significantly associated with survival for this unique patient population. Associations between patient characteristics and treatment outcomes are summarized in Table 1 and in supplemental Tables 6-8. Older age at cancer diagnosis had a significantly deleterious effect on survival (P = .019; Figure 4A) and was significantly associated with increased TRM rates (P = .029; Figure 4B). Advanced respiratory impairment (chronic bronchitis/bronchiectasis) was associated with a 4-year EFS of 28% (95% CI, 14.9-52.5) vs 44.9% (95% CI, 33-61.2) for those with recurrent respiratory exacerbations and 63% (95% CI, 51.7-76.8) for asymptomatic patients (P = .021; Figure 4C). Additionally, advanced respiratory impairment was significantly associated with increased TRM (P = .001; Figure 4D). The type of tumor significantly affected outcome: 4-year EFS was 31.1% (95% CI, 14.2-68) for patients with Hodgkin lymphoma, 38.9% (95% CI, 28.9-52.3) for those with mature B-cell lymphomas, and 63.9% (95 CI, 53.8-75.9) for patients with lymphoblastic malignancies (P = .004; supplemental Figure 4A). However, there were no significant differences between the different tumor types regarding rates of TRM, (P = .211; supplemental Figure 4B). Advanced neurological dysfunction (patients who are wheelchair bound) was associated with a 4-year EFS of 37.3% (95% CI, 26.4-52.8) compared with 50.8% (95% CI, 39-66.2) for those with an ataxic gait and 71.4% (95% CI. 56.5-90.3) for neurologically asymptomatic patients (P = .009; supplemental Figure 4C). However, the neurological status of patients was not significantly associated with TRM (P = .208; supplemental Figure 4D).
The association between demographics and treatment outcome
| Characteristic . | No. of pts . | No. of events . | 4-y EFS rate, % (95% CI) . | P value∗ . | No. of TRM events . | 4-y CI TRM, % (95% CI) . | P value∗ . | No. of progressive cancer events . | 4-y CI progressive cancer, % (95% CI) . | P value∗ . | No. of second malignancy events . | 4-y CI second malignancy, % (95% CI) . | P value∗ . | No. of deaths unrelated to cancer . | 4-y CI deaths unrelated to cancer, % (95% CI) . | P value∗ . |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Total | 185 | 90 | 47.9 (40.8-56.2) | — | 47 | 25.9 (19.5-32.4) | — | 26 | 14.5 (9.2-19.8) | 7 | 4.9 (1.3-8.4) | 10 | 6.5 (2.5-10.5) | |||
| Sex | .225 | .866 | .384 | .772 | .214 | |||||||||||
| Female | 72 | 40 | 41.9 (31.6-55.6) | 19 | 25.5 (17.3-33.7) | 12 | 14.9 (7.2-22.6) | 3 | 4.5 (0-9.5) | 6 | 9.7 (2.2-17.2) | |||||
| Male | 113 | 50 | 51.8 (42.7-62.8) | 28 | 26.6 (16.2-36.9) | 14 | 25.0 (11.9-38.1) | 4 | 5.4 (0.2-10.5) | 4 | 4.4 (0.1-8.8) | |||||
| Age at cancer diagnosis, y | .019 | .029 | .084 | .754 | <.001 | |||||||||||
| 0-4 | 40 | 12 | 69.1 (55.9-85.4) | 7 | 17.5 (5.5-29.4) | 2 | 6.3 (0-14.7) | 3 | 8.4 (0-17.7) | 0 | 0 | |||||
| 5-9 | 77 | 38 | 45.3 (34.6-59.4) | 15 | 19.8 (10.7-28.9) | 17 | 28.7 (15.8-41.7) | 2 | 3.6 (0-8.7) | 4 | 6.7 (0.2-13.3) | |||||
| 10-14 | 34 | 18 | 39.8 (25-63.3) | 10 | 31.1 (14.6-47.6) | 4 | 17.3 (1.1-33.4) | 1 | 4.1 (0-12.2) | 3 | 11.8 (0-25.2) | |||||
| ≤15 | 34 | 22 | 33.7 (20.8-54.6) | 15 | 44.6 (27.3-61.8) | 3 | 14.4 (0-30.5) | 1 | 3.1 (0-9.3) | 3 | 10.2 (0-21.5) | |||||
| Treatment era | .758 | .192 | .126 | <.001 | .426 | |||||||||||
| 1985-1999 | 28 | 16 | 41.6 (26.6-65) | 7 | 25.0 (8.5-41.4) | 4 | 15.9 (0.9-31.0) | 4 | 15.1 (1-29.3) | 1 | 3.8 (0-11.3) | |||||
| 2000-2009 | 66 | 33 | 49.6 (38.8-63.4) | 22 | 33.3 (21.8-44.8) | 5 | 9.4 (0.3-18.4) | 3 | 4.7 (0-10.1) | 3 | 4.5 (0-9.6) | |||||
| 2010-2023 | 91 | 41 | 48 (37.4-61.7) | 18 | 20.5 (11.9--29.1) | 17 | 26.3 (14.9-37.7) | 0 | 0 | 6 | 11 (1.8-20.2) | |||||
| Tumor type | .004 | .211 | .360 | .459 | <.001 | |||||||||||
| Mature B | 85 | 47 | 38.9 (28.9-52.3) | 22 | 26.5 (16.9-36.1) | 15 | 23.5 (11.7-35.3) | 1 | 1.9 (0-5.6) | 9 | 13.8 (5.1-22.4) | |||||
| Lymphoblastic | 79 | 27 | 63.9 (53.8-75.9) | 15 | 19.4 (10.5-28.2) | 8 | 13.0 (4.5-21.6) | 4 | 6.1 (0.2-11.9) | 0 | 0 | |||||
| Hodgkin lymphoma | 15 | 10 | 31.1 (14.2-68) | 6 | 40 (13.9-67.4) | 2 | 18.3 (0-43.9) | 1 | 7.8 (0-23.9) | 1 | 8.9 (0-26.9) | |||||
| Other | 6 | 6 | 4 | 1 | 1 | 0 | ||||||||||
| Baseline neurological status | .009 | .208 | .288 | .045 | .687 | |||||||||||
| Asymptomatic | 28 | 8 | 71.4 (56.5-90.3) | 5 | 17.8 (3.3-32.3) | 1 | 4.7 (0-14.1) | 1 | 3.6 (0-10.6) | 1 | 3.6 (0-10.6) | |||||
| Atactic gait | 64 | 28 | 50.8 (39-66.2) | 13 | 21.3 (10.8-31.7) | 8 | 17.1 (5.7-28.4) | 3 | 5.9 (0-12.5) | 4 | 8.4 (0.3-16.4) | |||||
| Wheelchair bound | 59 | 35 | 37.3 (26.4-52.8) | 19 | 33.0 (20.7-45.4) | 11 | 26.1 (10.9-41.2) | 0 | 0 | 5 | 10.2 (1.6-18.8) | |||||
| Unknown | 34 | 19 | 10 | 6 | 3 | 0 | ||||||||||
| Baseline respiratory status | .021 | .001 | .645 | <.001 | .374 | |||||||||||
| Asymptomatic | 66 | 22 | 63 (51.7-76.8) | 9 | 14.2 (5.5-22.9) | 9 | 18.2 (7.1-29.2) | 2 | 3.8 (0-9.0) | 2 | 3.9 (0-9.4) | |||||
| Recurrent respiratory infections (≥3 per y) | 60 | 30 | 44.9 (33-61.2) | 13 | 22.0 (11.3-32.8) | 9 | 18.4 (7.0-29.7) | 3 | 6.4 (0-13.8) | 5 | 11.1 (1.5-20.6) | |||||
| Chronic bronchitis/bronchiectasis | 25 | 18 | 28 (14.9-52.5) | 13 | 52 (31.8-72.2) | 2 | 7.7 (0-22.7) | 0 | 0 | 3 | 12 (6.8-25.4) | |||||
| Unknown | 34 | 20 | 12 | 6 | 2 | 0 | ||||||||||
| ATM mutation class | <.001 | .017 | .922 | .464 | <.001 | |||||||||||
| Absent ATM kinase activity | 77 | 43 | 39.4 (29-53.3) | 28 | 37.6 (26.4-48.7) | 10 | 17.2 (5.9-28.5) | 1 | 1.6 (0-4.7) | 4 | 7.6 (0.1-15) | |||||
| Residual ATM kinase activity | 25 | 5 | 78.7 (63.7-97.2) | 1 | 4.0 (0-11.8) | 3 | 13.8 (0-28.8) | 1 | 4.4 (0-13) | 0 | 0 | |||||
| Unknown | 83 | 42 | 18 | 13 | 5 | 6 |
| Characteristic . | No. of pts . | No. of events . | 4-y EFS rate, % (95% CI) . | P value∗ . | No. of TRM events . | 4-y CI TRM, % (95% CI) . | P value∗ . | No. of progressive cancer events . | 4-y CI progressive cancer, % (95% CI) . | P value∗ . | No. of second malignancy events . | 4-y CI second malignancy, % (95% CI) . | P value∗ . | No. of deaths unrelated to cancer . | 4-y CI deaths unrelated to cancer, % (95% CI) . | P value∗ . |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Total | 185 | 90 | 47.9 (40.8-56.2) | — | 47 | 25.9 (19.5-32.4) | — | 26 | 14.5 (9.2-19.8) | 7 | 4.9 (1.3-8.4) | 10 | 6.5 (2.5-10.5) | |||
| Sex | .225 | .866 | .384 | .772 | .214 | |||||||||||
| Female | 72 | 40 | 41.9 (31.6-55.6) | 19 | 25.5 (17.3-33.7) | 12 | 14.9 (7.2-22.6) | 3 | 4.5 (0-9.5) | 6 | 9.7 (2.2-17.2) | |||||
| Male | 113 | 50 | 51.8 (42.7-62.8) | 28 | 26.6 (16.2-36.9) | 14 | 25.0 (11.9-38.1) | 4 | 5.4 (0.2-10.5) | 4 | 4.4 (0.1-8.8) | |||||
| Age at cancer diagnosis, y | .019 | .029 | .084 | .754 | <.001 | |||||||||||
| 0-4 | 40 | 12 | 69.1 (55.9-85.4) | 7 | 17.5 (5.5-29.4) | 2 | 6.3 (0-14.7) | 3 | 8.4 (0-17.7) | 0 | 0 | |||||
| 5-9 | 77 | 38 | 45.3 (34.6-59.4) | 15 | 19.8 (10.7-28.9) | 17 | 28.7 (15.8-41.7) | 2 | 3.6 (0-8.7) | 4 | 6.7 (0.2-13.3) | |||||
| 10-14 | 34 | 18 | 39.8 (25-63.3) | 10 | 31.1 (14.6-47.6) | 4 | 17.3 (1.1-33.4) | 1 | 4.1 (0-12.2) | 3 | 11.8 (0-25.2) | |||||
| ≤15 | 34 | 22 | 33.7 (20.8-54.6) | 15 | 44.6 (27.3-61.8) | 3 | 14.4 (0-30.5) | 1 | 3.1 (0-9.3) | 3 | 10.2 (0-21.5) | |||||
| Treatment era | .758 | .192 | .126 | <.001 | .426 | |||||||||||
| 1985-1999 | 28 | 16 | 41.6 (26.6-65) | 7 | 25.0 (8.5-41.4) | 4 | 15.9 (0.9-31.0) | 4 | 15.1 (1-29.3) | 1 | 3.8 (0-11.3) | |||||
| 2000-2009 | 66 | 33 | 49.6 (38.8-63.4) | 22 | 33.3 (21.8-44.8) | 5 | 9.4 (0.3-18.4) | 3 | 4.7 (0-10.1) | 3 | 4.5 (0-9.6) | |||||
| 2010-2023 | 91 | 41 | 48 (37.4-61.7) | 18 | 20.5 (11.9--29.1) | 17 | 26.3 (14.9-37.7) | 0 | 0 | 6 | 11 (1.8-20.2) | |||||
| Tumor type | .004 | .211 | .360 | .459 | <.001 | |||||||||||
| Mature B | 85 | 47 | 38.9 (28.9-52.3) | 22 | 26.5 (16.9-36.1) | 15 | 23.5 (11.7-35.3) | 1 | 1.9 (0-5.6) | 9 | 13.8 (5.1-22.4) | |||||
| Lymphoblastic | 79 | 27 | 63.9 (53.8-75.9) | 15 | 19.4 (10.5-28.2) | 8 | 13.0 (4.5-21.6) | 4 | 6.1 (0.2-11.9) | 0 | 0 | |||||
| Hodgkin lymphoma | 15 | 10 | 31.1 (14.2-68) | 6 | 40 (13.9-67.4) | 2 | 18.3 (0-43.9) | 1 | 7.8 (0-23.9) | 1 | 8.9 (0-26.9) | |||||
| Other | 6 | 6 | 4 | 1 | 1 | 0 | ||||||||||
| Baseline neurological status | .009 | .208 | .288 | .045 | .687 | |||||||||||
| Asymptomatic | 28 | 8 | 71.4 (56.5-90.3) | 5 | 17.8 (3.3-32.3) | 1 | 4.7 (0-14.1) | 1 | 3.6 (0-10.6) | 1 | 3.6 (0-10.6) | |||||
| Atactic gait | 64 | 28 | 50.8 (39-66.2) | 13 | 21.3 (10.8-31.7) | 8 | 17.1 (5.7-28.4) | 3 | 5.9 (0-12.5) | 4 | 8.4 (0.3-16.4) | |||||
| Wheelchair bound | 59 | 35 | 37.3 (26.4-52.8) | 19 | 33.0 (20.7-45.4) | 11 | 26.1 (10.9-41.2) | 0 | 0 | 5 | 10.2 (1.6-18.8) | |||||
| Unknown | 34 | 19 | 10 | 6 | 3 | 0 | ||||||||||
| Baseline respiratory status | .021 | .001 | .645 | <.001 | .374 | |||||||||||
| Asymptomatic | 66 | 22 | 63 (51.7-76.8) | 9 | 14.2 (5.5-22.9) | 9 | 18.2 (7.1-29.2) | 2 | 3.8 (0-9.0) | 2 | 3.9 (0-9.4) | |||||
| Recurrent respiratory infections (≥3 per y) | 60 | 30 | 44.9 (33-61.2) | 13 | 22.0 (11.3-32.8) | 9 | 18.4 (7.0-29.7) | 3 | 6.4 (0-13.8) | 5 | 11.1 (1.5-20.6) | |||||
| Chronic bronchitis/bronchiectasis | 25 | 18 | 28 (14.9-52.5) | 13 | 52 (31.8-72.2) | 2 | 7.7 (0-22.7) | 0 | 0 | 3 | 12 (6.8-25.4) | |||||
| Unknown | 34 | 20 | 12 | 6 | 2 | 0 | ||||||||||
| ATM mutation class | <.001 | .017 | .922 | .464 | <.001 | |||||||||||
| Absent ATM kinase activity | 77 | 43 | 39.4 (29-53.3) | 28 | 37.6 (26.4-48.7) | 10 | 17.2 (5.9-28.5) | 1 | 1.6 (0-4.7) | 4 | 7.6 (0.1-15) | |||||
| Residual ATM kinase activity | 25 | 5 | 78.7 (63.7-97.2) | 1 | 4.0 (0-11.8) | 3 | 13.8 (0-28.8) | 1 | 4.4 (0-13) | 0 | 0 | |||||
| Unknown | 83 | 42 | 18 | 13 | 5 | 6 |
Data shown for 185 treated patients.
P values are based on a comparison performed at a predefined time point of 4 years from cancer diagnosis on the basis of log-log transformation.
Treatment outcomes according to patient characteristics. (A) EFS according to age group in 185 treated patients. (B) Cumulative incidence of TRM according to age group in 185 treated patients. (C) EFS according to respiratory status at cancer diagnosis in 151 treated patients with the available data. (D) Cumulative incidence of TRM according to respiratory status at cancer diagnosis in 151 treated patients with the available data. P values are based on a comparison performed at a predefined time point of 4 years from cancer diagnosis based on log-log transformation.
Treatment outcomes according to patient characteristics. (A) EFS according to age group in 185 treated patients. (B) Cumulative incidence of TRM according to age group in 185 treated patients. (C) EFS according to respiratory status at cancer diagnosis in 151 treated patients with the available data. (D) Cumulative incidence of TRM according to respiratory status at cancer diagnosis in 151 treated patients with the available data. P values are based on a comparison performed at a predefined time point of 4 years from cancer diagnosis based on log-log transformation.
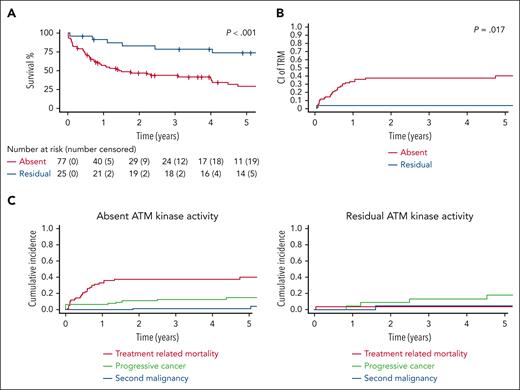
Finally, the germ line ATM PV class also showed a significant impact: 4-year EFS for patients with absent ATM kinase activity was 39.4% (95% CI, 29-53.3) vs 78.7% (95% CI, 63.7-97.2) for those with residual activity (P < .001; Figure 5A) and 4-year TRM rates were 37.6 % (95% CI, 26.4-48.7) and 4.0% (95% CI, 0-11.8), respectively (P = .017; Figure 5B). The patterns of therapy failure also differed considerably between these 2 groups. Although patients with absent ATM kinase activity demonstrate a therapy failure pattern similar to that described above for the entire AT cohort, with TRM as the major cause of failure (37.6%; 95% CI, 26.4-48.7), in patients with residual ATM kinase activity progressive cancer was the major event (13.8%; 95% CI, 0-28.8; Figure 5C).
Outcomes of patients with residual ATM kinase activity and with absent kinase activity. (A) EFS in patients with absent ATM kinase activity vs those with residual activity (102 treated patients with available data). (B) Cumulative incidence of TRM in patients with absent ATM kinase activity vs those with residual activity (102 treated patients with available data). (C) Patterns of cancer therapy failure for patients with absent ATM kinase activity vs those with residual activity. P values are based on a comparison performed at a predefined time point of 4 years from cancer diagnosis based on log-log transformation.
Outcomes of patients with residual ATM kinase activity and with absent kinase activity. (A) EFS in patients with absent ATM kinase activity vs those with residual activity (102 treated patients with available data). (B) Cumulative incidence of TRM in patients with absent ATM kinase activity vs those with residual activity (102 treated patients with available data). (C) Patterns of cancer therapy failure for patients with absent ATM kinase activity vs those with residual activity. P values are based on a comparison performed at a predefined time point of 4 years from cancer diagnosis based on log-log transformation.
As a preliminary analysis for the multivariate modeling, we conducted a series of univariate interactions between the research variables that were significantly associated with outcome, as summarized in supplemental Table 9. The germ line ATM PV class was significantly associated with tumor type (P < .001) and neurological status (P = .003) but not significantly associated with age at cancer diagnosis, sex, respiratory status or treatment era. The focus of this study was cancer-related events. As ATM PV class had a significant impact upon both EFS and TRM, whereas tumor type and neurological status were significantly associated with EFS alone, and because these differences in EFS may have been attributed to later events that were not cancer related (Table 1), we chose to include the germ line ATM PV class in our model. Notably, ATM PV class significantly affected patient outcome even when analyzed solely within the lymphoblastic malignancies group (supplemental Table 10).
In a multivariate analysis, the absence of ATM activity (HR, 14.11; 95% CI, 1.36-146.31; P = .029) and advanced respiratory status (HR, 2.86; 95% CI, 0.98-8.36; P = .035) were associated with an increased 4-year TRM, after adjusting for sex, age, treatment era, and respiratory status. Also, in a multivariate analysis, the absence of ATM activity was associated with a decreased 4-year EFS (HR, 0.362; 95% CI, 0.16-0.82; P = .009; Table 2 and supplemental Tables 11 and 12).
Multivariate models of EFS and TRM
| Characteristic∗ . | 4-y EFS . | 4-y CI of TRM . | ||
|---|---|---|---|---|
| HR (95% CI) . | P value† . | HR (95% CI) . | P value† . | |
| Sex, male vs female | 2.08 (0.95-4.54) | .053 | 0.90 (0.34-2.38) | .822 |
| Age, ≥10 y vs <10 y | 0.71 (0.32-1.60) | .383 | 1.29 (0.49-3.37) | .569 |
| Era, 2000-2009 vs 1985-1999 | 0.83 (0.16-4.24) | .798 | 0.91 (0.16-5.09) | .906 |
| Era, 2010-2023 vs 1985-1999 | 1.19 (0.26-5.51) | .797 | 0.39 (0.08-2.03) | .199 |
| Respiratory category, 3 vs 1+2‡ | 0.40 (0.12-1.33) | .098 | 2.86 (0.98-8.36) | .035 |
| ATM PV, absent vs residual | 0.36 (0.16-0.82) | .009 | 14.11 (1.36-146.31) | .029 |
| Characteristic∗ . | 4-y EFS . | 4-y CI of TRM . | ||
|---|---|---|---|---|
| HR (95% CI) . | P value† . | HR (95% CI) . | P value† . | |
| Sex, male vs female | 2.08 (0.95-4.54) | .053 | 0.90 (0.34-2.38) | .822 |
| Age, ≥10 y vs <10 y | 0.71 (0.32-1.60) | .383 | 1.29 (0.49-3.37) | .569 |
| Era, 2000-2009 vs 1985-1999 | 0.83 (0.16-4.24) | .798 | 0.91 (0.16-5.09) | .906 |
| Era, 2010-2023 vs 1985-1999 | 1.19 (0.26-5.51) | .797 | 0.39 (0.08-2.03) | .199 |
| Respiratory category, 3 vs 1+2‡ | 0.40 (0.12-1.33) | .098 | 2.86 (0.98-8.36) | .035 |
| ATM PV, absent vs residual | 0.36 (0.16-0.82) | .009 | 14.11 (1.36-146.31) | .029 |
The model includes 96 treated patients with available data on ATM PV class.
Patients with “other” tumor types were excluded.
To avoid multicollinearity, in models that included ATM PV class, we did not include tumor type and neurological status, which were highly associated with ATM PV class (Fisher test P values <.001 and .009, respectively).
P values are based on a comparison performed at a predefined time point of 4 years from cancer diagnosis on the basis of log-log transformation
Respiratory status: (1) asymptomatic; (2) recurrent respiratory exacerbations/infections (≥3 per year); and (3) chronic bronchitis/ bronchiectasis.
When the ATM PV class was excluded from the model and its associated risk factors, tumor type, and neurological status were included instead, advanced respiratory status was still independently associated with increased TRM (HR, 3.05; 95% CI, 1.16-7.98; P = .011), whereas tumor type and neurological status were not (supplemental Table 13).
Discussion
In this multinational study encompassing 202 pediatric and adolescent/young adult patients diagnosed with AT and hematological malignancies across 25 countries, we explore the spectrum of hematological cancers within this unique patient cohort and analyze survival rates and outcome data, with a stratified approach based on a functional classification of the patient germ line ATM PVs. Although outcomes of childhood leukemia and lymphoma in the general population have dramatically improved,40-43 our findings indicate that cure rates for patients with AT have not shown substantial improvement with the modernization of therapy over the past 4 decades. These findings underscore the pressing need to address this issue as an unmet challenge. Our study also reveals that the main cause of treatment failure in this population is TRM (26%).
We confirm that mature B-cell lymphomas are the most prevalent hematological malignancies in children with AT,14 followed by lymphoblastic malignancies, which are predominantly of T-lineage (85%).13 Interestingly, nearly two-thirds of T-ALLs (10 of 16 evaluated cases) in our cohort, belonged to the γδ lineage. This is noteworthy because these variants are usually rare in the non-AT population, accounting for only 8% to 10% of T-ALL cases.44,45 The enrichment of γδ T-ALL in patients with AT may reflect the relative increase of normal, nonmalignant circulating γδ T-cells at the expense of functional αβ T-cells, which was documented in these patients.46 Contrary to findings in a previous study suggesting lower survival rates among male patients with AT,47 our study demonstrates no significant discrepancy in outcomes between male and female patients with AT and hematological malignancies.
Our study reveals several important distinctions between patients with absent and residual ATM kinase activity. Patients with absent and residual ATM kinase activity present with a different spectrum of malignancy subtypes. Furthermore, 2 groups exhibit distinctly different patterns of cancer therapy failure: for patients with absent ATM kinase activity, TRM emerges as the major cause of failure, accounting for 36.7% of the cases. In contrast, progressive cancer constitutes the major cause of treatment failure in those with residual kinase activity, underscoring the “protective” effect of even residual amounts of active ATM kinase against treatment-related toxicity. Finally, our analysis demonstrates that the absence of ATM kinase activity independently correlates with reduced EFS. Additionally, both absence of kinase activity and advanced respiratory status at cancer diagnosis independently associate with elevated TRM rates.
There is a paucity of data from previous studies on cancer outcomes in patients with absent or residual ATM kinase activity. A study examining 46 pediatric and adult patients with AT, who developed solid and hematological cancers, found no significant differences in cancer incidence or survival rates based on ATM kinase activity.14 Another study involving 66 pediatric and adult patients reported a lower incidence of lymphoid tumors among children with residual ATM kinase activity but did not compare cancer therapy outcomes.31 Limitations in sample size and heterogeneity may have affected these findings, compounded by limited analyses of cancer therapy and the causes of death, underscoring the need for international collaborations to enhance the development of personalized anticancer therapies for rare patient subsets.
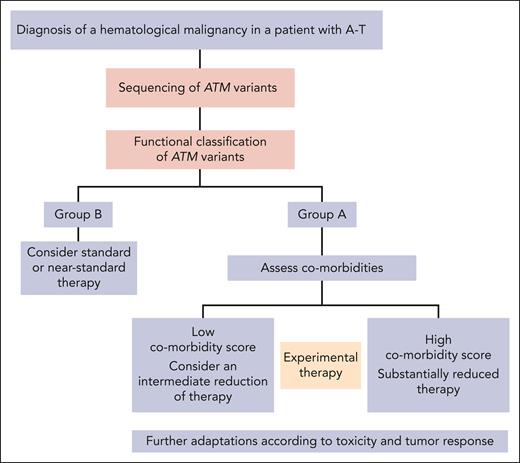
The findings of our study carry significant implications for refining patient management strategies. The standard treatment stratification system for non-A-T patients with hematological malignancies focuses upon cancer progression as the main cause of treatment failure. However, our findings demonstrate that in patients with AT the main cause of therapy failure is TRM, with the ATM PV class as a robust outcome predictor. Consequently, a specialized stratification system may be warranted for patients with AT, accounting for their unique risk factors associated with TRM. In patients with absent ATM kinase activity, treatment could be prospectively and systematically deescalated, to mitigate the elevated TRM rates. Conversely, patients with residual kinase activity who exhibit a “failure pattern” that is similar to that of the non-AT population,40-43 could be treated according to standard or near-standard therapy regimens. Moreover, baseline comorbidities, especially respiratory dysfunction, could also be used for further stratification (Figure 6). Regardless of their ATM PV class, all patients should be closely monitored for treatment toxicity and cancer response, with treatment adjustments made as necessary. There is an urgent imperative for the development of experimental innovative therapies for this unique patient population. Although less toxic treatment alternatives, such as certain immunotherapeutic modalities, have shown promise in specific instances of AT and hematological malignancies48-52 (primarily demonstrated through case studies) the issue remains complex and requires more substantial evidence to support widespread use. In addition to affecting anticancer therapeutic strategies, our findings have potential implications for enhancing patients' surveillance protocols. Given the heightened risk of hematological malignancies in individuals with AT, our research suggests that integrating genomic sequencing of germ line ATM variants with subsequent functional classification could offer a proactive approach. This strategy could allow for patient stratification based on ATM kinase activity, thus aiding in the selection of suitable cancer treatment strategies upon diagnosis.
Proposed stratification scheme for the treatment of patients with AT and hematological malignancies.
Proposed stratification scheme for the treatment of patients with AT and hematological malignancies.
The limitations of this study encompass its retrospective design and the heterogeneity in therapy approaches. Additionally, it lacks population-based representation and comprehensive self-reported ethnicity data for a significant portion of the cohort. Genetic data were incomplete, and functional information was lacking for some sequenced ATM PVs. TRM is a significant end point, but it lacks a standardized definition and may be subject to recall bias. The heterogeneity of the cohort may restrict the precision of stratified analyses conducted on particular subgroups, primarily because of inadequate statistical power. To establish a more precise assessment of the prevalence of hematological malignancies and their outcomes, larger prospective studies are necessary. However, despite these limitations, the study offers valuable data-driven guidelines for developing a novel risk stratification system and optimizing therapy selection for this unique patient population.
This multinational study serves as a prototype of the collaboration that is imperative in addressing rare CPS.53 Our study underscores the notable diversity in cancer patterns and therapy outcomes observed even within a single rare CPS, highlighting the influence of distinct germ line PVs. We have addressed several pivotal issues, including the challenges posed by a CPS accompanied by serious nonmalignant comorbidities and the balance between treatment efficacy and life-threatening cancer therapy-related toxicities. Our findings highlight the critical importance of collaborative studies conducted within international networks. Such efforts are crucial in generating data-driven treatment guidelines aimed at enhancing the care of these rare patients.
Acknowledgments
The authors thank the patients and their families for participating in this study. The authors thank David Zucker and Maor Moshe for their assistance with the statistical analyses, and Naomi Litichever and Dina Kugel for data management at the Department of Pediatric Hematology and Oncology, Schneider Children’s Medical Center of Israel. The authors extend their thanks to Melina Mescher, Danielle Brandes (Department of Pediatric Oncology, Hematology and Clinical Immunology, Heinrich Heine University Duesseldorf, Duesseldorf), and Netta Kasher for their assistance with the graphical elements.
This work was supported by the Israel Cancer Association, the Chaim Association, the Israel Children’s Cancer Foundation, and the Israeli Association for Fighting AT Disease. This publication is based upon work from COST Action LEGEND (CA16223), supported by COST (European Cooperation in Science and Technology). S.E. is supported by the Davidoff Foundation. S.K.T. is a Scholar of the Leukemia & Lymphoma Society and holds the Joshua Kahan Endowed Chair in Pediatric Leukemia Research at the Children’s Hospital of Philadelphia. S.I. is supported by the Israel Cancer Research Fund professorship. Y.S. is supported by the Dr Miriam and Sheldon G. Adelson Medical Research Foundation.
Authorship
Contribution: S.E., R.N., A.A., and A. Borkhardt conceptualized and designed the study; S.E. provided financial support; S.E. provided administrative support; S.E., J.L.C.L., A.P., M.T., S.B., A. Baruchel, T.L., S.K.T., O.A., N.A.-C., I.A, M.B.-H., N.B., T.B., F.C., L.C., L.D.P., S.D., G.E., R.F., A.G., H.H., J.H., E.J., J.J., A.K., J.L., L.L.N., N.M., L.M., V.P., L.P., M.P., I.S., J.S., E.S., M.S., T.H.T., M.U., J.V.-A., A.W., J.Z., V.M.-C., K.S., A.A., and A. Borkhardt provided study material or patient data; S.E., R.S., S.J., D.S.-L., M.T., and Y.S. analyzed ATM PVs; all authors were responsible for collection and assembly of data; S.E., R.S., T.L., D.S.-L., M.T., Y.S., K.S., R.N., A.A., and A. Borkhardt were responsible for data analysis and interpretation; S.E., R.S., Y.S., K.S., R.N., A.A., and A. Borkhardt were responsible for manuscript writing; and all authors approved the final version of the manuscript.
Conflict-of-interest disclosure: S.E. reports honoraria from Medison Pharma; and reports consulting or advisory role with Jazz Pharmaceuticals. A. Baruchel received honoraria for advisory board participation (to institution) from AstraZeneca,reports honoraria and research funding from Servier; received honoraria from Clinigen; and received honoraria for advisory board participation from Serb and Jazz. L.D.P. reports scientific advisory board membership with Amgen Australia. S.K.T. reports membership on an entity's board of directors or advisory committees with Syndax Pharmaceuticals; received research funding from Beam Therapeutics and Incyte Corporation; reports membership on an entity's board of directors or advisory committees with, and received research funding from, Kura Oncology; reports membership on an entity's board of directors or advisory committees with Aleta Biotherapeutics; received travel support from Amgen. O.A. received honoraria from, and reports consultancy with, Springworks Therapeutics. E.J. reports honoraria from Novartis; and reports speakers bureau membership with Novartis and Medison. L.L.N. reports consulting role with, and membership on an entity's board of directors or advisory committees of, Novartis, Amgen, Clinigen, and Jazz Pharmaceutical. N.M. The French Patient Association AT-Europe co-funds the Centre de Reference des Deficits Immunitaires Hereditaires (CEREDIH, the French national reference center for primary immunodeficiencies) and French A-T prospective subregistry. V.P. reports speaker honoraria from Recordati Rare Diseases. T.H.T. reports consultancy with, and received honoraria from, Servier and Jazz Pharmaceuticals. V.M.-C. reports consulting role with Adaptimmune Therapeutics PLC, Bristol Myers Squibb, Roche, and AstraZeneca. K.S. received speaker’s fee from Medscape and Amgen; received honoraria and educational grants from Servier; and received honoraria from Jazz Pharmaceuticals and Illumina. A.A. received honoraria from Jazz Pharmaceuticals, Amgen, Servier, and Novartis; reports consulting or advisory role with Jazz Pharmaceuticals, Amgen, Novartis, Takeda Science Foundation, and Roche; and received travel, accommodations, and expenses funding from Jazz Pharmaceuticals. The remaining authors declare no competing financial interests.
Correspondence: Sarah Elitzur, Department of Pediatric Hematology and Oncology, Schneider Children's Medical Center, 14 Kaplan St, Petah Tikva 4920235, Israel; email: sarahelitzur@gmail.com.
References
Author notes
R.N., A.A., and A.B. contributed equally as joint last authors.
Additional patient deidentified data may be found in a data supplement available with the online version of this article.
The online version of this article contains a data supplement.
There is a Blood Commentary on this article in this issue.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal