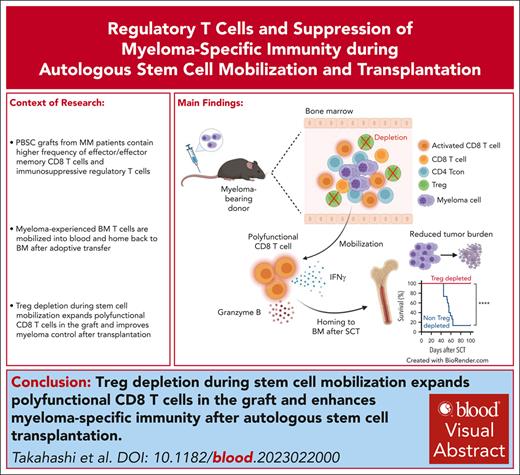
T cells from bone marrow egress to blood during SCM and preferentially home back to marrow after adoptive transfer.
Treg depletion during SCM expands polyfunctional effector T cells in mobilized grafts and enhances antimyeloma immunity after ASCT.
Visual Abstract
Autologous stem cell transplantation (ASCT) is the standard of care consolidation therapy for eligible patients with myeloma but most patients eventually progress, an event associated with features of immune escape. Novel approaches to enhance antimyeloma immunity after ASCT represent a major unmet need. Here, we demonstrate that patient-mobilized stem cell grafts contain high numbers of effector CD8 T cells and immunosuppressive regulatory T cells (Tregs). We showed that bone marrow (BM)-residing T cells are efficiently mobilized during stem cell mobilization (SCM) and hypothesized that mobilized and highly suppressive BM-derived Tregs might limit antimyeloma immunity during SCM. Thus, we performed ASCT in a preclinical myeloma model with or without stringent Treg depletion during SCM. Treg depletion generated SCM grafts containing polyfunctional CD8 T effector memory cells, which dramatically enhanced myeloma control after ASCT. Thus, we explored clinically tractable translational approaches to mimic this scenario. Antibody-based approaches resulted in only partial Treg depletion and were inadequate to recapitulate this effect. In contrast, a synthetic interleukin-2 (IL-2)/IL-15 mimetic that stimulates the IL-2 receptor on CD8 T cells without binding to the high-affinity IL-2Ra used by Tregs efficiently expanded polyfunctional CD8 T cells in mobilized grafts and protected recipients from myeloma progression after ASCT. We confirmed that Treg depletion during stem cell mobilization can mitigate constraints on tumor immunity and result in profound myeloma control after ASCT. Direct and selective cytokine signaling of CD8 T cells can recapitulate this effect and represent a clinically testable strategy to improve responses after ASCT.
Introduction
Multiple myeloma (MM) is a hematological malignancy characterized by the expansion of malignant plasma cells in the bone marrow (BM). The global incidence of myeloma has been increasing1 and despite the emergence of new therapeutics, MM remains incurable in all but a few patients who achieve dramatic responses to immunotherapy. Autologous stem cell transplantation (ASCT) is the standard of care consolidation therapy for eligible patients with myeloma, generating significantly longer progression-free survival (PFS) than chemotherapy alone.2,3 Improved PFS after ASCT is generally regarded as a result of cytoreduction from myeloablative chemotherapy, but the enhancement of myeloma-specific immunity is also increasingly being recognized.4 We previously demonstrated that ASCT has the potential to invoke T-cell–mediated myeloma control after transplantation using the Vk∗MYC myeloma mouse model and that immune escape is associated with T-cell dysfunction.5-8 Transplant-eligible patients with myeloma usually undergo granulocyte colony-stimulating factor (G-CSF)-based stem cell mobilization (SCM), followed by the collection of peripheral blood stem cells (PBSC). These mobilized grafts include hematopoietic stem cells and various types of conventional (Tcon) and regulatory (Treg) T cells, some of which may have originated in the BM9 and putatively include myeloma-specific T cells. In this study, we demonstrated that Treg depletion during SCM dramatically enhances antimyeloma immunity, resulting in potent myeloma control after ASCT. Although similar profound Treg depletion by antibody-based approaches was not achievable, approaches that directly stimulate CD8 T cells independently of Tregs represent a tractable approach to circumvent this inhibitory pathway.
Methods
Mice
Female 8 to 12-week-old C57BL/6 mice (B6.WT; CD45.2) were purchased from the Jackson Laboratory (Bar Harbor, ME). B6.PTPRCA mice (CD45.1), C57BL/6 × PTPRCA (CD45.1/45.2), and transgenic mice on a B6 background: FoxP3-GFP-DTR, CD11c-DOG, MataHari-BLITC, RAG2/IL2rg KO, and HULK reporter mice were bred in-house (Fred Hutchinson Cancer Center). Mice were housed in sterilized disposable cages and received acidified autoclaved water and normal chow.
Tumor inoculation
BM transplantation
Mice were transplanted as described previously.10 Briefly, MM-bearing recipient mice received 1000 cGy of total body irradiation on day −1. In FoxP3-GFP-DTR mice, Tregs were depleted by 4× intraperitoneal injection of 160 ng/body diphtheria toxin. For antibody-mediated depletion of Tregs, mice were treated with 500 μg anti-CD25 (rat IgG1, PC-61.5.3, BioXcell) twice a week, 100 μg anti-CTLA4 (Syrian hamster IgG, 9H10, BioXcell), and 100 μg anti-GITR (rat IgG2b, DTA-1, BioXcell) every other day via intraperitoneal injection. NL-201, provided by Neoleukin Therapeutics (Seattle, WA), was intraperitoneally administered at 225 μg/kg once a week. Donor mice were euthanized and the mobilized spleen was harvested. CD8 T cells in mobilized grafts were purified using magnetic-activated cell sorting microbeads (Miltenyi, Bergisch Gladbach, Germany). BM and CD4 T cells were harvested from the naïve C57Bl/6 mouse. CD4 T cells were isolated from peripheral lymph node and spleen using magnetic-activated cell sorting microbeads. Recipient mice received transplantation with BM and T cells (doses are detailed in the Figure 5 legend) and clinical scores and M-bands were measured after transplantation. Recipient mice were euthanized if paralysis occurred or the clinical score reached ≥6.11
Study approval
For human samples, ethical approval was obtained from the Fred Hutchinson Cancer Center/Seattle Cancer Care Alliance IRB and QIMR Berghofer Medical Research Institute Human Research Ethics Committees, with written informed consent from all participants. Cytogenetic risk in patients with myeloma was assessed as previously described.12 The number of months taken to develop progressive myeloma after transplantation was determined per physician documentation. All animal procedures were performed in accordance with protocols approved by the institutional animal ethics committee.
