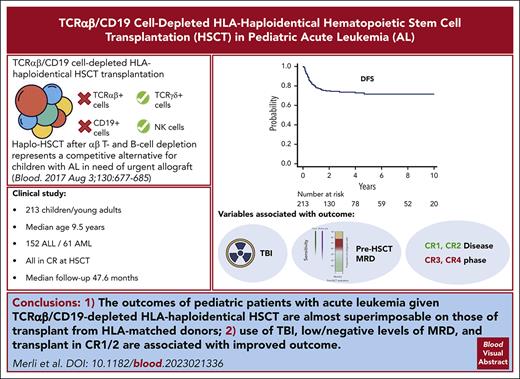
TCRαβ/CD19 cell–depleted haploHSCT is characterized by low NRM and acute/chronic GVHD, with OS and DFS similar to other transplants.
Use of TBI in the conditioning regimen, transplant in CR1/2, and low/negative levels of MRD are associated with improved outcome.
Visual Abstract
TCRαβ/CD19 cell depletion is a promising graft manipulation technique frequently used in the context of human leukocyte antigen (HLA)–haploidentical hematopoietic stem cell transplantation (HSCT). We previously reported the results of a phase I-II clinical trial (NCT01810120) to assess the safety and the efficacy of this type of exvivo T-cell depletion in 80 children with acute leukemia, showing promising survival outcomes. We now report an updated analysis on a cohort of 213 children with a longer follow-up (median, 47.6 months for surviving patients). With a 5-year cumulative incidence of nonrelapse mortality of 5.2% (95% confidence interval [CI], 2.8%-8.8%) and a cumulative incidence of relapse of 22.7% (95% CI, 16.9%-29.2%), projected 10-year overall and disease-free survival (DFS) were 75.4% (95% CI, 68.6%-80.9%) and 71.6% (95% CI, 64.4%-77.6%), respectively. Cumulative incidence of both grade II-IV acute and chronic graft-versus-host disease were low (14.7% and 8.1%, respectively). In a multivariable analysis for DFS including type of disease, use of total body irradiation in the conditioning regimen (hazard ratio [HR], 0.5; 95% CI, 0.26-0.98; P = .04), disease status at HSCT (complete remission [CR] ≥3 vs CR 1/2; HR, 2.23; 95% CI, 1.20-4.16; P = .01), and high levels of pre-HSCT minimal residual disease (HR, 2.09; 95% CI, 1.01-4.33; P = .04) were independently associated with outcome. In summary, besides confirming the good outcome results already reported (which are almost superimposable on those of transplant from HLA-matched donors), this clinical update allows the identification of patients at higher risk of treatment failure for whom personalized approaches, aimed at reducing the risk of relapse, are warranted.
Medscape Continuing Medical Education online

In support of improving patient care, this activity has been planned and implemented by Medscape, LLC and the American Society of Hematology. Medscape, LLC is jointly accredited with commendation by the Accreditation Council for Continuing Medical Education (ACCME), the Accreditation Council for Pharmacy Education (ACPE), and the American Nurses Credentialing Center (ANCC), to provide continuing education for the healthcare team.
Medscape, LLC designates this Journal-based CME activity for a maximum of 1.0 AMA PRA Category 1 Credit(s)™. Physicians should claim only the credit commensurate with the extent of their participation in the activity.
Successful completion of this CME activity, which includes participation in the evaluation component, enables the participant to earn up to 1.0 MOC points in the American Board of Internal Medicine's (ABIM) Maintenance of Certification (MOC) program. Participants will earn MOC points equivalent to the amount of CME credits claimed for the activity. It is the CME activity provider's responsibility to submit participant completion information to ACCME for the purpose of granting ABIM MOC credit.
All other clinicians completing this activity will be issued a certificate of participation. To participate in this journal CME activity: (1) review the learning objectives; (2) study the education content; (3) take the post-test with a 75% minimum passing score and complete the evaluation at https://www.medscape.org/journal/blood; and (4) view/print certificate. For CME questions, see page 291.
Disclosures
CME questions author Laurie Barclay, freelance writer and reviewer, Medscape, LLC, declares no competing financial interests.
Learning Objectives
Upon completion of this activity, participants will:
Describe safety and efficacy of TCRαβ/CD19-cell depletion in the setting of human leukocyte antigen (HLA)-haploidentical hematopoietic stem cell transplantation (HSCT) among children with pediatric acute leukemia, based on an updated analysis of longer-term follow-up data from a phase 1 to 2 clinical trial (NCT01810120)
Determine factors associated with outcomes of TCRαβ/CD19-cell depletion in the setting of HLA-haploidentical HSCT among children with pediatric acute leukemia, based on an updated analysis of longer-term follow-up data from a phase 1 to 2 clinical trial (NCT01810120)
Identify clinical implications of safety and efficacy of TCRαβ/CD19-cell depletion in the setting of HLA-haploidentical HSCT among children with pediatric acute leukemia, based on an updated analysis of longer-term follow-up data from a phase 1 to 2 clinical trial (NCT01810120)
Release date: January 18, 2024; Expiration date: January 18, 2025
Introduction
T-cell receptor (TCR)αβ/CD19 cell depletion has emerged as an effective graft manipulation strategy for preventing graft-versus-host disease (GVHD) in patients lacking a human leukocyte antigen (HLA)–matched donor or in need of an urgent transplant and, thus, candidates to receive an HLA-haploidentical related hematopoietic stem cell transplantation (haploHSCT).1 Indeed, up to 70% to 80% of patients of some ethnic groups, less represented in the international donor registries, do not find an HLA-matched suitable donor.2 To date, the most promising data after TCRαβ/CD19 cell depleted haploHSCT have been obtained in the pediatric population, although results in adults are encouraging as well.3 We previously reported the results of a phase I-II clinical trial (NCT01810120) to assess the safety and the efficacy of TCRαβ/CD19-depleted haploHSCT in 80 children with acute leukemia (AL).4 We showed that this type of transplant is characterized by a low incidence of both acute and chronic GVHD and nonrelapse mortality (NRM), translating into survival outcomes comparable to those of patients receiving an unmanipulated graft from an HLA-compatible donor.4
Other groups have reported similar data after TCRαβ/CD19-depleted haploHSCT,3,5-7 making this approach a valuable alternative in patients with AL in need of an allograft and even challenging the “classic hierarchy” of donor choice.
Herein, we report an update on a cohort of children with AL of our original study, which includes a much larger number of patients treated and with a significantly longer follow-up.
Materials and methods
This prospective clinical trial, approved by the local Ethical Committee and registered at ClinicalTrial.gov (NCT01810120), enrolled children and young adults with either lymphoblastic or myeloid AL (ALL and AML, respectively), who received TCRαβ/CD19-depleted haploHSCT between September 2011 and January 2022. Written informed consent was obtained from either patients or their legal guardians in accordance with the Declaration of Helsinki.
Patients and conditioning
All patients were in morphologic complete remission (CR) at time of transplantation; pre-HSCT minimal residual disease (MRD) was assessed in the 30 days before transplantation and was defined as negative/low levels if <10−4 for ALL and <10−3 for AML. We considered both flow cytometry and molecular MRD, when both available, choosing the highest value in case of discrepancy. As per Center policy, considering the detrimental effect of high MRD levels before transplantation, we aimed at reducing as much as possible MRD levels before HSCT without delaying the transplant. All patients received a fully myeloablative conditioning regimen based on a combination of total body irradiation (TBI) and/or cytotoxic drugs (details on doses/timing are reported in the supplemental Data [available on the Blood website]). The reason for choosing a given combination of drugs was mainly based on: (1) age; (2) previous therapies received/exposure; (3) type of disease; and (4) comorbidities. For example, fludarabine was avoided if a patient had already received >300 mg/m2 of the drug in previous cycles of chemotherapy. Together with chemotherapy/radiotherapy, patients received anti–T-lymphocyte globulin (Grafalon; Neovii Biotech) before transplantation (12 mg/kg total dose, from day −5 to day −3) to modulate bidirectional donor/recipient alloreactivity. Finally, rituximab (200 mg/m2) was included in the preparative regimen on day −1 to prevent post-transplantation Epstein-Barr virus–induced lymphoproliferative disorders. No patient received any post-transplant pharmacologic GVHD prophylaxis.
At Bambino Gesù Children’s Hospital, the following donor hierarchy policy was adopted: (1) matched family donor; (2) 8 of 8 matched unrelated donor; (3) haploidentical donor; and (4) mismatched unrelated donor or unrelated umbilical cord blood.
For T-cell–depleted transplant, in case of availability of multiple haploidentical donors, the following criteria were used for the donor selection8: (1) presence of natural killer (NK) alloreactivity (killer inhibitory receptor [KIR]//KIR-ligand [KIR-L]) mismatch in GVHD direction model); (2) presence of KIR B haplotype; (3) higher B content value in KIR B haplotype donors; (4) larger size of NK alloreactive subset; (5) donor/recipient human cytomegalovirus (HCMV) serology; (6) donor age; (7) donor/recipient body weight; (8) presence of educated KIR2DS1 in case of C2+ patient; (9) higher percentage of NK cells and γδ T lymphocytes; and (10) higher expression of NKp46/presence of NKG2C.
Haploidentical related donors underwent CD34+ HSC peripheral blood mobilization with granulocyte colony-stimulating factor (10-12 μg/kg/day) from day −5 to day −1. Poor CD34+ cell mobilizers received plerixafor (240 μg/kg) in addition to granulocyte colony-stimulating factor. Large-volume apheresis (LA) was performed with the Spectra Optia Cell Separator (Terumo BCT, Leuven, Belgium). LA products were stored overnight at 4°C before processing to remove αβ T cells and B cells.
The depletion procedure was performed in a closed bag system using clinical-grade reagents, disposable kits, and instrumentation obtained from Miltenyi Biotec (Bergish-Gladbach, Germany), as previously described.9 Briefly, LA products, after washing, were tagged with biotin-conjugated anti-TCRαβ antibody. After incubation and washing, the cells were labeled with paramagnetic beads conjugated to anti-biotin and anti-CD19 antibodies, for concomitant B-cell depletion. After washing, the cell suspension was loaded onto the CliniMACS device equipped with the depletion tubing set to automatically process cells through a magnetic column. Aliquots of the eluted cells (the graft) were used to enumerate CD34 HSC, TCRγδ cells, and NK cells in addition to residual TCRαβ T cells and B cells.9
Cells were analyzed for expression of CD3, αβ TCR, CD4, CD8, γδ TCR, CD19, CD20, CD16, CD56, and CD34 and for viability using 7-amino-actinomycin D (7-AAD). All antibodies were from BD Biosciences. The anti-αβ TCR was obtained from Miltenyi. CD34+ cell enumeration was performed using the BD Stem Cell Enumeration Kit, according to the International Society of Hematotherapy and Graft Engineering (ISHAGE) guidelines. Enumeration of residual αβ T cells and B cells (defined as CD20+ cells) was performed as recommended in the Miltenyi protocol.
TCR αβ/CD19 cell–depleted grafts were reinfused into the patients within 2 hours from the end of processing.
All patients received the following prophylaxes: antiviral with acyclovir; antifungal with agents active on both yeast and molds (ie, liposomal amphotericin B twice a week); and against Pneumocystis jirovecii pneumonia with cotrimoxazole twice a week.10
Chimerism and immune reconstitution
Chimerism analysis, evaluated through short tandem repeat (STR), was performed biweekly for the first 3 months and monthly thereafter. Immune recovery (count of TCR αβ CD3+, TCR γδ CD3+, CD4+, CD8+, NK, and CD19+ cells) was evaluated at 1, 3, 6, and 12 months after transplantation. Comparison with other type of transplant performed at our Center and for whom immune reconstitution data were available (namely, 50 T-replete HSCT from matched family donors and 92 T-replete HSCT from unrelated donors [UDs]) was performed.
KIR gene profile, KIR-L, and NK alloreactivity analyses
Donor KIR repertoires were analyzed for the presence/absence of NK alloreactivity combining genetic and phenotypic approaches.
DNA of the tested donors was extracted using the QIAamp DNA Blood Mini kit (Qiagen, Hilden, Germany). The KIR gene profiles were analyzed using a commercial kit based on the sequence-specific primer polymerase chain reaction approach (GenoVision, Saltsjoebaden, Sweden) following the manufacturer’s instructions. On the basis of the results, KIR genotypes (A/A or B/X)11-13 and B content values were assigned.14 The presence of KIR-L mismatch in GvH direction was evaluated by analyzing high-resolution HLA class I typing using the KIR-L calculator free tool (http://www.ebi.ac.uk/ipd/kir/ligand.html). The C1 epitope of HLA-B∗46:01 and HLA-B∗73:01, and the Bw4 epitope present on HLA-A∗23, HLA-A∗24, and HLA-A∗32 were also considered.15,16 Moreover, among the Bw4+ allotypes, HLA-B∗13:01, HLA-B∗13:02, and HLA-A∗25 were ignored, because they are not KIR3DL1 ligands.16 Surface phenotype of NK cells from donors was analyzed on freshly derived peripheral blood mononuclear cells using appropriate combinations of specific antibodies to identify inhibitory and activating KIRs, and NK cell alloreactive subsets.8,17 Multiparametric flow cytometry was performed on a Gallios flow cytometer (Beckman Coulter, Brea, CA) or MACSQuant-analyser (Miltenyi-Biotec, Bergisch Gladbach, Germany).
Statistical analysis
Patient, disease, and transplantation characteristics were summarized using descriptive statistics. Categorical variables are reported as absolute value and percentage, whereas quantitative variables are described as median value and range. Categorical variables were compared using the Fisher exact test; the Mann-Whitney rank sum test and Kruskal-Wallis test were used for continuous variables, as appropriate. Overall survival (OS) was defined as the probability of being alive and calculated starting from the time of HSCT to death or last follow-up; disease-free survival (DFS) was defined as the probability of survival without evidence of disease from the time of HSCT to death or last follow-up; GVHD/relapse-free survival was defined as the probability of survival without occurrence of grade 3 to 4 acute GVHD or chronic GVHD requiring treatment or relapse, whichever occurred first, from the time of HSCT to death or last follow-up. Survival probability was estimated by the Kaplan-Meier method, and differences between groups were calculated using the log-rank test. The Cox proportional hazard regression model was used to assess the association between patient-, disease-, and transplantation-related factors with survival outcomes.
Neutrophil engraftment was defined as time from HSCT to the first of 3 consecutive days with an absolute neutrophil count ≥0.5 × 109/L; platelet engraftment as the first of 7 consecutive days with an unsupported platelet count ≥20 × 109/L. Lack of engraftment or transient engraftment followed by peripheral blood count decline, with pancytopenia, absence of detectable donor cells in the recipient blood, or autologous hematopoietic reconstitution was considered as graft failure, primary and secondary, respectively.
Cumulative incidence of relapse, NRM, and acute and chronic GVHD were calculated using the method of Fine and Gray, taking into account the respective competitive risks (namely, death in remission for relapse, disease recurrence for NRM, and disease recurrence and death for GVHD); comparison between groups was performed with the Gray test.
Statistical analysis was performed using EZR version 1.32 (Saitama Medical Centre, Jichi Medical University),18 which is a graphical user interface for R (The R Foundation for Statistical Computing, Vienna, Austria). Data were analyzed as of November 2022.
The data that support the findings of this study are available from the corresponding author (P.M.) on reasonable request.
Results
Table 1, supplemental Table 1, and supplemental Figure 1 report details on characteristics of patients and donors included in the analysis, as well as the comparison of the features of the new patients analyzed compared with those of patients already reported.4 As expected in a pediatric cohort, most children had ALL in CR2; on the contrary, patients with AML underwent transplant more frequently in CR1, reflecting current HSCT indications.19,20 All patients received the transplant while in morphologic CR, with most patients having a negative MRD (the highest values being 1-1.5 × 10−2 in 2 patients). The median follow-up of surviving patients is 47.6 months (range, 6 months-11.7 years). Compared with our first report,4 in the additional cohort more patients were female, and in the ALL subgroup, more patients had a B-cell precursor origin and underwent transplant in CR3 or more advanced phase of disease. Notably, of 16 children with ALL who did not receive TBI in the conditioning regimen, 4 were infant mixed linkage leukemia (MLL)-rearranged, and 1 each were infant not MLL-rearranged and MLL-rearranged not infant. The reason for which the 11 noninfant patients with ALL did not receive TBI varied: (1) physician choice (eg, young age); and (2) concerns of the parents regarding long-term effects of TBI because of previous radiation therapies (eg, central nervous system irradiation during frontline protocols) and/or comorbidities.
Characteristics of patients, donors, and grafts
| Variable . | All . | Locatelli et al (2017)4 . | Additional 133 patients . | P value . | |||
|---|---|---|---|---|---|---|---|
| No. . | %/Range . | No. . | %/Range . | No. . | %/Range . | ||
| 213 | 100 | 80 | 133 | ||||
| Sex | |||||||
| Male | 105 | 49 | 55 | 69 | 50 | 37.5 | <.001 |
| Female | 108 | 51 | 25 | 31 | 83 | 62.5 | |
| Age at transplant, median (range), y | 9.5 | 0.7-24.6 | 9.7 | 0.9-20.9 | 9.3 | 0.7-24.6 | N.S. |
| ALL/AML | 152/61 | 66/34 | 56/24 | 70/30 | 96/37 | 72/28 | N.S. |
| ALL phenotype | |||||||
| BCP | 124 | 81.5 | 41 | 73 | 83 | 87 | .02 |
| T | 26 | 17 | 15 | 27 | 11 | 11 | |
| Mixed | 2 | 1.5 | 0 | 2 | 2 | ||
| Disease status at transplantation | |||||||
| ALL | |||||||
| CR1 | 40 | 19 | 15 | 19 | 25 | 19 | .02 |
| CR2 | 85 | 40 | 37 | 46 | 48 | 36 | |
| ≥CR3 | 27 | 12 | 4 | 5 | 23 | 17 | |
| Pre-HSCT MRD+∗ | 11 | 7 | |||||
| AML | |||||||
| CR1 | 40 | 19 | 16 | 20 | 24 | 18 | N.S. |
| CR2 | 21 | 10 | 8 | 10 | 13 | 10 | |
| Pre-HSCT MRD+† | 7 | 11.5 | |||||
| Conditioning regimen | N.S. | ||||||
| TBI + TT + Flu | 100 | 47 | 40 | 50 | 60 | 45 | |
| TBI + TT + L-PAM | 61 | 28.5 | 20 | 25 | 41 | 31 | |
| Bu + TT + Flu | 13 | 6 | 13 | 16 | 0 | ||
| Bu + Cy + L-PAM | 21 | 10 | 7 | 9 | 14 | 10.5 | |
| Others | 18 | 8.5 | 0 | 18 | 13.5 | ||
| CMV serology (donor/recipient) | N.S. | ||||||
| Neg/neg | 12 | 6 | 5 | 6 | 7 | 5 | |
| Neg/pos | 20 | 9 | 7 | 9 | 13 | 10 | |
| Pos/neg | 17 | 8 | 11 | 14 | 6 | 4.5 | |
| Pos/pos | 164 | 77 | 57 | 71 | 107 | 80.5 | |
| Donor characteristics | |||||||
| Age, y | 34.5 | 19-59 | 41.5 | 27-55 | 38 | 19-59 | .01 |
| Type of donor | .01 | ||||||
| Mother | 109 | 51 | 46 | 58 | 63 | 47.5 | |
| Father | 92 | 43 | 34 | 42 | 58 | 43.5 | |
| Brother/sister | 12 | 6 | 0 | 12 | 9 | ||
| Sex mismatch | 118 | 55 | 49 | 61 | 69 | 52 | N.S. |
| Female donor → male recipient | 75 (/118) | 64 | 35 (/49) | 71 | 40 (/69) | 58 | N.S. |
| NK alloreactivity (KIR/KIR-L model) yes/no | 75/127‡ | 37/63 | 36/44 | 45/55 | 39/83‡ | 32/68 | N.S. |
| KIR genotype A/A vs B/X | 47/155‡ | 23/77 | 16/64 | 20/80 | 31/91‡ | 25/75 | N.S. |
| Donor B content value 0-1 vs ≥2 | 122/80‡ | 60/40 | 44/36 | 55/45 | 79/43‡ | 65/35 | N.S. |
| Donor KIR2DS1 “educated and useful”‖ yes/no | 62/140‡ | 30/70 | 28/52 | 35/65 | 34/88‡ | 28/72 | N.S. |
| Cell dose infused, median (range) | |||||||
| CD34+ cells × 106/kg | 14.8 | 6.0- 40.4 | 13.9 | 6.0-40.4 | 15.53 | 6.1-33.8 | N.S. |
| αβ+ T cells × 106/kg | 0.04 | 0.001-0.099 | 0.047 | 0.002-0.099 | 0.033 | 0.001-0.099 | .005 |
| γδ+ T cells × 106/kg | 7.9 | 0.6-60.8 | 8.1 | 0.86-56.7 | 8.2 | 0.6-60.8 | N.S. |
| NK cells × 106/kg | 33.1 | 3.84-203.0 | 34.6 | 3.84-146.1 | 32.8 | 5.4-203.0 | N.S. |
| Variable . | All . | Locatelli et al (2017)4 . | Additional 133 patients . | P value . | |||
|---|---|---|---|---|---|---|---|
| No. . | %/Range . | No. . | %/Range . | No. . | %/Range . | ||
| 213 | 100 | 80 | 133 | ||||
| Sex | |||||||
| Male | 105 | 49 | 55 | 69 | 50 | 37.5 | <.001 |
| Female | 108 | 51 | 25 | 31 | 83 | 62.5 | |
| Age at transplant, median (range), y | 9.5 | 0.7-24.6 | 9.7 | 0.9-20.9 | 9.3 | 0.7-24.6 | N.S. |
| ALL/AML | 152/61 | 66/34 | 56/24 | 70/30 | 96/37 | 72/28 | N.S. |
| ALL phenotype | |||||||
| BCP | 124 | 81.5 | 41 | 73 | 83 | 87 | .02 |
| T | 26 | 17 | 15 | 27 | 11 | 11 | |
| Mixed | 2 | 1.5 | 0 | 2 | 2 | ||
| Disease status at transplantation | |||||||
| ALL | |||||||
| CR1 | 40 | 19 | 15 | 19 | 25 | 19 | .02 |
| CR2 | 85 | 40 | 37 | 46 | 48 | 36 | |
| ≥CR3 | 27 | 12 | 4 | 5 | 23 | 17 | |
| Pre-HSCT MRD+∗ | 11 | 7 | |||||
| AML | |||||||
| CR1 | 40 | 19 | 16 | 20 | 24 | 18 | N.S. |
| CR2 | 21 | 10 | 8 | 10 | 13 | 10 | |
| Pre-HSCT MRD+† | 7 | 11.5 | |||||
| Conditioning regimen | N.S. | ||||||
| TBI + TT + Flu | 100 | 47 | 40 | 50 | 60 | 45 | |
| TBI + TT + L-PAM | 61 | 28.5 | 20 | 25 | 41 | 31 | |
| Bu + TT + Flu | 13 | 6 | 13 | 16 | 0 | ||
| Bu + Cy + L-PAM | 21 | 10 | 7 | 9 | 14 | 10.5 | |
| Others | 18 | 8.5 | 0 | 18 | 13.5 | ||
| CMV serology (donor/recipient) | N.S. | ||||||
| Neg/neg | 12 | 6 | 5 | 6 | 7 | 5 | |
| Neg/pos | 20 | 9 | 7 | 9 | 13 | 10 | |
| Pos/neg | 17 | 8 | 11 | 14 | 6 | 4.5 | |
| Pos/pos | 164 | 77 | 57 | 71 | 107 | 80.5 | |
| Donor characteristics | |||||||
| Age, y | 34.5 | 19-59 | 41.5 | 27-55 | 38 | 19-59 | .01 |
| Type of donor | .01 | ||||||
| Mother | 109 | 51 | 46 | 58 | 63 | 47.5 | |
| Father | 92 | 43 | 34 | 42 | 58 | 43.5 | |
| Brother/sister | 12 | 6 | 0 | 12 | 9 | ||
| Sex mismatch | 118 | 55 | 49 | 61 | 69 | 52 | N.S. |
| Female donor → male recipient | 75 (/118) | 64 | 35 (/49) | 71 | 40 (/69) | 58 | N.S. |
| NK alloreactivity (KIR/KIR-L model) yes/no | 75/127‡ | 37/63 | 36/44 | 45/55 | 39/83‡ | 32/68 | N.S. |
| KIR genotype A/A vs B/X | 47/155‡ | 23/77 | 16/64 | 20/80 | 31/91‡ | 25/75 | N.S. |
| Donor B content value 0-1 vs ≥2 | 122/80‡ | 60/40 | 44/36 | 55/45 | 79/43‡ | 65/35 | N.S. |
| Donor KIR2DS1 “educated and useful”‖ yes/no | 62/140‡ | 30/70 | 28/52 | 35/65 | 34/88‡ | 28/72 | N.S. |
| Cell dose infused, median (range) | |||||||
| CD34+ cells × 106/kg | 14.8 | 6.0- 40.4 | 13.9 | 6.0-40.4 | 15.53 | 6.1-33.8 | N.S. |
| αβ+ T cells × 106/kg | 0.04 | 0.001-0.099 | 0.047 | 0.002-0.099 | 0.033 | 0.001-0.099 | .005 |
| γδ+ T cells × 106/kg | 7.9 | 0.6-60.8 | 8.1 | 0.86-56.7 | 8.2 | 0.6-60.8 | N.S. |
| NK cells × 106/kg | 33.1 | 3.84-203.0 | 34.6 | 3.84-146.1 | 32.8 | 5.4-203.0 | N.S. |
Data are given for the whole cohort and comparison between 80 already described patients and 133 new ones.
BCP, B-cell precursor; Bu, busulfan; CMV, cytomegalovirus; Cy, cyclophosphamide; Flu, fludarabine; L-PAM, melphalan; Neg, negative; N.S., not significant; Pos, positive; TT, thiotepa.
MRD+ if >10−4.
MRD+ if >10−3.
Data from 122 of 133 donors.
All patients but 5 engrafted with a median time to neutrophil and platelet recovery of 13 (range, 9-24) and 11 (range, 7-23) days, respectively. All the 5 patients experiencing graft failure (3 primary and 2 secondary graft failure) were rescued with a second allograft from the same or the other parent.
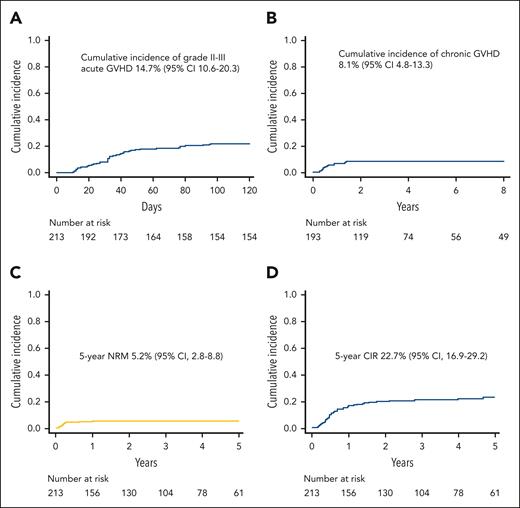
Cumulative incidence of grade II-III acute GVHD was 14.7% (95% confidence interval [CI], 10.6%-20.3%) (Figure 1A): notably, only 4 patients had visceral GVHD (3 gut and 1 liver), whereas for all other patients, the skin was the sole organ involved. No significant difference in the graft TCRαβ CD3+ cell content was present between patients with visceral, skin-only, or no GVHD (supplemental Figure 2). For patients with skin-only GVHD, front-line treatment was based on extracorporeal photoapheresis (plus topic treatment), whereas steroids (and/or further treatments) were employed in those who did not improve after 2 weeks (50% of patients). Conversely, patients with visceral GVHD received full-dose steroids already at onset. Chronic GVHD developed in 14 of the 193 patients (7%) at risk, and it was of limited severity in all cases, with the cumulative incidence of this complication being 8.1% (95% CI, 4.8%-13.3%) (Figure 1B). NRM was recorded in 11 patients (causes are detailed in supplemental Table 2), with the 5-year cumulative incidence being 5.2% (95% CI, 2.8%-8.8%) (Figure 1C). Of the 4 patients who died of interstitial pneumonia syndrome, 3 had received TBI (with lung shielding) in the conditioning regimen.
Cumulative incidence of acute and chronic GVHD, NRM and relapse. (A) Cumulative incidence of grade 2 to 3 acute GVHD in the whole cohort of patients. (B) Cumulative incidence of chronic GVHD (all grades; only patients at risk). (C) NRM of the whole cohort. (D) Cumulative incidence of relapse (CIR) of the whole cohort.
Cumulative incidence of acute and chronic GVHD, NRM and relapse. (A) Cumulative incidence of grade 2 to 3 acute GVHD in the whole cohort of patients. (B) Cumulative incidence of chronic GVHD (all grades; only patients at risk). (C) NRM of the whole cohort. (D) Cumulative incidence of relapse (CIR) of the whole cohort.
Overall, 129 patients developed a viral infection from cytomegalovirus, adenovirus (ADV), or human herpesvirus 6 (22 had ≥2 infections) at a median time of 24 days after HSCT (range, 0-376 days), with the 1-year cumulative incidence being 61.9% (95% CI, 55.1%-68.7%). The cumulative incidences of cytomegalovirus, ADV, and human herpesvirus 6 infections were 33.5% (95% CI, 27.6%-40.3%), 17.2% (95% CI, 12.7%-23.0%), and 11.4% (95% CI, 7.8%-16.6%), respectively. Notably, patients with viral infection had similar survival rates, as well as relapse incidence, compared with those without this kind of complication (data not shown). In addition, patients with viral infections had a slightly longer (although not statistically significant) time of hospitalization (median, 39 days; range, 25-277 days) compared with patients without this kind of complication (median, 36 days; range, 24-322 days; P = .07).
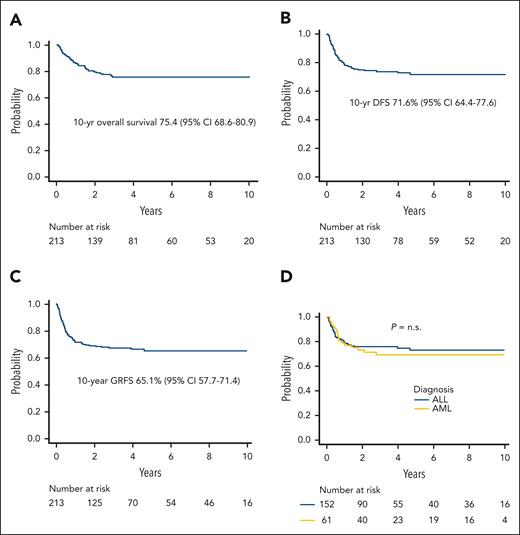
Relapse was the main cause of treatment failure, occurring in 44 patients at a median of 192 days after HSCT (range, 56-1711 days); the 5-year cumulative incidence of relapse was 22.7% (95% CI, 16.9%-29.2%) (Figure 1D). The projected 10-year probabilities of OS and DFS were 75.4% (95% CI, 68.6%-80.9%) (Figure 2A) and 71.6% (95% CI, 64.4%-77.6%) (Figure 2B), respectively. Finally, the 10-year GVHD-free/relapse-free survival was 65.1% (95% CI, 57.7%-71.4%) (Figure 2C). The DFS of patients with either ALL or AML is reported in Figure 2D.
Survival outcomes. (A) Projected 10-year overall survival of the whole cohort. (B) DFS of the whole cohort of patients analyzed. (C) Projected 10-year GVHD/relapse-free survival (GRFS) of the whole cohort. (D) DFS by type of disease. n.s., not significant.
Survival outcomes. (A) Projected 10-year overall survival of the whole cohort. (B) DFS of the whole cohort of patients analyzed. (C) Projected 10-year GVHD/relapse-free survival (GRFS) of the whole cohort. (D) DFS by type of disease. n.s., not significant.
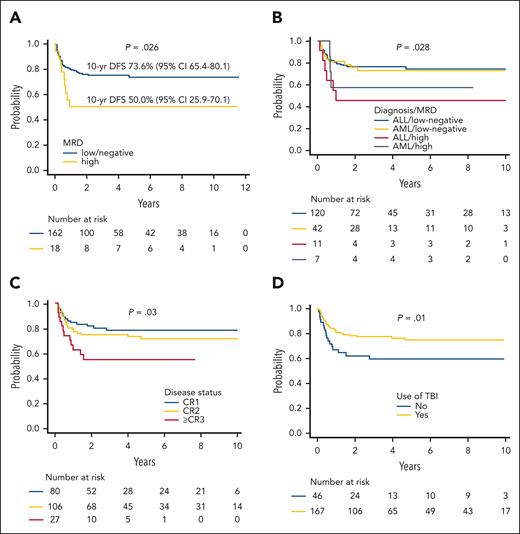
Variables predicting better DFS in univariate analysis were as follows: (1) low/negative MRD before HSCT (Figure 3A-B) (19% of patients, with either AML and ALL, did not have an available pre-HSCT evaluation); (2) early disease status at transplantation (CR1 vs CR2 vs CR >3) (Figure 3C); (3) use of TBI during the preparative regimen (Figure 3D) (the benefit deriving from the use of TBI is observed exclusively in patients with ALL; supplemental Figure 3A); and (4) an age at transplant above the median value (9.5 years). In a post hoc analysis on the TBI-based conditioning regimen used, the use of thiotepa and fludarabine was associated with an improved DFS compared with other combinations (82.4% vs 63.9%; P = .01) (supplemental Figure 3B). Given the discrepancies with the recently published Pediatric Transplantation & Cellular Therapy Consortium (PTCTC) ONC1401 trial regarding the use of TBI in the conditioning regimen,6 we analyzed the effect of radiation after removing patients who were infants or had high pre-HSCT MRD levels; also in these analyses, the positive effect of TBI on patient’s outcome was confirmed (supplemental Figure 3C). Another post hoc analysis was performed on patients with pretransplant high levels of MRD; notably, in this group, those who received >33 000 residual TCRαβ CD3+ cells/kg with the graft showed a significantly improved DFS (supplemental Figure 4). This was not true in patients with ALL receiving a non–TBI-based regimen, although the numbers are too small to draw any firm conclusion (data not shown).
Survival univariable analyses. (A) DFS by MRD of the evaluable patients. (B) DFS by MRD levels, stratified by type of disease. (C) DFS by disease phase at HSCT. (D) DFS by use of TBI in the conditioning regimen.
Survival univariable analyses. (A) DFS by MRD of the evaluable patients. (B) DFS by MRD levels, stratified by type of disease. (C) DFS by disease phase at HSCT. (D) DFS by use of TBI in the conditioning regimen.
As previously reported,3,4 neither NK alloreactivity (determined through the KIR/KIR-L mismatch in graft-versus-host direction model), nor B-content score, nor other NK-related variables (reported in Table 1) influenced outcome. Notably, also stratifying patients according to the use of a “KIR favorable” donor, as defined by Pulsipher and colleagues6 (ie, B content according to the Cooley Scale of ≥214 or inhibitory ligand mismatch by DNA typing or both), we were unable to identify a favorable clinical effect (supplemental Figure 3D). In a multivariable model for DFS including also type of disease, disease status at HSCT (reference CR ≥3: hazard ratio [HR], 2.23; 95% CI, 1.20-4.16; P = .01), pre-HSCT MRD (reference high levels: HR, 2.09; 95% CI, 1.01-4.33; P = .04), and TBI (reference received radiotherapy: HR, 0.50; 95% CI, 0.26-0.98; P = .04) remained statistically significant.
Immune reconstitution was fast, especially after the third month after HSCT. As already reported, TCRγδ lymphocytes and NK cells were the predominant lymphoid population in the first month after transplantation, whereas TCRαβ became predominant thereafter. CD3+ cell counts >500/μl were reached between 3 and 6 months after transplantation. As expected in a T-cell depleted (TCD) (and B-cell–depleted) setting, the comparison with the immune reconstitution of T-cell replete transplants, either from matched family donors or UDs performed at our center in the same time period, showed higher T and B cell counts in the first 6 months after transplants. Further details on immune recovery are reported in Table 2.
Details on immune reconstitution of the patients given TCRαβ/CD19-depleted haploHSCT
| Variable . | 1 mo . | 3 mo . | 6 mo . | 12 mo . | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| HaploHSCT . | MFD . | UD . | P value . | HaploHSCT . | MFD . | UD . | P value . | HaploHSCT . | MFD . | UD . | P value . | HaploHSCT . | MFD . | UD . | P value . | |
| CD3+ T cells/μl | 150 (1-1680) | 401 (55-1148) | 239 (24-1776) | <.01 | 234 (19-1727) | 495 (95-2254) | 323 (18-4766) | <.01 | 544 (110-2640) | 719 (210-3561) | 678 (131-8439) | .01 | 1040 (206-5045) | 1477 (305-3469) | 1279 (81-5072) | N.S. |
| CD4+ T cells/μl | 18 (0-542) | 150 (13-471) | 70 (1-863) | <.01 | 57 (2-615) | 165 (19-904) | 87 (0-1139) | <.01 | 169 (51-1186) | 258 (110-1027) | 204 (22-1796) | <.01 | 494 (110-2009) | 565 (117-1537) | 438 (77-1676) | N.S. |
| CD8+ T cells/μl | 29 (0-1024) | 229 (35-766) | 119 (2-1470) | <.01 | 62 (3-1110) | 281 (38-1328) | 191 (2-4118) | <.01 | 208 (25-1602) | 384 (58-2372) | 327 (78-6454) | <.01 | 392 (94-3975) | 625 (82-2286) | 615 (48-4065) | <.01 |
| αβ T cells/μl | 45 (0-812) | 343 (52-963) | 217 (1-1616) | <.01 | 174 (11-1340) | 476 (86-2062) | 290 (5-4274) | <.01 | 452 (78-2146) | 682 (190-3048) | 584 (122-8312) | <.01 | 959 (215-4621) | 1359 (270-3265) | 1052 (110-4853) | N.S. |
| γδ T cells/μl | 93 (0-1335) | 36 (3-176) | 14 (1-352) | <.01 | 50 (1-1313) | 37 (3-253) | 19 (1-320) | <.01 | 78 (4-1865) | 68 (7-482) | 36 (1-487) | <.01 | 97 (4-1305) | 103 (12-1136) | 92 (6-1044) | N.S. |
| CD3-CD56+ NK cells/μl | 176 (10-1813) | 220 (24-1010) | 143 (19-718) | N.S. | 147 (5-1448) | 137 (12-576) | 127 (4-880) | N.S. | 199 (31-2120) | 203 (72-1125) | 155 (8-1535) | N.S. | 237 (43-3116) | 278 (67-1369) | 223 (15-2314) | N.S. |
| CD19+ B cells/μl | 0 (0-110) | 5 (0-328) | 4 (0-251) | <.01 | 1 (0-475) | 110 (1-582) | 47 (0-461) | <.01 | 133 (0-1652) | 301 (0-2794) | 189 (0-2664) | <.01 | 288 (0-1723) | 506 (0-2629) | 355 (0-2199) | .02 |
| Variable . | 1 mo . | 3 mo . | 6 mo . | 12 mo . | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| HaploHSCT . | MFD . | UD . | P value . | HaploHSCT . | MFD . | UD . | P value . | HaploHSCT . | MFD . | UD . | P value . | HaploHSCT . | MFD . | UD . | P value . | |
| CD3+ T cells/μl | 150 (1-1680) | 401 (55-1148) | 239 (24-1776) | <.01 | 234 (19-1727) | 495 (95-2254) | 323 (18-4766) | <.01 | 544 (110-2640) | 719 (210-3561) | 678 (131-8439) | .01 | 1040 (206-5045) | 1477 (305-3469) | 1279 (81-5072) | N.S. |
| CD4+ T cells/μl | 18 (0-542) | 150 (13-471) | 70 (1-863) | <.01 | 57 (2-615) | 165 (19-904) | 87 (0-1139) | <.01 | 169 (51-1186) | 258 (110-1027) | 204 (22-1796) | <.01 | 494 (110-2009) | 565 (117-1537) | 438 (77-1676) | N.S. |
| CD8+ T cells/μl | 29 (0-1024) | 229 (35-766) | 119 (2-1470) | <.01 | 62 (3-1110) | 281 (38-1328) | 191 (2-4118) | <.01 | 208 (25-1602) | 384 (58-2372) | 327 (78-6454) | <.01 | 392 (94-3975) | 625 (82-2286) | 615 (48-4065) | <.01 |
| αβ T cells/μl | 45 (0-812) | 343 (52-963) | 217 (1-1616) | <.01 | 174 (11-1340) | 476 (86-2062) | 290 (5-4274) | <.01 | 452 (78-2146) | 682 (190-3048) | 584 (122-8312) | <.01 | 959 (215-4621) | 1359 (270-3265) | 1052 (110-4853) | N.S. |
| γδ T cells/μl | 93 (0-1335) | 36 (3-176) | 14 (1-352) | <.01 | 50 (1-1313) | 37 (3-253) | 19 (1-320) | <.01 | 78 (4-1865) | 68 (7-482) | 36 (1-487) | <.01 | 97 (4-1305) | 103 (12-1136) | 92 (6-1044) | N.S. |
| CD3-CD56+ NK cells/μl | 176 (10-1813) | 220 (24-1010) | 143 (19-718) | N.S. | 147 (5-1448) | 137 (12-576) | 127 (4-880) | N.S. | 199 (31-2120) | 203 (72-1125) | 155 (8-1535) | N.S. | 237 (43-3116) | 278 (67-1369) | 223 (15-2314) | N.S. |
| CD19+ B cells/μl | 0 (0-110) | 5 (0-328) | 4 (0-251) | <.01 | 1 (0-475) | 110 (1-582) | 47 (0-461) | <.01 | 133 (0-1652) | 301 (0-2794) | 189 (0-2664) | <.01 | 288 (0-1723) | 506 (0-2629) | 355 (0-2199) | .02 |
Data are given as median (range). Data from other type of transplant performed in the same time period are also reported for comparison.
MFD, matched family donor; N.S., not significant; UD, matched unrelated donor.
Discussion
TCRαβ/CD19-depleted haploHSCT is becoming a widespread HLA-haploidentical platform for pediatric patients affected by both malignant3,5,6,21 and nonmalignant disorders.22-24 This cohort of homogeneously treated patients with AL, the largest reported so far, with a median follow-up of almost 4 years, confirms the effectiveness and safety of this approach. Both acute and chronic GVHD incidence is lower even than those usually reported in the T-cell–replete, HLA-matched setting; this observation is particularly important for pediatric patients, who have a long life expectancy and can be particularly penalized by late sequels. Because no difference in the residual TCRαβ CD3+ cell content in the graft was found between patients with visceral, skin-only, or no GVHD, other variables (such as anti–T-lymphocyte globulin levels at time of transplant or in the immediate posttransplant period) may play a role in the development of this complication.
NRM remained remarkably low, with main cause of death being either infectious (as expected in a T-cell–depleted setting) or toxic (interstitial pneumonia syndrome and transplant-associated thrombotic microangiopathy); thus, further insight in this latter type of complications25 (still poorly characterized) may help further reduce NRM. Viral infections were recorded in a remarkable proportion of patients. This observation can be explained by the lack of protective effect mediated by donor pathogen-specific T lymphocytes transferred with the graft and indicates that strict monitoring and timely intervention are key for managing these infections. Disease recurrence remains the main cause of treatment failure (although in line with other types of transplant, it may also be due to the Center attitude to reduce pre-HSCT MRD levels as much as possible), particularly in patients in CR3 or more advanced CR and in those with high levels of pre-HSCT MRD. Thus, in these patient groups, pre-transplant and post-transplant strategies aimed at reducing the risk of relapse are desirable; indeed, different interventions can be considered, including pharmacologic (eg, zoledronic acid26 or monoclonal antibodies27) as well as cellular ones. In this regard, because no post-transplant pharmacologic GVHD prophylaxis is used, this depletion strategy represents the ideal platform for T-cell add-back to further improve outcomes, also in light of the well-known lymphopenia, typical of T-cell–depleted transplants, particularly evident during the first 3 months after HSCT (especially if compared with T-cell–replete transplant). Unmanipulated donor lymphocyte infusions (DLI,) infusion of selected lymphocyte populations, such as CD45RA-depleted cells,28 and genetically modified T cells29 are potentially valuable approaches deserving further investigations. In addition, given the promising results, in terms of both safety and efficacy, of post-transplant anti-CD19 allogeneic chimeric antigen receptor T cells from different donor types, including haploidentical ones, we recently reported,30 prophylactic/preemptive approaches for preventing relapse in high-risk B-lineage patients with ALL can be hypothesized.
Several other groups have reported encouraging results with this type of graft manipulation,3,5-7 confirming low rates of NRM and acute and chronic GVHD, with survival probabilities almost superimposable on those of transplant from other type of donors, including matched family or unrelated donors, thus challenging the “classic” donor hierarchy.
In this study, we found that a TBI-containing conditioning regimen was associated with a significantly better outcome in the 152 patients with ALL. This finding is consistent with the data obtained in the For Omitting Radiation Under Majority age (FORUM) randomized clinical trial, in which children with ALL who underwent transplant with unmanipulated cells from either an HLA-identical sibling or an unrelated donor had a much lower risk of disease recurrence, translating into a better DFS if prepared to the allograft with TBI compared with those receiving a myeloablative, chemotherapy-based conditioning regimen.31 The discrepancy between our results and those of the PTCTC ONC1401 trial6 regarding the effect of TBI/myeloablative conditioning may have several explanations. First, the number of patients with ALL investigated (and thus the statistical power) is different (152 versus 30); thus, the lack of a favorable effect displayed by TBI in the US report can simply be attributed to the limited number of patients analyzed by Pulsipher and colleagues.6 In addition, different radiation technique adopted and different study population (eg, the ethnic origin of the 2 populations is significantly different) may also have contributed. Finally, NRM in the myeloablative conditioning (MAC)/TBI group in the PTCTC ONC1401 report was remarkably high (18.5%) compared with that of patients receiving reduced toxicity regimen.
Our results differ from those reported by Pulsipher and colleagues6 also for what concerns a more favorable outcome conferred by the use of a “KIR favorable” donor. Although we used exactly the same criteria for the donor classification, we could not find any benefit deriving from the choice of a KIR favorable donor. This discrepancy can be explained by the different number of patients analyzed (only 46 children had ALL or AML in the PTCTC ONC1401 report). The lack of benefit of a donor with NK cell characteristics predicting a better anti-leukemia effect may be due to the interfering graft-versus-leukemia effect displayed by γδ T cells that can obscure/compensate that mediated by NK lymphocytes. In addition, it cannot be excluded that immunogenetic NK cell characteristics may have more relevance when a reduced toxicity regimen is employed. Finally, the presence of (unquantified) myeloid-derived suppressor cells, as previously demonstrated, may differently interfere with NK cell activity.32 Interestingly, in patients with pretransplant high levels of MRD, it seems that TCRαβ+ cells >33 000/kg in the graft be associated with improved outcome.
Direct comparisons between the outcomes observed in children and young patients given the TCRαβ/CD19-depleted haploHSCT and those reported after posttransplant cyclophosphamide (PT/Cy) haplo platform, the most used approach for haploidentical transplantation in adults, are not available. Results from cohorts smaller than that reported here have shown DFS probabilities ranging from 55% to 79% in children and younger patients treated with myeloablative approaches.33-35 These findings seem to indicate slightly inferior or similar survival outcomes, although chronic GVHD incidence, especially when peripheral blood was used as stem cell source, looks to be higher. Interestingly, a recent retrospective registry analysis of the Inborn Error Working Party of European Society for Blood and Marrow Transplantation (EBMT) in the setting of nonmalignant disorders showed higher OS and EFS with the use of TCRαβ/CD19-depleted HLA-haploidentical grafts.36
The costs associated with the use of haploidentical donors (compared with other alternative donors) are a matter of debate; however, these studies are made difficult by the different organization of national health systems, as well as by diversity of reimbursement models. Most of economic studies have compared the cost of haploidentical HSCT using PT/Cy (which is far cheaper than exvivo TCD) to those of other graft sources (mainly cord blood), with conflicting results,37-39 also considering different age groups.40 Reagents and personnel costs associated with TCRαβ/CD19 procedure are relevant. So far, few studies have investigated the cost burden with this kind of technique. Van Sambeek and colleagues reported a slightly higher cost for TCRαβ/CD19 depletion compared with use of unrelated donor grafts. However, given the lower incidence of GVHD, they hypothesized a cost-effectiveness of TCRαβ/CD19 depletion over a patient’s lifetime.41 Notably, the Children’s Oncology Group study ASCT2031 (NCT05457556), which will randomize haploidentical approaches (TCD and PT/Cy) with fully matched UD, will investigate resource use as an exploratory end point.
In summary, this clinical trial update confirms the good outcome results already reported in patients given TCRαβ/CD19-depleted haploHSCT, which are almost superimposable on those of transplant from HLA-matched donors.4,42 Moreover, in this study, we identified patients at higher risk of treatment failure for whom personalized approaches, aimed at reducing the risk of relapse, are warranted and we confirmed that a TBI-containing preparative regimen should be chosen whenever possible in children with ALL. In light of our results, the potential benefit suggested to be associated with the use of a KIR favorable donor24 needs to be further investigated and validated with robust numbers of patients analyzed.
Acknowledgments
This study was supported by grants from Associazione Italiana Ricerca sul Cancro (AIRC) Special Project 5×1000 No. 9962 (F.L.); AIRC IG 2018 identifier 21724 (F.L.); Ministero dell'Università e della Ricerca (grants PRIN 2017 and PRIN 2020 to F.L.); Ministero della Salute (GR-2018-12365485 to P.M.; Ricerca Corrente IRCCS G. Gaslini to M.F.; and IRCCS Ospedale Policlinico San Martino to R.M. and D.P.).
Authorship
Contribution: F.L. designed the study and supervised the project; P.M., M. Algeri, F.G., V.B., E.B., M.B., C.R., and F.Q. collected the data; P.M., M.A., F.G., E.B., M.B., C.R., F.Q., M.L.C., R.C., L.S., D. Pagliara, and F.L. were involved in the clinical management of patients; R.M.P. and M. Andreani performed human leukocyte antigen typing; A.M. and G.D.P. performed donor apheresis; S.B., E.G., and G.L.P. performed graft manipulation and graft characterization; V.B., R.S., M.F., R.M., and D. Pende performed immune monitoring; M.F., R.M., and D. Pende performed natural killer immunogenetic characterization; P.M. and F.L. analyzed and interpreted the data and wrote and edited the manuscript; and all authors had access to primary clinical trial data, contributed to the intellectual content of this article, and reviewed and approved the final manuscript.
Conflict-of-interest disclosure: P.M. reports personal fees from Sobi and Jazz, outside the submitted work. M. Algeri served on Scientific Advisory Board for Vertex Pharmaceuticals and as Steering Committee member for Vertex Pharmaceuticals, outside the submitted work. F.L. reports personal fees from Amgen, personal fees from Novartis, other from Bellicum Pharmaceutical, other from Neovi, personal fees from Miltenyi, personal fees from Medac, personal fees from Jazz Pharmaceutical, and personal fees from Takeda, outside the submitted work. The remaining authors declare no competing financial interests.
Correspondence: Pietro Merli, IRCCS Bambino Gesù Children’s Hospital, Piazza S. Onofrio 4, 00165 Rome, Italy;.
References
Author notes
Presented as an oral presentation at the 65th annual meeting of the American Society of Hematology, New Orleans, LA, 12 December 2022.
The online version of this article contains a data supplement.
There is a Blood Commentary on this article in this issue.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal