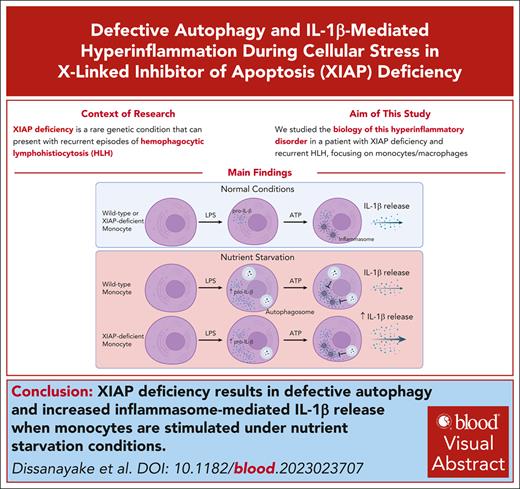
Key Points
Activation of the NLRP3 inflammasome during nutrient starvation results in excessive interleukin-1β production by XIAP-deficient cells.
XIAP-deficient monocytes exhibit defective autophagic flux during nutrient starvation.
Visual Abstract
Deficiency of X-linked inhibitor of apoptosis protein (XIAP) is a rare genetic condition that can present with recurrent episodes of hemophagocytic lymphohistiocytosis (HLH), though the exact mechanisms leading to this hyperinflammatory disorder are unclear. Understanding its biology is critical to developing targeted therapies for this potentially fatal disease. Here, we report on a novel multiexonic intragenic duplication leading to XIAP deficiency with recurrent HLH that demonstrated complete response to interleukin (IL)-1β blockade. We further demonstrate using both primary patient cells and genetically modified THP-1 monocyte cell lines that, contrary to what has previously been shown in mouse cells, XIAP-deficient human macrophages do not produce excess IL-1β when stimulated under standard conditions. Instead, nucleotide-binding oligomerization domain–like receptor family pyrin domain containing 3 (NLRP3) inflammasome–mediated hyperproduction of IL-1β is observed only when the XIAP-deficient cells are stimulated under autophagy-promoting conditions and this correlates with defective autophagic flux as measured by decreased accumulation of the early autophagy marker LC3-II. This work, therefore, highlights IL-1β blockade as a therapeutic option for patients with XIAP deficiency experiencing recurrent HLH and identifies a critical role for XIAP in promoting autophagy as a means of limiting IL-1β–mediated hyperinflammation during periods of cellular stress.
Introduction
X-linked lymphoproliferative syndrome type 2 (XLP-2; Online Mendelian Inheritance in Man #300635) is caused by deficiency of X-linked inhibitor of apoptosis protein (XIAP, also known as BIRC4) and is characterized by immune dysregulation that can manifest in a variety of ways, including inflammatory bowel disease, arthritis, uveitis, and recurrent infections.1,2 One of the most severe manifestations is the development of hemophagocytic lymphohistiocytosis (HLH), a hyperinflammatory state that occurs in ∼60% of individuals with XLP-2 with a fatality rate of >20%.2,3 Individuals with severe or treatment-refractory disease often undergo hematopoietic stem cell transplantation but are prone to high rates of posttransplantation complications, including graft-versus-host-disease.4 Given the rates of morbidity and mortality associated with the disease and its current treatment options, new targeted therapeutic options are needed for patients with XLP-2.
The precise molecular links between XIAP deficiency and the immune dysregulation observed in XLP-2 remain unclear.2,5 The finding that HLH is associated with elevated proinflammatory cytokines, such as interleukin (IL)-1β and IL-18, suggests that XIAP deficiency may be associated with dysregulated production of inflammasome-derived cytokines. The canonical pathway for release of these IL-1 family cytokine members requires 2 signals.6 The first is a priming signal that results in nuclear factor kappa B–mediated production of a “pro” form of the cytokine. The second signal activates an inflammasome, a multimeric complex consisting of a sensor, the apoptosis–associated speck-like protein containing a caspase-recruitment domain adaptor protein, and caspase-1, which can cleave the procytokine to its bioactive form.
Previous work using murine bone marrow–derived myeloid cells has suggested that XIAP may limit the activation of the nucleotide-binding oligomerization domain–like receptor family pyrin domain containing 3 (NLRP3) inflammasome by inhibiting a complex consisting of receptor-interacting protein kinase 3 (RIPK3) and caspase-8 downstream of the tumor necrosis factor (TNF) receptor 1.7-10 In this model, the absence of XIAP allows TNF-α production after a priming signal, such as lipopolysaccharide (LPS) ligation of toll-like receptor-4, to act in an autocrine/paracrine manner to mediate RIPK3/caspase-8–dependent NLRP3 activation, thereby circumventing the need for a second signal, such as adenosine triphosphate (ATP), to activate NLRP3.