Statistics
Welch t test or Mann-Whitney U test for 2 samples, Wilcoxon matched-pairs signed rank test for paired samples and one-way analysis of variance (ANOVA) or Kruskal-Wallis test for multiple samples were used to compare data. Survival curves were plotted using Kaplan-Meier estimates and compared using the log-rank test. Data are presented as the mean ± standard error of the mean (SEM); P < .05 was considered statistically significant. All tests were performed using GraphPad Prism 9 (La Jolla, CA).
Results
Mobilized stem cell grafts from patients with myeloma contain high numbers of effector/effector memory CD8 T cells and Tregs
Myeloma is a BM-based disease and we have recently shown that myeloma-specific immunity can be seen in the BM after ASCT but not in the peripheral blood (PB).13 Therefore, we examined T-cell composition in the BM at steady state and in PBSC grafts from patients with myeloma. We collected samples from patients with myeloma who underwent ASCT (supplemental Table 2, available on the Blood website). To minimize the phenotypic alterations in T cells driven by myeloma itself, we collected BM samples within 1 month before harvesting PBSC, when patients had completed myeloma-targeted cytoreductive induction therapy. We performed fluorescence-activated cell sorting (FACS) analysis to compare the phenotype of T cells in BM and PBSC and then performed unbiased clustering using FlowSOM. In the CD8 T-cell compartment, PBSC had fewer central memory T cell (TCM) and more CD45RA+ effector memory T cells (TEMRA) than BM (Figure 1A). When we compared subpopulations of CD8 T cells, we noted that the proportion of differentiated effector TEMRA (Pop0) and activated TEM (Pop 4 and 5) was significantly increased in PBSCs (Figure 1B). CD69+ populations (Pop 1,7,9) remained in the BM even after SCM in humans and mice (supplemental Figure 1), suggesting that CD69+ cells are potentially resistant to SCM using G-CSF and CCR4 receptor antagonists. CD4 Tcon and CD4 TEM cells were significantly increased in the PBSC (Figure 1C). In contrast to CD8 T cells, Granzyme B+ CD4 T cells were not significantly higher in PBSC than in the BM (Figure 1D). The proportion of TIGIT+CD4+ Tcon was also significantly higher in PBSC (Figure 1D), consistent with previous data.14,15 Next, we compared the frequency of Tregs expressing known immunosuppressive molecules in the BM and PBSC. PBSC had higher proportions of TIGIT+CD39+ Tregs, which were enriched for FoxP3highCD45RA− activated Treg subsets16 (supplemental Figure 2), suggesting that Tregs in PBSC grafts were enriched for highly immunosuppressive subsets (Figure 1E). These phenotypically highly suppressive Tregs were more frequent in PBSC from patients with myeloma than in those from healthy donors (Figure 1F). Higher proportions of suppressive TIGIT+CD39+ Treg in the stem cell graft correlated with earlier progressive myeloma after transplantation (Figure 1G). Thus, human PBSC from patients with myeloma have a higher frequency of both effector/effector memory CD8 T cells and immunosuppressive Tregs.
Mobilized grafts from patients with myeloma contain high numbers of effector/effector memory CD8 T cells and Tregs. BM and PBSC were collected from the same patients with myeloma (n = 14-18). We performed FACS analysis to determine the phenotype of T cells in the BM and PBSC. Data from the BM and PBSC were combined and unbiased clustering was performed to visualize each population using FlowSOM. A heatmap was generated based on the expression levels of the flow cytometry markers. (A) Representative FACS plots and frequency of naïve and memory subsets in CD8 T cells. (B) Heatmap of marker expression and the frequency of each CD8 T-cell population. (C) Representative FACS plots and frequency of naïve and memory subset of conventional CD4 T cells. (D) Heatmap of marker expression and the frequency of each CD4 T-cell population. (E) Representative FACS plots of CD25+CD127−CD4 Tregs and the proportion of TIGIT+ Treg and TIGIT+CD39+ Treg. Each red dot and blue square represent a single BM and PBSC sample, respectively. Paired BM and PBSC are connected by solid line. (F) Frequency of TIGIT+ Treg and TIGIT+CD39+ Treg in PBSC from healthy donors (HD) and patients with myeloma (Pts). Wilcoxon matched pairs signed rank test for paired comparison and Welch test for 2 samples comparison (n = 18 per group). (G) Months to progressive myeloma after stem cell transplantation in cytogenetic high-risk patients with at least 12 months of follow-up relative to Treg within the stem cell grafts. ∗P < .05, ∗∗P < .01, ∗∗∗P < .001, ∗∗∗∗P < .0001.
Mobilized grafts from patients with myeloma contain high numbers of effector/effector memory CD8 T cells and Tregs. BM and PBSC were collected from the same patients with myeloma (n = 14-18). We performed FACS analysis to determine the phenotype of T cells in the BM and PBSC. Data from the BM and PBSC were combined and unbiased clustering was performed to visualize each population using FlowSOM. A heatmap was generated based on the expression levels of the flow cytometry markers. (A) Representative FACS plots and frequency of naïve and memory subsets in CD8 T cells. (B) Heatmap of marker expression and the frequency of each CD8 T-cell population. (C) Representative FACS plots and frequency of naïve and memory subset of conventional CD4 T cells. (D) Heatmap of marker expression and the frequency of each CD4 T-cell population. (E) Representative FACS plots of CD25+CD127−CD4 Tregs and the proportion of TIGIT+ Treg and TIGIT+CD39+ Treg. Each red dot and blue square represent a single BM and PBSC sample, respectively. Paired BM and PBSC are connected by solid line. (F) Frequency of TIGIT+ Treg and TIGIT+CD39+ Treg in PBSC from healthy donors (HD) and patients with myeloma (Pts). Wilcoxon matched pairs signed rank test for paired comparison and Welch test for 2 samples comparison (n = 18 per group). (G) Months to progressive myeloma after stem cell transplantation in cytogenetic high-risk patients with at least 12 months of follow-up relative to Treg within the stem cell grafts. ∗P < .05, ∗∗P < .01, ∗∗∗P < .001, ∗∗∗∗P < .0001.
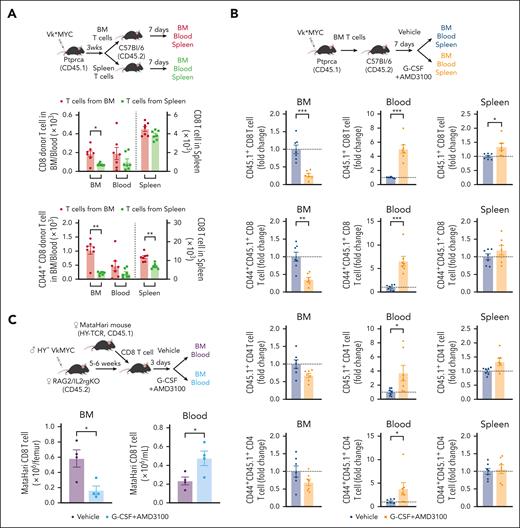
BM resident CD8 T cells are mobilized into the PB during SCM
If MM immunity resides primarily in the BM rather than PB,13 the use of PBSC rather than BM may represent a suboptimal graft source to maximize MM-specific immunity after ASCT. Conversely, MM-specific immunity may be mobilized into PB during SCM in a manner similar to that of stem cells. To study this, we analyzed the distribution of spleen- or BM-derived CD8 T cells after adoptive transfer into secondary CD45 congenic mice. We analyzed CD8 T cells from the BM, blood, and spleen 1 week after adoptive transfer. Although transferred CD45.1+ CD8 T cells were observed in all 3 organs, BM CD8 T cells preferentially migrated to the BM compared with those from the spleen. This was also true for the BM-derived CD44+ memory CD8 T cells (Figure 2A). Next, we examined whether these BM-resident CD8 T cells were mobilized into the blood during SCM with G-CSF and AMD3100, a CXCR4 antagonist (Figure 2B). Transferred BM T cells were indeed efficiently mobilized from BM to blood, including CD44+ memory fractions and Treg (Figure 2B, supplemental Figure 3). We also confirmed that clonally restricted myeloma-specific T cells were mobilized from the BM to the blood using MataHari HY-TCR transgenic mice17 and HY+ Vk∗MYC (Figure 2C). These results confirm that BM-resident T cells, which include myeloma-specific CD8 T cells, can be mobilized into the PB during SCM.
CD8 T cells from the BM egress into the PB during stem cell mobilization. (A) T cells were harvested from the BM and spleen of MM-bearing mice (CD45.1) and transferred to naïve B6 mice (CD45.2). Recipient mice were euthanized 1 week after adoptive transfer for analysis. The absolute count of total and CD44+ memory CD8 T cells (CD45.1) in the BM, blood, and spleen (n = 7 per group from 2 independent experiments). (B) BM T cells from MM-bearing mice (CD45.1) were harvested and transferred to naïve B6 mice (CD45.2). B6 mice were treated with G-CSF+AMD3100 or PBS 1 week after T-cell transfer and then euthanized to measure the absolute counts of transferred T cells (CD45.1) in the BM, blood, and spleen. Donor T cells are shown as fold change compared with the number in the nonmobilized group (n = 7 per group from 2 independent experiments). (C) Female MataHari HY-specific TCR transgenic CD8 T cells (CD45.1) were transferred into male (HY+) VkMYC (Vk28158)-bearing female RAG2/IL2rg KO mice, followed by G-CSF+AMD3100 or control vehicle injection. Total number of CD45.1+ MataHari CD8 T cells is shown (n = 4 per group from 2 independent experiments). Data represents mean ± SEM. Welch t test or Mann-Whitney test for 2 samples comparison. ∗P < .05, ∗∗P < .01, ∗∗∗P < .001.
CD8 T cells from the BM egress into the PB during stem cell mobilization. (A) T cells were harvested from the BM and spleen of MM-bearing mice (CD45.1) and transferred to naïve B6 mice (CD45.2). Recipient mice were euthanized 1 week after adoptive transfer for analysis. The absolute count of total and CD44+ memory CD8 T cells (CD45.1) in the BM, blood, and spleen (n = 7 per group from 2 independent experiments). (B) BM T cells from MM-bearing mice (CD45.1) were harvested and transferred to naïve B6 mice (CD45.2). B6 mice were treated with G-CSF+AMD3100 or PBS 1 week after T-cell transfer and then euthanized to measure the absolute counts of transferred T cells (CD45.1) in the BM, blood, and spleen. Donor T cells are shown as fold change compared with the number in the nonmobilized group (n = 7 per group from 2 independent experiments). (C) Female MataHari HY-specific TCR transgenic CD8 T cells (CD45.1) were transferred into male (HY+) VkMYC (Vk28158)-bearing female RAG2/IL2rg KO mice, followed by G-CSF+AMD3100 or control vehicle injection. Total number of CD45.1+ MataHari CD8 T cells is shown (n = 4 per group from 2 independent experiments). Data represents mean ± SEM. Welch t test or Mann-Whitney test for 2 samples comparison. ∗P < .05, ∗∗P < .01, ∗∗∗P < .001.
BM Tregs are characterized by a high expression of immunosuppressive molecules and are mobilized into the PB during SCM
Because Treg proportions were significantly increased in PBSC grafts relative to the BM, we next examined whether they were also mobilized from the BM to the PB during SCM in mice. FoxP3-GFP mice were mobilized with G-CSF, AMD3100, or vehicle controls, and Tregs in the BM and blood were analyzed. The numbers of both Treg and CD4 Tcon decreased in the BM and increased in the blood after mobilization (Figure 3A). As previously shown, Tregs expressing TIGIT and KLRG-1 were uniquely observed in the BM but not in the blood of vehicle-treated mice.18,19 Neuropilin-1 (Nrp-1) is required to maintain Treg stability and function and to limit antitumor immune responses.20 We noted higher proportions of TIGIT+Nrp-1+ Tregs in the BM than in the blood in the absence of SCM (Figure 3B). Importantly, this Treg subset decreased in the BM and increased in the blood after SCM (Figure 3C). We also performed detailed unbiased clustering using FlowSOM, based on the expression levels of Treg-associated molecules (Figure 3D). The proportion and number of the TIGIT+Nrp-1+KLRG-1+ Treg subset (Pop3) was significantly higher in mobilized blood than in blood from nonmobilized mice. This population also expressed CTLA-4 and ATP-converting enzymes CD39 and CD73. Although the CD8/total Treg ratio was not different, the CD8/TIGIT+Nrp-1+KLRG-1+ Treg (Pop3) ratio was significantly lower in mobilized blood (Figure 3E). An in vitro Treg suppression assay demonstrated that Treg from mobilized spleen preferentially suppressed CD8 T-cell proliferation (supplemental Figure 4). Based on these data, unique immunosuppressive BM Treg were mobilized into PBSC grafts.
Bone marrow Tregs with an immunosuppressive phenotype are mobilized into the blood during SCM. FoxP3-GFP-DTR mice were treated with G-CSF and AMD3100 or PBS for 5 days. BM and blood were collected from the treated mice after mobilization. The number and phenotype of Tregs in the BM, blood, and spleen were assessed by flow cytometry. (A) Total number of CD8 T cells, conventional CD4 T cell, and Tregs in the BM and blood. Tregs were identified using expression of green fluorescent protein (GFP). (B) Representative FACS plots of Tregs in the BM and blood from naïve mice. The proportion of TIGIT+Nrp-1+ and TIGIT+KLRG-1+ are shown. (C) Total number of TIGIT+Nrp-1+ and TIGIT+KLRG-1+ Tregs in the BM and blood with and without mobilization. (D) Heatmap depicts the expression levels of each marker on Tregs in mobilized and nonmobilized blood. The total number of each population is shown. (E) CD8/Treg and CD8/immunosuppressive Tregs (Pop3) with or without mobilization. Data are combined from 2 independent experiments. Data represents mean ± SEM. Mann-Whitney test for 2 sample comparison. ∗P < .05, ∗∗P < .01, ∗∗∗P < .001, ∗∗∗∗P < .0001.
Bone marrow Tregs with an immunosuppressive phenotype are mobilized into the blood during SCM. FoxP3-GFP-DTR mice were treated with G-CSF and AMD3100 or PBS for 5 days. BM and blood were collected from the treated mice after mobilization. The number and phenotype of Tregs in the BM, blood, and spleen were assessed by flow cytometry. (A) Total number of CD8 T cells, conventional CD4 T cell, and Tregs in the BM and blood. Tregs were identified using expression of green fluorescent protein (GFP). (B) Representative FACS plots of Tregs in the BM and blood from naïve mice. The proportion of TIGIT+Nrp-1+ and TIGIT+KLRG-1+ are shown. (C) Total number of TIGIT+Nrp-1+ and TIGIT+KLRG-1+ Tregs in the BM and blood with and without mobilization. (D) Heatmap depicts the expression levels of each marker on Tregs in mobilized and nonmobilized blood. The total number of each population is shown. (E) CD8/Treg and CD8/immunosuppressive Tregs (Pop3) with or without mobilization. Data are combined from 2 independent experiments. Data represents mean ± SEM. Mann-Whitney test for 2 sample comparison. ∗P < .05, ∗∗P < .01, ∗∗∗P < .001, ∗∗∗∗P < .0001.
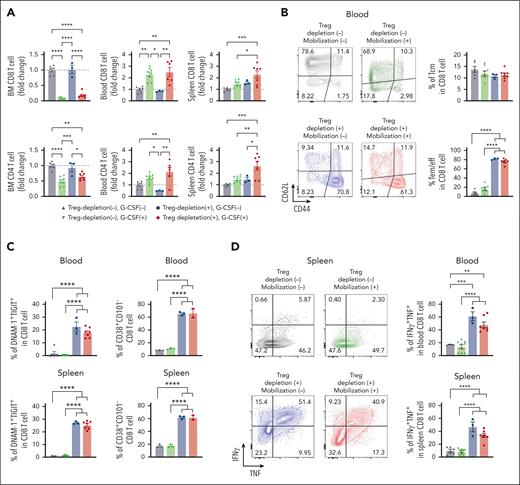
Treg depletion during SCM results in grafts containing a high number of polyfunctional CD8 T cells
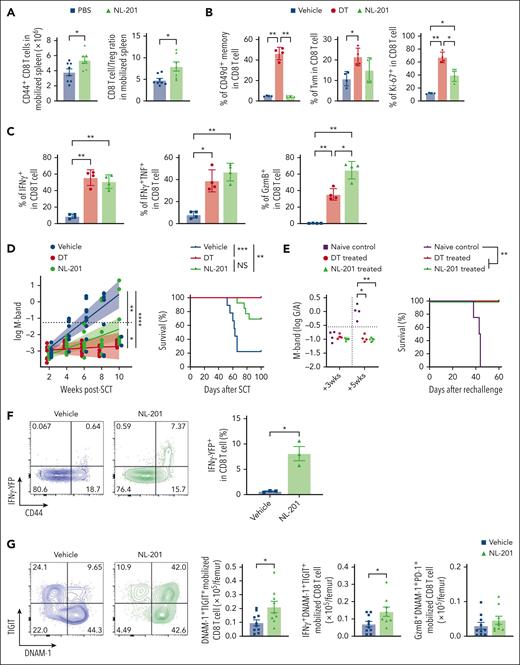
We previously showed that grafts containing myeloma-experienced memory CD8 T cells can mediate myeloma-specific immunity after ASCT.8 Although these myeloma-experienced CD8 T cells can be mobilized into PBSC grafts, this beneficial effect may be negated by the high number of concurrently mobilized highly immunosuppressive Tregs. We hypothesized that Treg depletion during SCM is highly beneficial. To test this, we depleted Tregs during SCM in FoxP3-DTR mice. We confirmed complete depletion of Treg in the BM, blood, and spleen (supplemental Figure 5A). As expected, the number of BM CD8 and CD4 T cells decreased in the BM and increased in the blood after SCM. Treg depletion itself did not impact the number of T cells in the BM or the blood (Figure 4A). In the spleen, only the Treg-depleted groups showed a significantly increased number of T cells after SCM. Treg depletion promoted CD8 T-cell differentiation, increasing effector memory CD8 T cells in the blood, an effect that was independent of SCM (Figure 4B). CD8 T cells showed increased proportions of DNAM-1+TIGIT+ and CD38+ activated CD8 T cells in the blood and spleen after Treg depletion (Figure 4C). Consistent with this, the Treg-depleted groups had higher proportions of T cells with IFNγ+TNF+ multicytokine-producing capacity (Figure 4D). To rule out the possibility that diphtheria toxin (DT)-induced cell death and associated inflammation were responsible for these observations, we repeated the same experiment using CD11c-DTR mice in which dendritic cells were depleted by DT. CD11c+ DC were efficiently depleted by the same schedule as DT administration (supplemental Figure 5B), but an increase in effector memory CD8 T cells and DNAM-1+TIGIT+ CD8 T cells was observed only in DT-treated FoxP3-DTR mice. Thus, Treg depletion itself was responsible for phenotypic and functional changes in CD8 T cells.
Treg depletion during SCM increases the total number of polyfunctional CD8 T cells in donor grafts. FoxP3-DTR mice were treated with diphtheria toxin (DT) or vehicle control every other day for 1 week. Mice in the mobilization group were treated with G-CSF and AMD3100. The mice were euthanized and T cells in the BM, blood, and spleen were harvested and analyzed by flow cytometry. (A) Fold changes in the number of CD8 and CD4 T cells. The fold change was calculated using the mean T-cell numbers from the non-Treg-depleted, nonmobilized group (grey triangles). (B) Representative FACS plots and frequency of memory CD8 T cells in the blood. (C) Percentage of activated DNAM-1+TIGIT+ and CD38+CD101− CD8 T cells in the blood and spleen. (D) Frequency of IFNγ+TNF+ CD8 T cells in the blood and spleen (n = 3-8 per group from 2 independent experiments). Grey triangle: non-Treg depleted and nonmobilized, Green triangle: non-Treg depleted and mobilized, Blue square: Treg depleted and nonmobilized, Red circle: Treg depleted and mobilized. Data represents mean ± SEM. One-way ANOVA for multiple sample comparisons. ∗P < .05, ∗∗P < .01, ∗∗∗P < .001, ∗∗∗∗P < .0001.
Treg depletion during SCM increases the total number of polyfunctional CD8 T cells in donor grafts. FoxP3-DTR mice were treated with diphtheria toxin (DT) or vehicle control every other day for 1 week. Mice in the mobilization group were treated with G-CSF and AMD3100. The mice were euthanized and T cells in the BM, blood, and spleen were harvested and analyzed by flow cytometry. (A) Fold changes in the number of CD8 and CD4 T cells. The fold change was calculated using the mean T-cell numbers from the non-Treg-depleted, nonmobilized group (grey triangles). (B) Representative FACS plots and frequency of memory CD8 T cells in the blood. (C) Percentage of activated DNAM-1+TIGIT+ and CD38+CD101− CD8 T cells in the blood and spleen. (D) Frequency of IFNγ+TNF+ CD8 T cells in the blood and spleen (n = 3-8 per group from 2 independent experiments). Grey triangle: non-Treg depleted and nonmobilized, Green triangle: non-Treg depleted and mobilized, Blue square: Treg depleted and nonmobilized, Red circle: Treg depleted and mobilized. Data represents mean ± SEM. One-way ANOVA for multiple sample comparisons. ∗P < .05, ∗∗P < .01, ∗∗∗P < .001, ∗∗∗∗P < .0001.
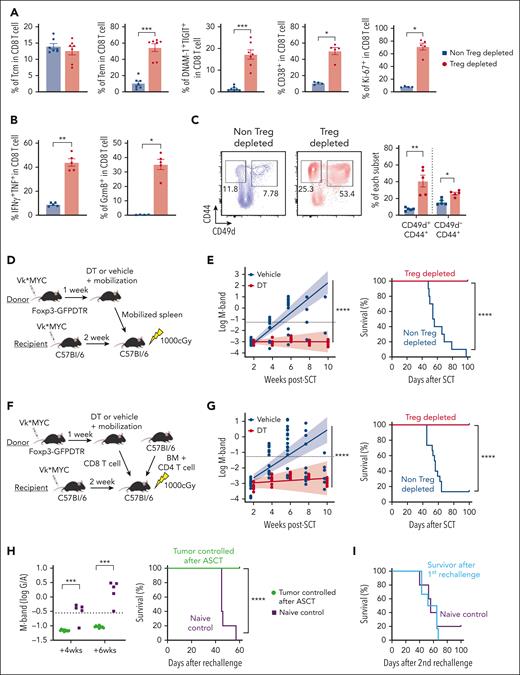
Myeloma-experienced CD8 T cells in Treg-depleted mobilized grafts generate long-term myeloma control after ASCT
We next examined whether Treg depletion during mobilization could enhance antimyeloma immunity after ASCT and contribute to long-term tumor control. CD8 T cells in Treg-depleted grafts again had more effector memory CD8 T cells with an activated and proliferative phenotype, together with a high cytokine and granzyme secreting capacity (Figure 5A-B). Memory CD8 T cells from mobilized grafts included CD49d+ antigen-experienced memory T cells and CD49d– virtual memory T cells (Tvm). Both memory subsets were increased in Treg-depleted mobilized grafts, although the former predominated (Figure 5C). Next, we transplanted unseparated Treg-depleted grafts (Figure 5D) and quantified myeloma progression by measuring M-bands (G/A ratio) and survival as previously described.7 ASCT recipients receiving Treg-depleted grafts were dramatically protected from disease progression and mortality (Figure 5E). To clarify whether this outcome depended on CD8 T cells, we repeated these transplants with grafts in which only CD8 T cells differed (ie, were from mobilized donors with or without Treg depletion) and were transplanted with identical BM and CD4 T cells from naïve donor mice (Figure 5F). CD8 T cells from Treg-depleted donors were indeed capable of mediating protection from myeloma progression after ASCT in isolation (Figure 5G). We also examined the efficacy of complete Treg depletion after ASCT, which resulted in lethal autoimmunity within 5 weeks of ASCT (supplemental Figure 6). These results confirm that the improved antimyeloma immunity derived from Treg depletion during SCM is dependent on downstream effects on CD8 T cells rather than a deficiency of Treg in the graft and after transplantation.
Myeloma-experienced CD8 T cells generated during SCM with Treg depletion mediate potent myeloma-specific immunity after ASCT. MM-bearing FoxP3-DTR donor mice were treated with DT or vehicle control during mobilization with G-CSF and AMD3100. T cells were harvested from spleens containing mobilized cells unless otherwise specified. (A-C) Phenotype and function of antigen-experienced memory (CD44+CD49d+) and virtual memory (TVM, CD44+CD49d–) CD8 T cells in spleens from mice treated with DT (Treg-depleted) or vehicle control (non-Treg-depleted) during mobilization (n = 5 per group from 2 independent experiments). (D-G) MM-bearing recipient mice were lethally irradiated and transplanted with mobilized splenocytes including 5 × 106 T cells from Treg-depleted or non-Treg-depleted donors (D-E; n = 10 per group) or 10 × 106 BM cells and 2 × 106 CD4 T cells from nonmobilized B6 donors and 2 × 106 CD8 T cells from mobilized Treg-depleted or non-Treg-depleted MM-bearing donors (F,G; n = 15 per group from 2 independent experiments). Recipient mice were monitored for survival and tumor burden using M-band (G/A ratio) level. Dotted line indicates a statistically determined threshold for myeloma relapse. (H) M-band and survival after tumor rechallenge in long-term survivors (>100 days after ASCT) who were transplanted with CD8 T cells from Treg-depleted donors. Naïve B6 mice were injected with the same tumor as a nonimmune control. (I) Mice from (H) were then rechallenged with a different clone of Vk∗MYC (Vk12598) and their survival was monitored. Data represents mean ± SEM. Mann-Whitney test for 2 sample comparison and log-rank test for survival data. ∗P < .05, ∗∗P < .01, ∗∗∗P < .001, ∗∗∗∗P < .0001.
Myeloma-experienced CD8 T cells generated during SCM with Treg depletion mediate potent myeloma-specific immunity after ASCT. MM-bearing FoxP3-DTR donor mice were treated with DT or vehicle control during mobilization with G-CSF and AMD3100. T cells were harvested from spleens containing mobilized cells unless otherwise specified. (A-C) Phenotype and function of antigen-experienced memory (CD44+CD49d+) and virtual memory (TVM, CD44+CD49d–) CD8 T cells in spleens from mice treated with DT (Treg-depleted) or vehicle control (non-Treg-depleted) during mobilization (n = 5 per group from 2 independent experiments). (D-G) MM-bearing recipient mice were lethally irradiated and transplanted with mobilized splenocytes including 5 × 106 T cells from Treg-depleted or non-Treg-depleted donors (D-E; n = 10 per group) or 10 × 106 BM cells and 2 × 106 CD4 T cells from nonmobilized B6 donors and 2 × 106 CD8 T cells from mobilized Treg-depleted or non-Treg-depleted MM-bearing donors (F,G; n = 15 per group from 2 independent experiments). Recipient mice were monitored for survival and tumor burden using M-band (G/A ratio) level. Dotted line indicates a statistically determined threshold for myeloma relapse. (H) M-band and survival after tumor rechallenge in long-term survivors (>100 days after ASCT) who were transplanted with CD8 T cells from Treg-depleted donors. Naïve B6 mice were injected with the same tumor as a nonimmune control. (I) Mice from (H) were then rechallenged with a different clone of Vk∗MYC (Vk12598) and their survival was monitored. Data represents mean ± SEM. Mann-Whitney test for 2 sample comparison and log-rank test for survival data. ∗P < .05, ∗∗P < .01, ∗∗∗P < .001, ∗∗∗∗P < .0001.
Treg-depleted stem cell grafts mediate myeloma-specific immunity
We previously demonstrated that long-term tumor control after ASCT in these systems requires tumor-specific memory CD8 T cells with the capacity for self-renewal and the generation of differentiated effector T-cell progeny.7,8,13 Importantly, the control of myeloma by CD8 T cells is myeloma clone-specific.8 To assess tumor-specific memory T-cell function after ASCT, we rechallenged long-term survivors (>100 days after ASCT, myeloma-controlled mice) with the original myeloma clone (Vk12653). Naïve nontransplanted mice that received the same myeloma served as nonimmune controls. Notably, myeloma-controlled mice were protected from myeloma, whereas naïve mice relapsed within 4 weeks and died of tumor progression within 60 days (Figure 5H). To clarify whether myeloma immunity in the mice that received transplantation was clone-specific, we injected a different myeloma clone (Vk12598) into the same mice that had rejected the original clone. In this instance, long-term survivors had no capacity to control a second unrelated myeloma clone, and myeloma progressed at the same rate as in naïve mice that did not receive transplantation (Figure 5I). Thus, Treg depletion during SCM generated highly potent myeloma-specific CD8 T-cell immunity without severe toxicity.
An IL-2/IL-15 mimetic generates protective myeloma-specific CD8 T cells during SCM
To recapitulate the effect of Treg depletion in FoxP3-DTR transgenic mice by DT, we attempted to deplete BM Treg using anti-CD25, CTLA-4, and GITR antibodies that are all described to be capable of varying degrees of activity against Treg. Although the number of BM Tregs was reduced by ∼75% by antibody treatment, activated effector memory CD8 T cells were not generated, as observed after complete Treg depletion in transgenic mice (supplemental Figure 7). Next, we focused on alternative methods to activate CD8 T cells independently of Treg. NL-201 is a synthetic IL-2/IL-15 mimetic that can bind to the beta and gamma chains of the IL-2 receptor, but not to the high-affinity IL-2R alpha (CD25)21 that Treg are critically dependent on. Thus, we treated mice with NL-201 during SCM and analyzed its effects on donor CD8 T cells.
NL-201 increased the number of memory CD8 T cells and the CD8 T-cell/Treg ratio in mobilized grafts, consistent with the selective expansion of CD8 T cells rather than Treg (Figure 6A). Although NL-201 did not expand antigen-experienced CD49d+ memory T cells (supplemental Figure 8) or virtual memory T cells, it generated activated and proliferating CD8 T cells (Figure 6B) with significantly higher expression of granzyme B and cytokines (Figure 6C), the latter being similar to that observed after stringent Treg depletion. We next studied the effect of NL-201 administration during SCM on CD8 T-cell–dependent myeloma control after ASCT. Recipient mice transplanted with CD8 T cells from NL-201 treated stem cell donors showed improved disease control and survival, which was similar to that mediated by CD8 T cells from Treg-depleted stem cell donors (Figure 6D). Myeloma rechallenge in long-term survivors after ASCT demonstrated that recipients of NL-201 treated CD8 T cells had sustained myeloma-specific immunity and were protected from disease progression (Figure 6E). We also examined the phenotype and function of NL-201 treated CD8 T cells early after ASCT to understand the effect of activation by NL-201 during SCM. We transplanted CD8 T cells from HULK reporter mice expressing IFNγ-YFP, IL-10-GFP, and FoxP3-RFP to evaluate cytokine production in recipient mice without ex-vivo stimulation. In corroboration with the restimulation data (Figure 6C), CD8 T cells expressed more IFNγ in mice mobilized with NL-201 than in vehicle-treated mice (Figure 6F). We next assessed the phenotype of BM T cells 2 weeks after ASCT at a time when tumor burdens were not different between the NL-201 and control groups (supplemental Figure 9A). BM-resident CD8 T cells were predominantly derived from mature T cells in the donor graft (supplemental Figure 9B). CD8 T cells derived from grafts mobilized in the presence of NL-201 had an increased frequency of effector memory T cells early after transplantation compared with the control group, whereas memory differentiation of BM-derived CD8 T cells was not different (supplemental Figure 9C). CD8 T cells from mobilized grafts were CD49d+ in both groups, indicative of an antigen-experienced memory phenotype (supplemental Figure 9D). Notably, CD8 T cells in recipients of NL-201-treated grafts had more DNAM-1+TIGIT+ activated CD8 T cells. The total number of activated CD8 T cells producing IFNγ in vivo was also significantly increased in recipients of NL-201-treated grafts, but Granzyme B expression was not altered (Figure 6G). Together, these data demonstrate that NL-201 treatment during stem cell mobilization expands polyfunctional CD8 T cells that express antigen-experienced markers and increased secretion of in vivo IFNγ early after transplantation. Thus, direct cytokine signaling of donor CD8 T cells, independent of Treg, during SCM could recapitulate the effect of Treg depletion and generate potent and long-lasting myeloma-specific immunity after ASCT.
An IL-2/IL-15 mimetic generated protective myeloma-specific CD8 T cells during SCM. MM-bearing B6 or FoxP3-GFP-DTR mice were treated with DT, NL-201 or vehicle control during mobilization with G-CSF and AMD3100. CD8 T cells were harvested from mobilized spleen and transplanted with BM and CD4 T cells from naïve B6 into irradiated MM-bearing recipient mice. (A) Total memory CD8 and CD8 T-cell/Treg ratio in the spleen of mice treated with NL-201 and vehicle during mobilization (n = 6-9 per group from 3 independent experiments). (B-C) Phenotype and cytokine production of antigen-experienced memory (CD44+CD49d+) and virtual memory (TVM, CD44+CD49d–) CD8 T cells in mobilized grafts (n = 4 /group from 2 independent experiments). (D) M-band and survival after ASCT (n = 9-13 per group from 2 independent experiments). (E) Mice with long-term tumor control after ASCT were rechallenged with the same tumor clone. Naïve B6 mice were injected with tumor cells as controls. Mice were monitored for tumor burden using M-band and survival (n = 4-6 per group from 1 experiment). (F-G) MM-bearing HULK donor mice (IFNγ-YFP × IL-10-GFP × FoxP3-RFP reporter) were treated with NL-201 or the control vehicle during mobilization. Transplantation was performed as above. (F) Representative FACS plots and the frequency of IFNγ-YFP+ CD8 T cells in the mobilized graft (n = 3 per group from 2 independent experiments). (G) Representative FACS plots and the total number of each subset of CD8 T cells from the mobilized graft 2 weeks after transplantation (n = 9-10 per group from 2 independent experiments). Mann-Whitney test for 2 sample comparison, One-way ANOVA for multiple samples comparison, and log-rank test for survival data. ∗P < .05, ∗∗P < .01, ∗∗∗P < .001.
An IL-2/IL-15 mimetic generated protective myeloma-specific CD8 T cells during SCM. MM-bearing B6 or FoxP3-GFP-DTR mice were treated with DT, NL-201 or vehicle control during mobilization with G-CSF and AMD3100. CD8 T cells were harvested from mobilized spleen and transplanted with BM and CD4 T cells from naïve B6 into irradiated MM-bearing recipient mice. (A) Total memory CD8 and CD8 T-cell/Treg ratio in the spleen of mice treated with NL-201 and vehicle during mobilization (n = 6-9 per group from 3 independent experiments). (B-C) Phenotype and cytokine production of antigen-experienced memory (CD44+CD49d+) and virtual memory (TVM, CD44+CD49d–) CD8 T cells in mobilized grafts (n = 4 /group from 2 independent experiments). (D) M-band and survival after ASCT (n = 9-13 per group from 2 independent experiments). (E) Mice with long-term tumor control after ASCT were rechallenged with the same tumor clone. Naïve B6 mice were injected with tumor cells as controls. Mice were monitored for tumor burden using M-band and survival (n = 4-6 per group from 1 experiment). (F-G) MM-bearing HULK donor mice (IFNγ-YFP × IL-10-GFP × FoxP3-RFP reporter) were treated with NL-201 or the control vehicle during mobilization. Transplantation was performed as above. (F) Representative FACS plots and the frequency of IFNγ-YFP+ CD8 T cells in the mobilized graft (n = 3 per group from 2 independent experiments). (G) Representative FACS plots and the total number of each subset of CD8 T cells from the mobilized graft 2 weeks after transplantation (n = 9-10 per group from 2 independent experiments). Mann-Whitney test for 2 sample comparison, One-way ANOVA for multiple samples comparison, and log-rank test for survival data. ∗P < .05, ∗∗P < .01, ∗∗∗P < .001.
Discussion
ASCT significantly prolongs PFS and may improve overall survival among patients with high-risk cytogenetic abnormalities2 but almost all patients eventually relapse. Here, we demonstrate an innovative approach to optimize antimyeloma immunity during the collection of stem cell grafts for ASCT.
Tumor-specific memory T cells play a significant role in sustaining long-term tumor control.22,23 Because there is no established tumor-specific peptide or multimer staining for these tumor clones, it is not possible to detect tumor-specific T cells directly. However, we and others have shown that BM T cells but not those in the PB, display hallmarks of T-cell exhaustion in mice and patients with progressive myeloma, which is often associated with tumor recognition.13,24 Furthermore, studies have demonstrated that CD8 T cells from the BM of patients with myeloma recognize and kill autologous myeloma cells ex vivo.25-27 Together, these studies indicate that myeloma-reactive T cells are present in the BM tumor microenvironment (TME). Although tumor-infiltrating T cells in solid tumors are often exhausted and dysfunctional in the TME,28,29 myeloma before ASCT is typically well controlled by induction chemotherapy. Indeed, in our study, most BM-resident T cells had a memory phenotype rather than an exhausted phenotype before mobilization in patients, regardless of prior treatment and cytogenetic abnormalities. BM is known to be a reservoir for memory CD8 T cells and is the preferred site for the accumulation of actively dividing memory CD8 T cells.30,31 Importantly, we confirmed that memory CD8 T cells in the marrow were mobilized into the PB by treatment with G-CSF and a CXCR4 receptor antagonist. These are important observations, as we have previously demonstrated that myeloma experienced, memory CD8 T cells in the donor graft are key mediators of tumor-specific immunity after ASCT in mice.8 The preferential migration of tumor-specific T cells into tumors is a crucial factor that underpins the success of immune cell therapy.32 Here, we show that CD8 T cells from the BM preferentially migrated back to the BM after transfer, consistent with BM homing signatures. Thus, PBSC grafts likely contain myeloma-experienced CD8 T cells that can reinfiltrate the BM after ASCT to promote myeloma immunity.
Despite the presence of myeloma-specific memory T cells in the BM, their proliferation and function are modulated by the BM microenvironment. The BM is a Treg-rich organ in which Tregs constitute 20% to 60% of CD4 T cells, a proportion that is higher than that in other peripheral organs in which it is typically 5%.15,19 Tregs are one of the main contributors to an immunosuppressive TME and high infiltration of Tregs into tumor sites results in poor survival in various types of cancers.33,34 In myeloma, Tregs are associated with disease progression35,36 and poor prognosis.36,37 In addition to suppressing effector T-cell function, Tregs are involved in the IL-2-mediated inhibition of memory CD8 T-cell division38 and induce apoptosis of memory CD8 T cells in a CD30-dependent manner.39 Furthermore, the consumption of IL-2 is an important in vivo mechanism by which Tregs control CD8 T-cell expansion and effector differentiation.40 BM Tregs are particularly suppressive and have a memory phenotype with higher expression of immune inhibitory molecules and IL-10 production.41,42 Recent studies have indicated that BM Tregs express Nrp-1, which is required to maintain Treg stability and to limit antitumor immunity.18,20,43 Our data demonstrated that G-CSF expanded TIGIT+ CD39+ activated Treg in human PBSC grafts. We also confirmed that G-CSF mobilization increased the number of BM-derived Treg expressing high levels of immunosuppressive molecules in mouse blood, including the Nrp-1+ population. Given this highly active Treg network in the BM, local myeloma-specific CD8 T cells are likely to be suppressed in the marrow during SCM. In line with this, Treg depletion during SCM promoted effector differentiation and activation of CD8 T cells with polyfunctionality in the mobilized graft. These activated polyfunctional CD8 T cells generated potent myeloma-specific immunity after ASCT. Thus, Treg depletion during SCM is a novel approach to maximize antimyeloma immunity after ASCT. We noted an inverse correlation between the proportions of TIGIT+CD39+ Treg in the stem cell graft and time to progressive disease after ASCT in patients at high cytogenetic risk. It is tempting to speculate that removing Treg from stem cell grafts ex vivo after collection may be an alternative approach but several lines of evidence suggest that this is likely to be less efficacious. Firstly, recipient Tregs are relatively chemoradiotherapy resistant and preferentially expand after ASCT in the absence of donor Tregs.44 Second, the profound lymphodepletion present after ASCT is permissive to the generation of induced Tregs from conventional donor T cells.45,46 Finally, our data suggest that the critical step is the expansion of activated BM-derived and myeloma-specific memory T cells during SCM rather than after transplantation. In these transplant systems, the depletion of all Tregs after transplantation resulted in fatal autoimmunity. Thus, the specific and transient depletion of only donor Tregs during SCM is likely the optimal strategy peritransplant, thereby balancing antitumor efficacy and autoimmunity.
In our study, we used a DT-driven Treg depletion model to obtain highly activated polyfunctional CD8 T cells in the mobilized graft; however, this approach is not clinically translatable. Antibody-mediated depletion of Tregs using anti-CD25 or anti-CTLA-4 is a common clinical approach;47-49 however, this does not result in robust Treg depletion. In addition, these markers are expressed on activated T cells and the potential depletion of these cells is likely counterproductive to tumor immunity. Indeed, antibody-mediated Treg depletion only partially depleted BM Tregs in our system and was insufficient to activate and differentiate CD8 T cells during SCM. Thus, we focused on a synthetic CD122-biased, IL-2/IL-15 mimetic to directly expand effector T cells, as competition for IL-2 consumption between Tregs and effector T cells is a key mechanism of Treg-mediated immunosuppression in vivo.40 NL-201 acts as an agonist for IL-2 and IL-15 receptors, which share beta and gamma subunits,21 and expands proliferating polyfunctional CD8 T cells, including virtual memory T cells, but not Tregs. CD8 T cells from mice treated with NL-201 during SCM had a less differentiated phenotype than those from Treg-depleted mice and lacked CD49d expression. NL-201 has the capacity to activate antigen-specific CD8 T cells and antigen-nonspecific innate-like virtual memory CD8 T cells, consistent with previous literature using IL-15 mimetics.50-52 The latter bystander-activated CD8 T cells have been described to mediate immunity in the absence of TCR engagement, typically via the NKG2D pathway.50-52 Myeloma rechallenge experiments in our study confirmed that the protective immunity generated in the context of NL-201 was completely antigen-dependent. Given this, myeloma-specific CD8 T cells rather than bystander CD8 innate-like virtual memory T cells are the predominant cell population mediating myeloma immunity after NL-201 treatment during stem cell mobilization. Recent studies exploring optimal CAR T-cell manufacturing conditions demonstrated that IL-15 signaling preserved T cells in a less differentiated, stem cell-like phenotype and improved in vivo antitumor activity and long-term persistence.53,54 Therefore, NL-201 expanded the ideal CD8 T-cell population for adoptive T-cell therapies during SCM.
IL-2 promotes potent antitumor activity against various cancers; however, considerable toxicity is an obstacle to the clinical success of the native cytokine in cancer treatment.55,56 Immunological mechanisms underpinning this toxicity include vascular leakage caused by proinflammatory cytokine production by activated NK cells57 and pulmonary edema caused by the direct binding of IL-2 to CD25+ endothelial cells in the lung.58 Our short-term use of NL-201 during SCM and the lack of CD25-binding by NL-201 may overcome these obstacles. Additional CD122-biased IL-2 formulations are being actively developed and several clinical trials for solid tumors are ongoing.59,60 Thus, the peritransplant use of CD122-biased IL-2 mimetics represents a promising tractable approach to improve patient outcomes after ASCT for myeloma.
In conclusion, SCM caused egress of BM residing, myeloma-experienced T cells into PBSC grafts. However, CD8 T-cell responses were constrained by concurrently mobilized BM Tregs. Treg depletion during SCM mitigated this effect and resulted in the expansion of polyfunctional CD8 T cells, which generated profound myeloma control after ASCT. Direct and selective cytokine signaling of CD8 T cells using an IL-2/IL-15 mimetic could recapitulate this effect and represent a clinically testable strategy to improve responses after ASCT.
Acknowledgments
Neoleukin therapeutics provided the NL-201 used in this study. The visual abstract was created using BioRender.com.
This work was supported by a research grant from the National Institutes of Health (NIH), National Cancer Institute (U01 CA244291).
The content is solely the responsibility of the authors and does not necessarily represent the official views of the NIH.
Authorship
Contribution: S.T. and S.A.M. designed and performed the experiments, analyzed the data, and wrote the manuscript; K.S.E., C.R.S., T.S., S.R.W.L., S.D.O., and A.D.C performed experiments; A.V., S.K., M.L.C., and D.J.G. provided and coordinated the clinical samples; A.V. supervised experiments conducted in Brisbane, Australia; P.Z. and M.K. edited and wrote the manuscript; G.R.H. supervised the study and wrote the manuscript; and all the authors approved the final manuscript.
Conflict-of-interest disclosure: D.J.G. has received research funding from Bristol Myers Squibb, Cellectar Biosciences, Janssen Biotech, Juno Therapeutics, Seattle Genetics, and Springworks Therapeutics; consulted for Legend Biotech; is a member of the board or advisory committee for Bristol Myers Squibb, GSK, Janssen Biotech, Neoleukin Therapeutics, and Seattle Genetics; and has patents for Juno therapeutics. G.R.H. has consulted for Generon Corporation, NapaJen Pharma, iTeos Therapeutics, Neoleukin Therapeutics, Commonwealth Serum Laboratories, and Cynata Therapeutics and has received research funding from Compass Therapeutics, Syndax Pharmaceuticals, Applied Molecular Transport, Serplus Technology, Heat Biologics, Laevoroc Oncology, iTEOS therapeutics, and Genentech. The remaining authors declare no competing financial interests.
Correspondence: Geoffrey R. Hill, Fred Hutchinson Cancer Center, 1100 Fairview Ave N, Seattle, WA 98109; email: grhill@fredhutch.org.
References
Author notes
For original data, please contact the corresponding author, Geoffrey R. Hill (grhill@fredhutch.org)
The online version of this article contains a data supplement.
There is a Blood Commentary on this article in this issue.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.



This feature is available to Subscribers Only
Sign In or Create an Account Close Modal