XIAP has also recently been implicated as a modulator of autophagy. Autophagy is a process by which intracellular components are targeted for degradation through engulfment in double-membrane structures, termed autophagosomes, and subsequent transport to lysosomes.11 During this process, the cytosolic protein LC3-I is lipidated to LC3-II and incorporated into the developing autophagosome, thereby serving as a useful biomarker for the early steps of autophagic flux.12 Autophagy is constitutively present at basal levels but is dramatically upregulated during periods of cellular stress, such as nutrient starvation, and its function is crucial to cellular homeostasis as disruption is associated with a variety of diseases.13
Although several studies have suggested a role for XIAP in the regulation of autophagy, the direction of its involvement remains unclear. For example, XIAP was reported to be an inhibitor of autophagy through a pathway involving mouse double minute 2 and p53 in the HCT116 colon cancer cell line.14 Conversely, overexpression of XIAP in HeLa cells is associated with increased autophagosome formation through increased expression of Beclin-1, whereas chemical or small interfering RNA–mediated XIAP inhibition in mouse embryonic fibroblasts results in decreased fusion of autophagosomes with lysosomes.15,16 It is therefore possible that XIAP may have differing roles in driving or inhibiting autophagy depending on the cellular context.
Here, we report a patient with recurrent HLH who was found to have XIAP deficiency secondary to a previously undescribed intragenic multiexonic duplication. His HLH episodes showed complete response to IL-1β blockade, lending further evidence to the concept of XLP-2–associated HLH as a disease of inflammasome dysregulation. However, contrary to previous studies, we demonstrate using both primary patient cells and XIAP-knockout (KO) THP-1 monocyte cell lines that XIAP deficiency is not associated with increased IL-1β production with either signal 1 alone or in conjunction with signal 2. Instead, we find that XIAP-deficient cells show defective autophagic flux, which is associated with increased IL-1β release in a RIPK3-independent manner. This work therefore links HLH in XLP-2 specifically to IL-1β overproduction and presents conditions of cellular stress as a novel inciting factor that augments IL-1β production by XIAP-deficient cells owing to defective autophagy.
Methods
Genome sequencing
Polymerase chain reaction (PCR)–free genome sequencing on DNA extracted from whole blood was performed at the Centre for Applied Genomics using 2 × 150 bp paired end sequencing with the Illumina Hiseq X platform to an average depth of coverage >35×. Genome analysis was performed as previously described.17 Briefly, Burrows-Wheeler Aligner was used for genome alignment (Build GRCh37/hg19) with small sequence variation (substitutions, insertions, duplications, and deletions) called using Genome Analysis Tool Kit Version 3.6. Copy number variant (CNV) analysis was performed with the read depth algorithms ERDs (Estimation by Read Depth with SNVs) and CNVnator as previously described.18
Peripheral blood samples
Peripheral blood mononuclear cells (PBMCs) were collected using venous blood sampling into Becton Dickinson (BD) Vacutainer CPT tubes (catalog no. 362753) and processed according to manufacturer’s instructions before storage in liquid nitrogen.
Monocyte isolation
Monocytes were isolated from PBMC by negative selection using the Classical Monocyte Isolation Kit (Miltenyi Biotec, catalog no. 130-117-337), LS columns (Miltenyi Biotec, catalog no. 130-042-401) and QuadroMACS separator (Miltenyi Biotec, catalog no. 130-090-976) according to manufacturer’s instructions. Purity of the isolated population was confirmed to be >90% using flow cytometry for CD14-positive cells.
THP-1 cell line generation
Cas9 stably expressing THP-1 cells (Applied Biological Materials, catalog no. T3274) were cultured and passaged in RPMI medium supplemented with 10% fetal bovine serum and 1.2 μg/mL puromycin. Alt-R CRISPR-Cas9 crRNA/tracrRNA gene editing system (Integrated DNA Technologies) was used to KO the XIAP gene. Three crRNAs were designed (supplemental Figure 2A, available on the Blood website) and an equimolar mix of all 3 crRNAs was used to target the XIAP gene in exon 1. The guide RNA was created according to manufacturer instructions (https://www.idtdna.com/pages/support/guides-and-protocols). Three hundred picomoles of the XIAP-guide RNAs were transfected to THP-1/Cas9 (1 × 106) cells using Lonza 2D nucleofector program V-01. Three days after transduction, live single cells were seeded in each well of 96-well plates. Three weeks after sorting, 65 μL of culture medium of the grown transfected THP-1/Cas9 cell clones were passaged into 24-well plates. The remaining 35 μL were lysed using Direct PCR Lysis Reagent (Cedarlane, catalog no. 301-C). DNA was subjected to genomic PCR using DreamTaq Hot Start Green PCR master mix (ThermoFisher, catalog no. K9021) and hXIAP-EX1 primers (forward: AGGTGGACAAGTCCTATTTTCA, reverse: TCCGTGCTTCATAATCTGCCA). Five μL of PCR products was run on 1.5% agarose gel to ensure amplification of the genomic region of XIAP, and the rest of the PCR products were purified using QIAQuick kit (Qiagen, catalog no. 28106). Twenty nanograms of purified PCR products were sequenced to confirm the deletions in the targeted region of XIAP. Western blotting was performed to confirm absence of XIAP protein. Three clones that underwent the above protocol without editing in the region of interest were used as control cells for off-target effects.
THP-1 cell macrophage differentiation
THP-1 monocytes were differentiated with phorbol 12-myristate 13 acetate (Sigma, catalog no. P1585-1MG) for 48 hours and then rested in phorbol 12-myristate 13 acetate–free media for 72 hours before being used as macrophages in experiments.
Muramyl dipeptide (MDP) stimulation assay
A functional assay for XIAP was adapted from a previously established protocol.19 Briefly, PBMC or THP-1 monocytes were incubated in medium alone or stimulated with 200 ng/mL LPS (Sigma) or 200 ng/mL lipidated MDP (Invivogen) for 5 to 6 hours in the presence of GolgiPlug (BD Biosciences). Cells were then harvested using Accutase (ThermoFisher) and placed on ice. Surface staining was performed for CD14 (BD Biosciences, catalog no. 561383) and human leukocyte antigen (HLA)-DR (BD Biosciences, catalog no. 560651). The cells were fixed and permeabilized using Cytofix/Cytoperm (BD Biosciences) and intracellular cytokine staining was performed for TNF-α (BD Biosciences, catalog no. 554513). Flow cytometry was acquired on a LSRII Analyzer (BD Biosciences) and analyzed with Cytobank software (Beckman Coulter).
Cell culture and serum starvation
For standard conditions, cells were incubated at 37°C at 500 000 cells per well in a 12-well plate containing 1 mL of RPMI 1640 (VWR) supplemented with 10% LPS-free fetal calf serum, 1 mM sodium pyruvate, 0.1 mM nonessential amino acids, 50 μM 2-mercaptoethanol, 2mM L-glutamine, and 10 mM HEPES (N-2-hydroxyethylpiperazine-N′-2-ethanesulfonic acid). For serum starvation, RPMI 1640 containing 1% LPS-free fetal calf serum was used in place of 10%. The autophagosome-lysosome fusion inhibitor bafilomycin A1 (InvivoGen, catalog no. tlrlbaf1) was added (50 nM) at the start of the serum starvation for detection of LC3-II accumulation by western blotting and LC3 puncta by immunofluorescence.
NLRP3 activation and cytokine quantitation by enzyme-linked immunosorbent assay
Monocytes isolated from PBMC or THP-1 macrophages were plated at 500 000 cells/mL in media alone or containing LPS (10 ng/mL). Where indicated, ATP (5 mM) was added for the last hour of the incubation. For NLRP3 inhibition, 500 nM MCC950 (InvivoGen, catalog no. inh-mcc) was added 1 hour before addition of ATP. For RIPK3 inhibition, 5 μM GSK-872 (MedChemExpress, catalog no. HY-101872) was added 30 minutes before the start of the stimulation with LPS.
Supernatants were harvested at 4 hours or 24 hours as indicated for enzyme-linked immunosorbent assay detection of IL-1β (Invitrogen, catalog no. 88-7261-88) or TNF-α (Invitrogen, catalog no. 88-7346-88) according to manufacturer’s instructions.
Cell death measurement
THP-1 macrophages were serum-starved and stimulated as indicated before staining with annexin V (STEMCELL Technologies, catalog no. 100-0331) and 7-aminoactinomycin D (STEMCELL Technologies, catalog no. 75001) per manufacturer’s instructions. Flow cytometry samples were acquired as detailed above.
Cell lysis and western blotting
Cell lysis for protein isolation was performed using RIPA buffer containing halt protease and phosphatase inhibitor and EDTA (ThermoFisher). Protein concentration was determined using the DC protein assay (Bio-Rad). Immunoblots for XIAP and IL-1β were prepared with Bolt 4% to 12% Bis-Tris gels (Invitrogen) and anti-XIAP mouse monoclonal antibody (BD Biosciences, catalog no. 610716) or anti-IL-1β mouse monoclonal antibody (Cell Signaling Technology, catalog no. 12242S). Immunoblots for LC3 were prepared using handcasted 15% bis-acrylamide gels and anti-LC3 mouse monoclonal antibody (Cell Signaling Technology, catalog no. 83506S). Glyceraldehyde-3-phosphate dehydrogenase (GAPDH) was used as a loading control (Invitrogen, catalog no. AM4300). Relative protein levels were normalized to GAPDH by densitometry using ImageJ software (National Institutes of Health).
Immunofluorescence imaging
THP-1 macrophages underwent methanol fixation and permeabilization with phosphate-buffered saline containing 10% fetal calf serum and 0.2% saponin. Immunostaining was performed with anti-LC3B antibody (Cell Signaling Technology, catalog no. 3868S) along with DAPI (4′,6-diamidino-2-phenylindole) counterstaining. Z-stack images were acquired on a Zeiss Axiovert 200 M confocal microscope equipped with a cooled ORCA-Fusion BT, Hamamatsu CCD camera, 5-line laser module (Spectral Applied Research) for 405-, 443-, 491-, 561-, and 655-nm lines, and a MAC5000, Ludl filter wheel. Images were acquired using a Zeiss ×63/1.4 NA oil objective. Image analysis was performed using Volocity v6.3.1. Objects between 0.5 and 3.0 μm3 and shape factor >0.3 were identified and counted by the software over individual cell regions of interest. Threshold for identifying objects was set based on cytosolic signal intensity of each individual cell.
Quantitative real-time PCR
RNA extraction was performed using RNeasy Mini kit (Qiagen, catalog no. 74104) according to manufacturer instructions. Complemntary DNA (cDNA) was synthesized using High-Capacity cDNA Reverse Transcription Kit (ThermoFisher, catalog no. 4368814) and random hexamer primer according to manufacturer’s instructions. For each quantitative reverse transcription polymerase chain reaction, the reverse-transcribed cDNA (5 ng) was amplified using PCR by mixing it with 5 μL of TaqMan Universal PCR Master Mix Power SYBR Green Master Mix (2×; Applied Biosystems, catalog no. 4364340) and 0.5 μM of each primer (IL1b: Hs01555410_m1, GAPDH Hs02786624_g1) to a total volume of 10 μL. The threshold cycle (Ct) of each target gene was normalized to the geometric means of the Ct values for human housekeeping gene GAPDH. The 2−ΔΔCt method was used to present the relative expression.
Statistical analysis
Two-tailed paired t-tests were used for analysis using GraphPad Prism v9.0. A P value of <.05 was considered statistically significant. Experiments with THP-1 cells were each conducted with 3 independent XIAP-deficient clones and 3 control clones as biological replicates.
Study approval
Experiments were carried out with research ethics board approval from The Hospital for Sick Children. Informed written consent to participate in research and for publication was obtained from the participant and his parents.
Results
Clinical description
Our male patient aged 6 years presented with prolonged fever and 3 additional HLH-2004 criteria (splenomegaly, hypofibrinogenemia, and hyperferritinemia with a peak level of 14 158 mg/L). He also demonstrated pancytopenia and elevated soluble CD25 (2374 U/mL) though not meeting the thresholds specified in the HLH-2004 criteria.20 He received combination treatment of corticosteroids, cyclosporine, and anakinra with good response. Corticosteroids were weaned off over the course of 4 months, and cyclosporine and anakinra were both tapered off during the subsequent 6 months. Within 4 months of stopping medications, he presented again with fever, hyperferritinemia, and cytopenias, necessitating another 2-month course of corticosteroids, after which he again flared 3 months later and was started on anakinra (3 mg/kg). Infectious disease testing, including Epstein-Barr virus, was consistently negative during this time. He was subsequently maintained on anakinra monotherapy for 1.5 years before transitioning to canakinumab owing to intolerance of the daily injections. Since starting IL-1β blockade over 5 years ago, he had no further episodes of HLH (supplemental Figure 1).
Identification of genetic variant associated with absence XIAP protein and function
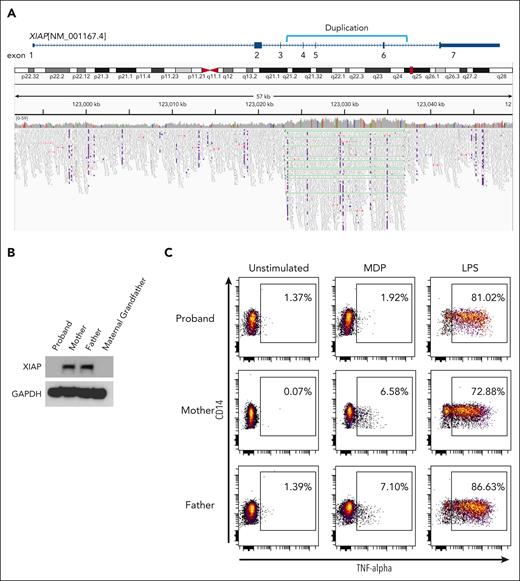
Genetic panel testing and exome sequencing did not initially identify a contributory variant in genes associated with HLH, including XIAP. However, genome sequencing demonstrated an intragenic duplication spanning XIAP (NM_001167.4) exons 4 to 6. The duplication was tandem in orientation and predicted to produce a stop codon after the fifth position in the new reading frame (E434Gfs∗5) (Figure 1A). The duplication was confirmed to be inherited from his asymptomatic heterozygous mother (data not shown).
Novel genetic duplication in XIAP leading to absence of protein and function. (A) Genome sequencing read mapping alignment data from proband revealing intragenic multiexonic duplication in the XIAP gene (NM_001167.4). (B) Western blotting analysis of PBMC from the proband, his mother, his father, and maternal grandfather with anti-XIAP and anti-GAPDH (loading control). (C) PBMC from the proband, his mother, and his father were stimulated with media alone (unstimulated), muramyl dipeptide or LPS and intracellular cytokine production was measured using anti-TNFα. Plots are gated on HLA-DR–positive, CD14-positive monocytes. Western blots and flow cytometry plots are representative of 2 independent experiments.
Novel genetic duplication in XIAP leading to absence of protein and function. (A) Genome sequencing read mapping alignment data from proband revealing intragenic multiexonic duplication in the XIAP gene (NM_001167.4). (B) Western blotting analysis of PBMC from the proband, his mother, his father, and maternal grandfather with anti-XIAP and anti-GAPDH (loading control). (C) PBMC from the proband, his mother, and his father were stimulated with media alone (unstimulated), muramyl dipeptide or LPS and intracellular cytokine production was measured using anti-TNFα. Plots are gated on HLA-DR–positive, CD14-positive monocytes. Western blots and flow cytometry plots are representative of 2 independent experiments.
Absence of XIAP protein in PBMC was confirmed using western blot (Figure 1B). Previous work has established a functional assay for XIAP deficiency based on the requirement of XIAP for signal transduction after ligation of the nucleotide-binding oligomerization domain 2 receptor by MDP.19 As shown in Figure 1C, PBMC from our patient demonstrated defective TNF-α production by intracellular cytokine staining after MDP-stimulation but not after stimulation of an XIAP-independent pathway by LPS. These studies established the absence of functional XIAP protein in our patient and confirmed a diagnosis of XLP-2 secondary to a previously undescribed form of genetic variant consisting of a multiexonic intragenic duplication in the XIAP gene.
Serum starvation is associated with IL-1β hyperproduction in XIAP deficiency
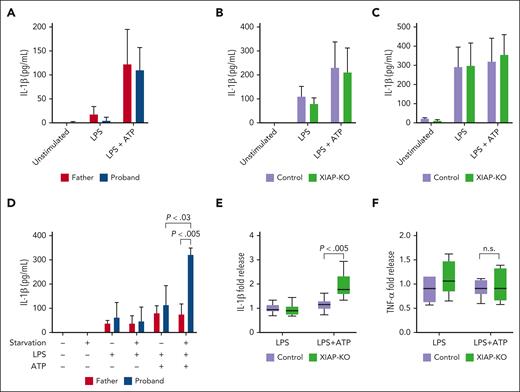
Given previous suggestions of inflammasome dysregulation in XIAP deficiency and the complete response of our patient’s recurrent episodes of HLH to IL-1β blockade, we next sought to assess for dysregulated IL-1β production by his monocytes. Unexpectedly, stimulation with either LPS alone or with the addition of ATP as a second signal to activate the inflammasome did not result in increased IL-1β release from the proband’s cells compared with those of his father (Figure 2A).
XIAP-deficient cells produce excess IL-1β when stimulated under serum starvation. (A) Monocytes were isolated from PBMC obtained from the proband and his healthy father and stimulated with media alone (unstimulated), LPS, or LPS with ATP added for the last hour of incubation. After 4 hours, supernatants were taken for IL-1β measurement using enzyme-linked immunosorbent assay (ELISA). (B) Control or XIAP-KO THP-1 macrophages were stimulated as indicated in panel A, followed by supernatant retrieval for IL-1β measurement using ELISA. (C) Control or XIAP-KO THP-1 macrophages were stimulated with media alone (unstimulated), LPS, or LPS with ATP added for the last hour of incubation. After 24 hours, supernatants were taken for IL-1β measurement using ELISA. (D) Monocytes isolated from PBMC obtained from the proband and his healthy father were stimulated as indicated in panel A under standard (10% FCS) or starvation (1% FCS) conditions, after which supernatants were taken for IL-1β measurement by ELISA. Panels A-D are representative of >3 independent experiments and indicate mean with standard deviation. (E) Control or XIAP-KO THP-1 macrophages were stimulated with LPS or LPS with ATP added for the last hour of incubation in serum starvation (1% FCS) media for 4 hours, after which supernatants were harvested for IL-1β measurement using ELISA. (F) Supernatants obtained as in panel E were subjected to ELISA for TNF-α measurement. For panels E-F, results are pooled from 3 independent experiments and normalized using measurements from supernatants of cells incubated with the same stimuli under nonserum starvation conditions. The median value is indicated with a line, the box indicates 25th to 75th percentile, and the whiskers indicate the minimum and maximum values.
XIAP-deficient cells produce excess IL-1β when stimulated under serum starvation. (A) Monocytes were isolated from PBMC obtained from the proband and his healthy father and stimulated with media alone (unstimulated), LPS, or LPS with ATP added for the last hour of incubation. After 4 hours, supernatants were taken for IL-1β measurement using enzyme-linked immunosorbent assay (ELISA). (B) Control or XIAP-KO THP-1 macrophages were stimulated as indicated in panel A, followed by supernatant retrieval for IL-1β measurement using ELISA. (C) Control or XIAP-KO THP-1 macrophages were stimulated with media alone (unstimulated), LPS, or LPS with ATP added for the last hour of incubation. After 24 hours, supernatants were taken for IL-1β measurement using ELISA. (D) Monocytes isolated from PBMC obtained from the proband and his healthy father were stimulated as indicated in panel A under standard (10% FCS) or starvation (1% FCS) conditions, after which supernatants were taken for IL-1β measurement by ELISA. Panels A-D are representative of >3 independent experiments and indicate mean with standard deviation. (E) Control or XIAP-KO THP-1 macrophages were stimulated with LPS or LPS with ATP added for the last hour of incubation in serum starvation (1% FCS) media for 4 hours, after which supernatants were harvested for IL-1β measurement using ELISA. (F) Supernatants obtained as in panel E were subjected to ELISA for TNF-α measurement. For panels E-F, results are pooled from 3 independent experiments and normalized using measurements from supernatants of cells incubated with the same stimuli under nonserum starvation conditions. The median value is indicated with a line, the box indicates 25th to 75th percentile, and the whiskers indicate the minimum and maximum values.
To further explore this result, we used CRISPR gene editing to generate a XIAP-KO monocyte/macrophage THP-1 cell line (supplemental Figure 2A). THP-1 cells have been used extensively for studying both inflammasome activation and inflammasome–mediated monogenic diseases.21-23 Successful deletion of XIAP was confirmed by protein level using western blot and by functional activity through MDP-stimulation (supplemental Figure 2B-C). Recapitulating the results using the patient cells, XIAP-KO THP-1 macrophages did not produce increased levels of IL-1β after LPS stimulation with or without ATP (Figure 2B). Because previous studies suggested a mechanism by which autocrine/paracrine TNF-α activity results in excess IL-1β production by XIAP-deficient cells, we extended the duration of LPS stimulation to allow more time for this feedback to occur. As shown in Figure 2C, prolonged in vitro stimulation of THP-1 cells with LPS alone resulted in higher levels of IL-1β production, perhaps relating to release of endogenous ATP, as has previously been described.24 However, IL-1β release was not significantly different when comparing control and XIAP-KO cells stimulated over the course of 24 hours with or without exogenous ATP added. These findings are therefore contrary to the previous work showing increased IL-1β release by XIAP-deficient murine bone marrow–derived macrophages upon stimulation with LPS.7-10
Given the proposed involvement of XIAP in autophagy, we next sought to determine whether serum starvation could elicit differences in IL-1β production between control and XIAP-deficient cells. As shown in Figure 2D, unlike the monocytes from his healthy father, the proband’s monocytes released significantly increased amounts of IL-1β when stimulated with LPS and ATP under serum starvation conditions. The difference was less dramatic with XIAP-deficient THP-1 cells, perhaps owing to the higher background rates of IL-1β production by these cell lines, but we still observed an approximate 2-fold increase in IL-1β production by XIAP-deficient THP-1 cells when stimulated with LPS and ATP under serum starvation conditions (Figure 2E). Conversely, TNF-α levels were similar in the supernatants, indicating that serum starvation does not lead to a global increase in proinflammatory cytokine production (Figure 2F). XIAP-deficient cells did not exhibit increased survival compared with control cells upon serum starvation, as proportions of annexin V–positive apoptotic cells were similar (supplemental Figure 3A). The increased IL-1β release upon stimulation did, however, correlate with a trend toward increased pyroptosis in serum-starved stimulated XIAP-deficient cells, as measured by proportion of annexin V and 7-aminoactinomycin double-positive cells25 (supplemental Figure 3B).
IL-1β hyperproduction is caused by increased NLRP3 activity through a RIPK3-independent pathway
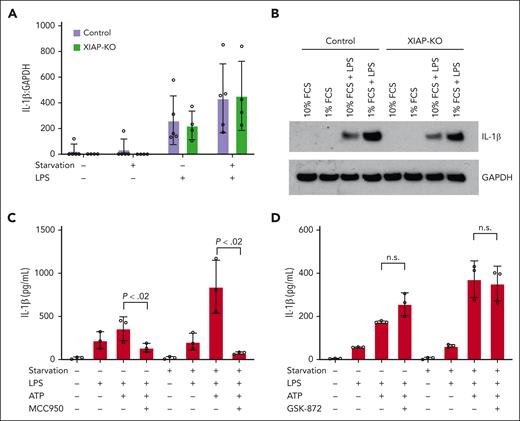
IL-1β production occurs through a 2-step process with translation of a procytokine followed by inflammasome-mediated processing into the active cytokine that is released from the cell. As shown in Figure 3A, both control and XIAP-KO cells exhibit increased levels of IL-1β transcript when stimulated with LPS under serum starvation. This correlates with increased levels of pro-IL-1β protein in the cytosol of these cells as measured using western blot (Figure 3B). As has been observed in other studies, this may represent a positive effect of serum starvation on nuclear factor kappa B activity.26,27 However, because this phenomenon was observed in both control and XIAP-KO cells, it alone does not account for the increased IL-1β that is measured from serum-starved XIAP-deficient cells only.
IL-1β hyperproduction is mediated by NLRP3 through a RIPK3-independent mechanism. (A) Control and XIAP-KO THP-1 cells were stimulated with LPS under standard (10% FCS) and serum starvation (1% FCS) conditions for 4 hours, followed by harvesting for RNA isolation and quantitative real-time PCR for IL-1β transcript levels. Results are normalized to GAPDH transcript levels. (B) Control and XIAP-knockout THP-1 cells were stimulated as indicated in panel A followed by protein extraction for western blotting using anti-IL-1β. Anti-GAPDH was used as loading control. (C) XIAP-KO THP-1 cells were stimulated as indicated under standard (10% FCS) or serum starvation (1% FCS) conditions with or without the addition of NLRP3 inhibitor MCC950. Supernatants were harvested at 4 hours for IL-1β measurement using ELISA. (D) XIAP-KO THP-1 cells were stimulated as indicated under standard (10% FCS) or serum starvation (1% FCS) conditions with or without the addition of RIPK3 inhibitor GSK872. Supernatants were harvested at 4 hours for IL-1β measurements by ELISA. All results are representative of 3 independent experiments. Graphs indicate the mean with standard deviation.
IL-1β hyperproduction is mediated by NLRP3 through a RIPK3-independent mechanism. (A) Control and XIAP-KO THP-1 cells were stimulated with LPS under standard (10% FCS) and serum starvation (1% FCS) conditions for 4 hours, followed by harvesting for RNA isolation and quantitative real-time PCR for IL-1β transcript levels. Results are normalized to GAPDH transcript levels. (B) Control and XIAP-knockout THP-1 cells were stimulated as indicated in panel A followed by protein extraction for western blotting using anti-IL-1β. Anti-GAPDH was used as loading control. (C) XIAP-KO THP-1 cells were stimulated as indicated under standard (10% FCS) or serum starvation (1% FCS) conditions with or without the addition of NLRP3 inhibitor MCC950. Supernatants were harvested at 4 hours for IL-1β measurement using ELISA. (D) XIAP-KO THP-1 cells were stimulated as indicated under standard (10% FCS) or serum starvation (1% FCS) conditions with or without the addition of RIPK3 inhibitor GSK872. Supernatants were harvested at 4 hours for IL-1β measurements by ELISA. All results are representative of 3 independent experiments. Graphs indicate the mean with standard deviation.
To confirm that the IL-1β hyperproduction is mediated by the NLRP3 inflammasome, experiments were conducted using the MCC950 inhibitor. As shown in Figure 3C, the addition of MCC950 during stimulation completely abrogated the increased IL-1β production observed in XIAP-KO THP-1 cells under serum starvation.
As mentioned above, previous studies in murine bone marrow–derived macrophages have demonstrated a role for XIAP in inhibiting RIPK3-mediated NLRP3 inflammasome activation.7-10 In this model, LPS alone results in IL-1β production by XIAP-deficient macrophages through a TNF-dependent mechanism. Conversely, we did not measure a difference in IL-1β release with LPS alone under standard or serum-starvation conditions, suggesting an alternate pathway for this observation. To further exclude a role for RIPK3 in the IL-1β hyperproduction that is observed upon starvation of XIAP-deficient cells, we used the compound GSK-872 to block RIPK3 during stimulation of THP-1 macrophages. As shown in Figure 3D, RIPK3 inhibition did not diminish the IL-1β hyperproduction observed upon stimulation of XIAP-KO THP-1 cells under serum starvation.
XIAP-deficient cells exhibit defective autophagic flux that correlates with IL-1β hyperproduction
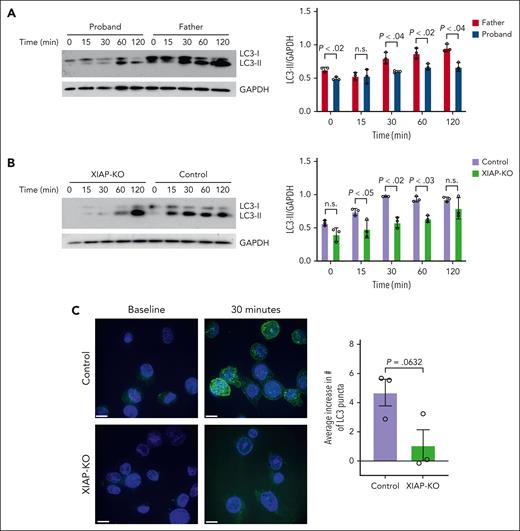
Having demonstrated increased IL-1β release by serum–starved XIAP-deficient cells, we sought to determine whether this dysregulation is associated with altered autophagic flux. PBMC from the proband and his father were serum-starved, and immunoblotting was performed to measure the level of LC3-II as an indicator of early autophagic flux. As shown in Figure 4A, the proband’s cells demonstrated decreased production of LC3-II compared with his healthy father upon serum starvation. Similarly, serum-starved XIAP-KO THP-1 cells showed a decreased rate of accumulation of LC3-II compared with control cells upon serum starvation (Figure 4B). This finding correlates with results from immunofluorescent microscopy demonstrating decreased formation of LC3 puncta in XIAP-deficient cells compared with control cells after serum starvation (Figure 4C). These results indicate that XIAP plays a role in promoting autophagy in monocytes and link the observed IL-1β hyperproduction upon stimulation of serum-starved XIAP-deficient cells to defective induction of autophagy.
XIAP-deficient cells exhibit defective autophagic flux upon serum starvation. Immunoblots and densitometry of LC3 protein from (A) PBMC of proband and his healthy father and (B) control and XIAP-KO THP-1 cells after starvation for the indicated time. GAPDH loading control was used for normalization of LC3-II. (C) Representative immunofluorescence images showing LC3 staining in control and XIAP-KO THP-1 cells at baseline and after 30 minutes of starvation and quantification of average increase in number of LC3 puncta upon starvation. Scale bars represent a distance of 10 μm. Results are representative of 3 independent experiments. Graphs indicate the mean and standard error of the mean.
XIAP-deficient cells exhibit defective autophagic flux upon serum starvation. Immunoblots and densitometry of LC3 protein from (A) PBMC of proband and his healthy father and (B) control and XIAP-KO THP-1 cells after starvation for the indicated time. GAPDH loading control was used for normalization of LC3-II. (C) Representative immunofluorescence images showing LC3 staining in control and XIAP-KO THP-1 cells at baseline and after 30 minutes of starvation and quantification of average increase in number of LC3 puncta upon starvation. Scale bars represent a distance of 10 μm. Results are representative of 3 independent experiments. Graphs indicate the mean and standard error of the mean.
Discussion
At least 90 different genetic variants have been reported to cause XIAP deficiency in XLP-2, including nonsense and missense mutations, small insertions and deletions, whole-exon deletions, and splice site variants.2,5 Here, we present a patient with XLP-2 secondary to a previously undescribed multiexonic duplication of the XIAP gene. Importantly, this was not detected on panel testing or whole-exome sequencing, underscoring the importance of deletion/duplication analysis or whole-genome sequencing when XLP-2 is highly suspected.
Treatment of XLP-2 has been challenging and is complicated by the diverse inflammatory manifestations that are variably expressed by individuals with XIAP deficiency. A recent study analyzed published data on 167 patients with XLP-2.5 In patients presenting with HLH in the absence of inflammatory bowel disease and managed conservatively without hematopoietic stem cell transplantation, reported treatments included steroids alone or in conjunction with intravenous immunoglobulin, cyclosporin A, etoposide, anti-CD20, anti-CD52, or TNF blockade. Our work suggests that IL-1β dysregulation plays a critical role in XLP-2–associated recurrent HLH and highlights IL-1β blockade as a potential additional therapeutic option. This is in keeping with current approaches to the management of secondary HLH, of which a subset respond to IL-1β blockade.28-30 Further study is required to determine the duration of this response, particularly whether it may be a life-long treatment option, and which other disease manifestations of XLP-2 are also responsive to IL-1β blockade.
In contrast to previous studies,7-10,31 we did not detect increased IL-1β production when either primary patient monocytes or XIAP-KO THP-1 cells were stimulated in vitro with LPS. This may reflect a difference between murine and human cells in the role of XIAP at inhibiting RIPK3-mediated NLRP3 activation downstream of the TNF receptor and is consistent with a recent study in which XIAP-deficient patient monocytes were stimulated directly with TNF-α and not observed to produce excess IL-1β compared with control cells.32 We also note significant differences in the concentration of LPS used in these studies, which were typically 10 to 50 times higher and may therefore, activate alternative pathways that are not observed in our system.
We propose here a model in which stimulation during cellular stress, such as nutrient starvation, causes increased levels of IL-1β transcript and pro-IL-1β levels independently of XIAP, with XIAP-dependent activation of autophagy playing a crucial role in limiting the processing of this excess substrate into active IL-1β by the NLRP3 inflammasome. This is in keeping with previous work that has demonstrated increased IL-1β release by macrophages after disruption of autophagy owing to deficiency of Atg16L1.33 Several mechanisms have been proposed for this observation. For example, macrophages have been shown to create autophagosomes that sequester and degrade inflammasome components after NLRP3 activation, thereby limiting IL-1β production.34 When autophagy was perturbed, inflammasome activity and IL-1β release were enhanced. Additionally, cellular stress has been suggested to result in mitochondrial damage that is associated with increased reactive oxygen species production. Inhibition of autophagic clearance of reactive oxygen species–producing mitochondria resulted in enhanced NLRP3 activation by secondary signals, such as ATP.35,36 Importantly, in these models impaired autophagy alone was not sufficient to drive IL-1β production in the absence of the NLRP3 stimulus, which is similar to the need for ATP to observe the effect in our system. Future work will determine the precise contributions of these and other functions of autophagy in limiting IL-1β in XIAP-deficient monocytes specifically.
Interestingly, infections are known triggers of HLH in individuals with XLP-2. Several mechanisms have been proposed for this predisposition, including microbial persistence and chronic stimulation of the immune system.2,37 However, it is notable that certain infections seem to be especially strong triggers of HLH in healthy individuals and even more so in XIAP-deficiency. Specifically, Epstein-Barr virus is found in 25% to 37% of cases of XLP2-HLH, whereas cytomegalovirus and human herpesvirus 6 are also associated to lesser degrees.2,5 A common feature of these herpesviruses is their ability to disrupt autophagy, in part because of their encoding of ubiquitin deconjugases that inhibit activation of the p62 autophagy receptor.38,39 Our work suggests that the impact of these viruses on decreasing autophagy may have direct impacts on the body’s ability to dampen inflammasome activity, thereby resulting in excessive IL-1β release that predisposes to the development of HLH.
In summary, we find that IL-1β is an integral driver and therapeutic target for patients with XLP-2 who have recurrent HLH and that XIAP-deficient monocytes/macrophages exhibit defective autophagic flux and increased IL-1β release when stimulated under serum starvation conditions. More broadly, this work presents autophagic defects as a new category of pathophysiological mechanisms that can contribute to HLH predisposition, which may provide novel therapeutic targets in this subset of patients.
Acknowledgments
The authors thank Bhooma Thiruvahindrapuram, Ted Young, Susan Walker, and the Centre for Applied Genomics for their expertise in genome analysis. The authors thank Annie Huang, Tania Watts, and Cynthia Guidos for their fruitful discussions regarding this project, and Michael B. Jordan for clinical guidance regarding the patient. The authors extend their heartfelt gratitude to the patient and his family for their trust and contributions to this work.
This independent research was supported by the Gilead Sciences Research Scholars Program in Rheumatology, The Hospital for Sick Children Department of Pediatrics, and the Childhood Arthritis and Rheumatic Diseases Biobank funded by Sick Kids Foundation.
The graphical abstract was created with BioRender.com.
Authorship
Contribution: D.D. designed the research studies; D.D., A.F., M.M., and G.A.d.P.L. conducted experiments and acquired the research data; D.D., S.A.F., and R.S.M.Y. analyzed the research data; D.D. and R.M.L. acquired and interpreted the clinical data; C.M. analyzed and interpreted the genetic data; D.D. and R.S.M.Y. wrote the manuscript; and all authors provided feedback and approved the manuscript.
Conflict-of-interest disclosure: R.M.L. serves as a consultant for Swedish Orphan Biovitrum (Sobi) and Novartis. The remaining authors declare no competing financial interests.
Correspondence: Dilan Dissanayake, Division of Rheumatology, The Hospital for Sick Children, 555 University Ave, Toronto, ON M5G 1X8, Canada; email: dilan.dissanayake@sickkids.ca.
References
Author notes
Original data are available on request from the corresponding author, Dilan Dissanayake (dilan.dissanayake@sickkids.ca).
The online version of this article contains a data supplement.
There is a Blood Commentary on this article in this issue.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal