Mutational screening after transplantation identifies rare relapse-initiating stem cells while patients remain in complete remission.
Mutational screening of HSPCs after stem cell transplantation enhances MRD sensitivity ∼100-fold.
Visual Abstract
Relapse after complete remission (CR) remains the main cause of mortality after allogeneic stem cell transplantation for hematological malignancies and, therefore, improved biomarkers for early prediction of relapse remains a critical goal toward development and assessment of preemptive relapse treatment. Because the significance of cancer stem cells as a source of relapses remains unclear, we investigated whether mutational screening for persistence of rare cancer stem cells would enhance measurable residual disease (MRD) and early relapse prediction after transplantation. In a retrospective study of patients who relapsed and patients who achieved continuous-CR with myelodysplastic syndromes and related myeloid malignancies, combined flow cytometric cell sorting and mutational screening for persistence of rare relapse-initiating stem cells was performed in the bone marrow at multiple CR time points after transplantation. In 25 CR samples from 15 patients that later relapsed, only 9 samples were MRD-positive in mononuclear cells (MNCs) whereas flowcytometric-sorted hematopoietic stem and progenitor cells (HSPCs) were MRD-positive in all samples, and always with a higher variant allele frequency than in MNCs (mean, 97-fold). MRD-positivity in HSPCs preceded MNCs in multiple sequential samples, in some cases preceding relapse by >2 years. In contrast, in 13 patients in long-term continuous-CR, HSPCs remained MRD-negative. Enhanced MRD sensitivity was also observed in total CD34+ cells, but HSPCs were always more clonally involved (mean, 8-fold). In conclusion, identification of relapse-initiating cancer stem cells and mutational MRD screening for their persistence consistently enhances MRD sensitivity and earlier prediction of relapse after allogeneic stem cell transplantation.
Introduction
A common feature of most cancers is that they respond well to cytotoxic treatments and go into complete remission (CR) but nevertheless frequently relapse, often years later. Myelodysplastic syndromes (MDS) and related myeloid malignancies like myelodysplastic/myeloproliferative neoplasms (MDS/MPN) and acute myeloid leukemia with myelodysplasia-related changes (AML-MRC) have an overall dismal prognosis,1,2 and for most patients allogeneic hematopoietic stem cell transplantation (allo-HSCT) remains the only curative treatment,3 although >30% of patients relapse.4 Risk factors for relapse include somatic genomic alterations,5,6 but most patients go into CR after transplantation. Although most relapses occur within a year, late relapses are not rare, with some occurring >10 years after transplantation,7 compatible with rare cancer stem cells escaping pretransplantation conditioning and posttransplantation graft-versus-leukemia effect. However, the identity of the relapse-initiating cells after transplantation remains to be established. Preemptive therapeutic intervention against an impending relapse (while tumor burden remains very low) is likely to improve outcome compared with treatment of overt clinical relapse.8-10 However, to explore this, it is critical to establish more sensitive and specific biomarkers for measurable residual disease (MRD) and early prediction of relapse after allo-HSCT.11,12 Platforms used to assess MRD in MDS and AML include sequencing-based tracking of recurrent mutations and flow cytometry (FCM) tracking of aberrant cell surface phenotypes11-14 but in particular FCM platforms have not been extensively validated in MDS after allo-HSCT.
MDS and related malignancies are propagated by recurrent somatic oncogenic mutations.15-17 Because progression to AML18,19 and relapse after allo-HSCT12,20 is predominantly derived from clones sustaining the same founder mutations,21 targeted screening for recurrent mutations identified at diagnosis could facilitate earlier detection of emerging relapse after allo-HSCT. A retrospective study demonstrated higher risk for disease progression upon detection of oncogenic mutations in the bone marrow (BM) after allo-HSCT.22 However, 20% of mutation-negative cases later progressed, and 30% of mutation-positive cases did not experience subsequent disease progression.22 Other MDS/AML MRD studies assessing post–allo-HSCT persistence of recurrent driver mutations had similar sensitivity and specificity limitations regarding predicting relapse after allo-HSCT,11,23-26 highlighting the need for the development of MRD screening platforms with higher sensitivity and relapse predictive value.
Much less focus has been directed at enhancing MRD sensitivity and specificity of relapse prediction by mutational screening of cell sources other than whole BM or blood, which are currently routinely used for MRD assessment. Recent studies have shown that MRD sensitivity can significantly be enhanced by analysis of total CD34+ cells.23 However, although MDS stem cells are CD34+, they often represent a minor fraction of total CD34+ cells.27,28 If maintenance of MDS clones are strictly dependent on identified rare MDS stem cells,27,28 they must, in cases of relapse, escape immune-surveillance after allo-HSCT, similar to normal HSCs capable of crossing allogeneic immune barriers.29 However, whether MDS stem/progenitor cells selectively escape conditioning and immune targeting after allo-HSCT, and, if so, could enhance MRD detection and potentially also relapse prediction, remains to be investigated. Identification of the relapse-initiating cells would also facilitate identification of novel therapeutic targets, specifically targeting the rare cells responsible for relapse after transplantation. Herein, mutational screening of FCM-purified hematopoietic stem and progenitor cells (HSPCs) was compared with the current standard using unfractionated mononuclear cells (MNCs), with the goal of identifying the relapse-initiating cells, enhancing MRD sensitivity and improving early prediction of relapse.
Methods
An overview of the study design is outlined in supplemental Figure 1 (available on the Blood website).
Patients
Patients with MDS, MDS/MPN, or AML-MRC with a <30% BM blast diagnosis according to World Health Organization 2016 classifications who had undergone allo-HSCT at the Karolinska University Hospital were included in the study. Samples were collected after informed consent and analyzed in accordance with ethical approval and the Declaration of Helsinki. Clinical information; treatment; and genetic features at diagnosis, disease progression, and after allo-HSCT are shown in supplemental Tables 1 and 2 and summarized in supplemental Table 3. Patients were grouped according to relapses (n = 16; patients 1-16) and continuous-CR without signs of recurring MDS or AML ≥66 months after allo-HSCT (n = 13; patients 17-29). As expected, relapse cases were enriched in patients with poor prognosis TP53 and RAS pathway mutations, complex karyotype, and −7/del(7q) (supplemental Table 3). For further information see supplemental Methods.
Identification of recurrent somatic mutations by targeted DNA sequencing
Candidate somatic mutations in genes recurrently mutated in MDS and related myeloid malignancies including AML15-19 were identified by targeted DNA sequencing of genomic DNA from BM MNCs (supplemental Table 4). At least 1 genetic lesion was identified at diagnosis as well as at relapse for each patient except for patients 1 and 17, and therefore whole-genome sequencing (WGS) was used to identify somatic clonal mutations to enable clonal tracking in remission BM samples for these 2 patients (see supplemental Methods for details). All candidate mutations detected were further validated, and variant allele frequency (VAF) quantified by digital droplet polymerase chain reaction (ddPCR) analysis of DNA from diagnosis and relapse, and of diagnostic T cells (isolated by CD3+ magnetic beads [Miltenyi] or fluorescence-activated cell sorting [FACS] of CD15/16/33−CD19−CD56−CD235a−CD3+CD8+ cells). Mutations used for further ddPCR MRD analysis were present in diagnostic and relapse BM MNCs at a VAF >5% higher than those detected by ddPCR in T cells (<2% VAF). For further details, see supplemental Methods.
Whole-genome DNA amplification
Whole-genome DNA amplification was performed using the REPLI-g single-cell kit (Qiagen) according to the manufacturer’s instructions. For further details, see supplemental Methods.
ddPCR
ddPCR with mutation-specific probes (supplemental Table 5) were prepared as described in supplemental Methods. Plates were read on a QX200 droplet reader (Bio-Rad), and results analyzed using QuantaSoft version 1.5.38.1118 software (Bio-Rad) generating VAFs as the fractional abundance (including upper and lower 95% confidential intervals) of the mutated allele based on Poisson distribution. The number of events in the different channels (Ch1, FAM; Ch2, HEX) are shown in the source data files 1 to 4 for the different ddPCR experiments and are further explained in supplemental Methods. We included a minimum of 1 mutated DNA control sample, 1 wild-type DNA healthy sample, and 1 no-template control (water) in every run to reliably define the gating strategy for each experiment as described.30 In our study, the limit of detection (LOD) for reliable detection of different mutations were, in part, set based on validation experiments for each specific primer-probe assay, establishing the specificity and LOD for each probe (supplemental Table 6), and, in part, on the number of cells analyzed, as described in supplemental Methods.
FCM and FACS
BM MNCs and CD34+ cells were prepared for FCM analysis and sorting (supplemental Table 7), as previously described31,32 using panel settings shown in supplemental Table 7A. Live cells were identified by DAPI (4',6-diamidino-2-phenylindole; Invitrogen) or 7-aminoactinomycin D (Sigma-Aldrich) exclusion. MRD analysis by FCM was based on recommendations from the European LeukemiaNet (ELN)13,33 and previously applied FCM-based panels,34 using 3 separate panels (supplemental Table 7A-C). Because the ELN specifies that there is no separate recommended panel for monitoring MRD in MDS,33,35 and most patients with MDS who receive transplantation have advanced disease and frequently progress or relapse as AML, we used protocols recommended for advanced MDS and AML.36 For more details, including on specific panels used for FCM-based MRD analysis, see supplemental Methods.
Separation of CD34+ cells
CD34+ cells were enriched from BM MNCs using magnetic beads conjugated to anti-human CD34 antibody (Miltenyi), resulting in 27% to 71% (median 54%) CD34 purity. VAFs in total CD34+-enriched cells were predicted as shown in supplemental Table 8. Samples subjected to FACS after CD34 enrichment were adjusted for purity obtained by CD34 immunomagnetic enrichment. In each case, the CD34+ VAF prediction was based on the non-DTA (DNMT3A, TET2, or ASXL1) recurrent mutation with the highest estimate CD34+ VAF used.
Single nucleotide polymorphism (SNP)-based MRD analysis
MRD was also assessed by ddPCR using patient-specific SNPs. SNPs were selected from targeted-capture sequencing data after performing mutation calling by EBCall37 and annotation by ANNOVAR. ddPCR probes and primers for selected (see supplemental Methods) SNPs in 6 patients who achieved continuous-CR (patients 18, 21, and 23-26) and 4 patients who relapsed (patients 5-8) were designed using Primer3Plus according to the Droplet Digital PCR Application Guide (Bio-Rad, supplemental Table 9).
Statistical analysis
Statistical analyses were conducted using R version 4.0.3 and GraphPad 7-9. Significances of differences and fold change between 2 groups were calculated by the paired Wilcoxon signed rank test and the binomial test with probability of success 0.5 using R, respectively, unless otherwise specified. A binomial test was performed to test whether the HSPC fraction with the largest frequency in pretransplantation samples relative to the mean frequency in normal age-matched controls showed the largest VAF in the earliest remission time point after transplantation with the probability of success of 1 in 6 (1 among 6 investigated HSPC fractions). Pearson correlation analysis was performed using GraphPad. For all reported P values using 2-sided tests, the significance level was set at .05. Allo-HSCT VAFs between continuous-CR and relapse groups were compared using the unpaired Wilcoxon signed rank by GraphPad.
Results
Clonal involvement of rare HSPCs precedes other evidence of impending relapse after allo-HSCT
We investigated 29 patients (MDS, n = 20; MDS/MPN, n = 5; and AML-MRC, n = 4; patients 1-29) that had obtained CR after allo-HSCT. In total, 16 patients (patients 1-16) experienced relapse between 4 and 33 months after transplantation, and 13 patients (patients 17-29) remained in continuous-CR ≥66 months after transplantation (supplemental Tables 1-3). In all but 1 relapse case (patient 1), 1 or multiple recurrent oncogenic mutations15,16 were identified in diagnostic BM MNCs (Figure 1A; supplemental Table 10) and confirmed by ddPCR (mean LOD ± standard error of mean [SEM]), with VAF for all investigated mutations of 0.09% (±0.01%) based on analysis of normal controls; supplemental Table 6. Clonally dominating diagnostic mutations also dominated at relapse, except for patient 1 for whom the dominating diagnostic clone was minor at relapse, in which case we tracked clonal mutations identified by WGS at diagnosis and relapse (supplemental Figures 2 and 3; supplemental Table 10). Although a previous and larger study found a small but significant increase in pretransplantation VAFs in patients who relapsed compared with those without disease progression,22 VAF before allo-HSCT was not significantly different between relapse and continuous-CR cohorts in this study (Figure 1B-C). In all patients, mutations were highly present in all FCM-purified HSPC populations28,38 before transplantation and at relapse (supplemental Figure 3, source data file 1).
Recurrent genetic lesions in patients who received allo-HSCT. (A) Mutational map of recurrent oncogenic mutations and truncating changes used for clonal tracking as well as chromosomal abnormalities (see “Methods” and supplemental Tables 1 and 10) identified before transplantation and/or at relapse in patients with MDS and related malignancies undergoing allo-HSCT. Hatched boxes indicate new genetic lesions identified at relapse and “X” indicates patients with biallelic TP53 mutations before transplantation based on either >60% VAF or loss of chromosome 17 at diagnosis (see supplemental Table 1 and source data file 1). “Months after Allo-HSCT” reflects the time of clinical relapse for relapse group and last observation time point for continuous-CR group. (B) ddPCR data in BM MNCs for the mutation within each patient with the highest VAF (%) at indicated days before allo-HSCT (last available BM sample before conditioning for allo-HSCT) in patients who relapsed (n = 14; all patients with available BM MNCs) 4 to 33 months after allo-HSCT compared with patients who remained in continuous CR (n = 10; all patients with available BM MNCs) for ≥66 months after allo-HSCT. (C) Violin plot of VAF for mutations shown in panel B in BM MNCs for all patients in continuous-CR and patients who relapsed before allo-HSCT. Dashed lines indicate the median and dotted lines indicate the quartiles. No statistical difference (P = .98, the Mann Whitney U test) was found between relapsed and continuous-CR groups. Pre–allo-HSCT samples correspond to pre–allo-HSCT samples in raw data source data file 1. CMML, chronic myelomonocytic leukemia; Del(5q), MDS with isolated del(5q); MDS-EB, MDS with excess blasts; MPN-u, myeloproliferative neoplasm unclassifiable; MDS-MLD, MDS with multilineage dysplasia; MDS-RS-MLD, MDS with multilineage dysplasia and ring sideroblasts; WHO, World Health Organization 2016 classification.
Recurrent genetic lesions in patients who received allo-HSCT. (A) Mutational map of recurrent oncogenic mutations and truncating changes used for clonal tracking as well as chromosomal abnormalities (see “Methods” and supplemental Tables 1 and 10) identified before transplantation and/or at relapse in patients with MDS and related malignancies undergoing allo-HSCT. Hatched boxes indicate new genetic lesions identified at relapse and “X” indicates patients with biallelic TP53 mutations before transplantation based on either >60% VAF or loss of chromosome 17 at diagnosis (see supplemental Table 1 and source data file 1). “Months after Allo-HSCT” reflects the time of clinical relapse for relapse group and last observation time point for continuous-CR group. (B) ddPCR data in BM MNCs for the mutation within each patient with the highest VAF (%) at indicated days before allo-HSCT (last available BM sample before conditioning for allo-HSCT) in patients who relapsed (n = 14; all patients with available BM MNCs) 4 to 33 months after allo-HSCT compared with patients who remained in continuous CR (n = 10; all patients with available BM MNCs) for ≥66 months after allo-HSCT. (C) Violin plot of VAF for mutations shown in panel B in BM MNCs for all patients in continuous-CR and patients who relapsed before allo-HSCT. Dashed lines indicate the median and dotted lines indicate the quartiles. No statistical difference (P = .98, the Mann Whitney U test) was found between relapsed and continuous-CR groups. Pre–allo-HSCT samples correspond to pre–allo-HSCT samples in raw data source data file 1. CMML, chronic myelomonocytic leukemia; Del(5q), MDS with isolated del(5q); MDS-EB, MDS with excess blasts; MPN-u, myeloproliferative neoplasm unclassifiable; MDS-MLD, MDS with multilineage dysplasia; MDS-RS-MLD, MDS with multilineage dysplasia and ring sideroblasts; WHO, World Health Organization 2016 classification.
In BM CR samples, MNCs were mutational MRD-positive in 9 of 16 patients who relapsed, preceding clinical relapse diagnosis by a mean of 5 months (range, 2-11 months; supplemental Figure 4A, source data file 1), whereas 7 patients were consistently MRD-negative, in multiple (2-4) consecutive BM remission samples (supplemental Figure 4B, source data file 1), in agreement with previous studies.22
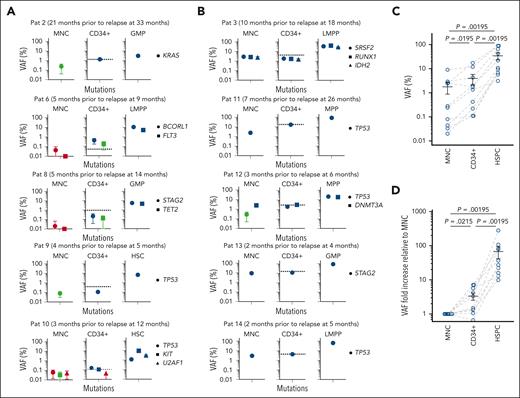
We hypothesized that relapses are initiated by rare therapy-resistant HSPCs,27,28,31 which we therefore purified (Figure 2A-B; supplemental Figure 3) from the same remission MNC samples. In 1 case (patient 16), the latest available remission sample was 11 months before relapse, at which time neither MNCs nor HSPCs were MRD-positive (source data file 1). In all remaining 15 patients MRD-positivity in HSPCs preceded diagnosis of relapse (Figure 2C-D; supplemental Figure 5A, source data file 1) by a mean of 10 months (P = .00501 compared with MNCs; Figure 3A), and in some cases by >2 years. Moreover, in all patients, clonal involvement was higher in HSPCs than in MNCs. In 10 patients (patients 1-10), MRD-positive HSPCs preceded MRD-positive MNCs and clinical relapse by a mean of 10 and 13 months, respectively (Figure 2C, source data file 1). In 5 additional patients (patients 11-15), MNCs were also MRD-positive in the first CR sample in which HSPCs were MRD-positive; but, in these CR samples, VAFs of the most clonally involved HSPCs were much higher than for MNCs (mean, 32-fold; Figure 2D; source data file 1). In total, mutational MRD was compared between MNCs and HSPCs in 25 remission samples from 15 patients in which MRD positivity was confirmed in either MNCs and/or HSPCs. None of these were MRD-negative in HSPCs whereas 17 samples were MRD-negative in MNCs, and in all 25 samples VAF was higher (mean, 97-fold) in HSPCs than in MNCs (P = 5.96 × 10−8; Figure 3B, source data file 1). In 8 remission samples confidently MRD-positive in both HSPCs and MNCs, VAF was 10- to 78-fold (mean, 30-fold) higher (P = .008; the Wilcoxon paired test) in HSPCs. In several patients who relapsed (patients 1-2, Patients 4-7) for whom multiple consecutive remission samples were MRD-negative in MNCs, HSPCs were MRD-positive, preceding relapse by 7-27 months (Figure 3C, source data file 1). Rather than presence or absence of poor prognosis genomic alterations, the timing of relapse was decisive for how much earlier after allo-HSCT HSPCs were MRD-positive before clinical detection of relapse (supplemental Figure 5B, source data file 1).
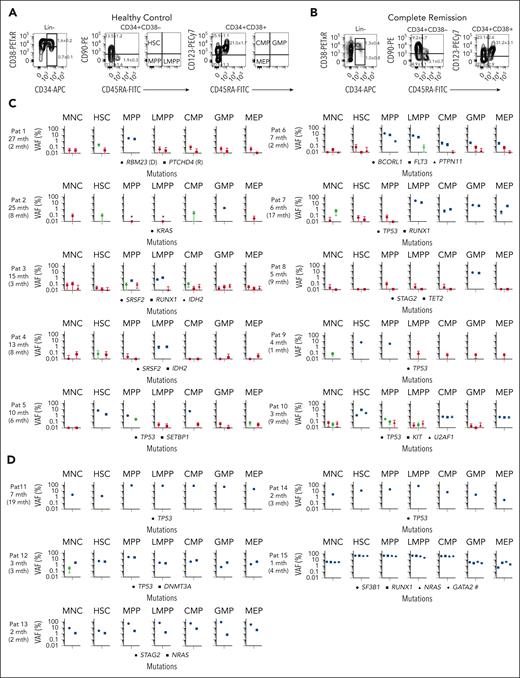
Clonal involvement of stem and progenitor cell compartments in patients with MDS in CR after allo-HSCT. (A-B) Representative FACS profiles of BM cells from an aged-matched healthy control (A) and from a patient during CR who had later relapsed (B; patient 8). Numbers in left panels indicate mean (± SEM) percentages of BM MNCs; in the second and third columns, percentages of total CD34+ cells for 12 age-matched normal controls (A) and 15 patients who relapsed (B). Also indicated are the quadrants from which investigated HSPC populations were sorted for mutational MRD analysis (for individual FACS profiles at diagnosis, CR, and relapse for all patients who experienced relapse, see supplemental Figure 3). (C-D) ddPCR screening for patient-specific mutations identified in BM MNCs before transplantation and still present at relapse was used to assess clonal involvement in remission BM MNCs and purified HSPCs. For each patient the number of months (mth) before relapse are shown, and in parenthesis months after transplantation. (C) Ten patients in whom MRD-positivity of HSPCs preceded MRD-positivity of BM MNCs and (D) 5 additional patients in whom MRD-positivity of distinct HSPCs was higher than MRD-positivity of BM MNC. For all patients (C-D), with the exception of patient 7, the earliest time point after allo-HSCT at which MNCs and/or HSPCs were found to be confidently MRD-positive, is shown. For patient 1, in whom the dominating diagnostic and relapse clones were mutually exclusive (see supplemental Figure 2), data are shown for the earliest time point after allo-HSCT when both the dominant diagnostic (D) and dominant relapse (R) mutation was detected in HSPCs. Error bars represent the 95% confidence interval of VAFs calculated according to Poisson distribution, as further specified in “Methods.” Blue color indicates highly confident clonal involvement, red indicates negative, and green indicates inconclusive data based on the LOD established in normal BM samples for each ddPCR probe (supplemental Table 6) as well as the number of cells analyzed as described in supplemental Methods. ∗Analysis negative for clonal involvement (red) but based on analysis of <50 purified cells. Hashtag (#): mutation of “unknown” significance as specified in supplemental Methods. Raw data including cell numbers analyzed can be found in source data file 1. HSC, hematopoietic stem cell (LIN−CD34+CD38low/−CD90+CD45RA−); MPP, multipotent progenitor (LIN−CD34+CD38low/−CD90−CD45RA−); LMPP, lymphoid-primed MPP (LIN−CD34+CD38low/−CD90−CD45RA+); CMP, common myeloid progenitor (LIN−CD34+CD38+CD90−CD123+CD45RA−); GMP, granulocyte-monocyte progenitor (LIN−CD34+CD38+CD90−CD123+CD45RA+); and MEP, megakaryocyte-erythroid progenitor (LIN−CD34+CD38+CD90−CD123−CD45RA−).
Clonal involvement of stem and progenitor cell compartments in patients with MDS in CR after allo-HSCT. (A-B) Representative FACS profiles of BM cells from an aged-matched healthy control (A) and from a patient during CR who had later relapsed (B; patient 8). Numbers in left panels indicate mean (± SEM) percentages of BM MNCs; in the second and third columns, percentages of total CD34+ cells for 12 age-matched normal controls (A) and 15 patients who relapsed (B). Also indicated are the quadrants from which investigated HSPC populations were sorted for mutational MRD analysis (for individual FACS profiles at diagnosis, CR, and relapse for all patients who experienced relapse, see supplemental Figure 3). (C-D) ddPCR screening for patient-specific mutations identified in BM MNCs before transplantation and still present at relapse was used to assess clonal involvement in remission BM MNCs and purified HSPCs. For each patient the number of months (mth) before relapse are shown, and in parenthesis months after transplantation. (C) Ten patients in whom MRD-positivity of HSPCs preceded MRD-positivity of BM MNCs and (D) 5 additional patients in whom MRD-positivity of distinct HSPCs was higher than MRD-positivity of BM MNC. For all patients (C-D), with the exception of patient 7, the earliest time point after allo-HSCT at which MNCs and/or HSPCs were found to be confidently MRD-positive, is shown. For patient 1, in whom the dominating diagnostic and relapse clones were mutually exclusive (see supplemental Figure 2), data are shown for the earliest time point after allo-HSCT when both the dominant diagnostic (D) and dominant relapse (R) mutation was detected in HSPCs. Error bars represent the 95% confidence interval of VAFs calculated according to Poisson distribution, as further specified in “Methods.” Blue color indicates highly confident clonal involvement, red indicates negative, and green indicates inconclusive data based on the LOD established in normal BM samples for each ddPCR probe (supplemental Table 6) as well as the number of cells analyzed as described in supplemental Methods. ∗Analysis negative for clonal involvement (red) but based on analysis of <50 purified cells. Hashtag (#): mutation of “unknown” significance as specified in supplemental Methods. Raw data including cell numbers analyzed can be found in source data file 1. HSC, hematopoietic stem cell (LIN−CD34+CD38low/−CD90+CD45RA−); MPP, multipotent progenitor (LIN−CD34+CD38low/−CD90−CD45RA−); LMPP, lymphoid-primed MPP (LIN−CD34+CD38low/−CD90−CD45RA+); CMP, common myeloid progenitor (LIN−CD34+CD38+CD90−CD123+CD45RA−); GMP, granulocyte-monocyte progenitor (LIN−CD34+CD38+CD90−CD123+CD45RA+); and MEP, megakaryocyte-erythroid progenitor (LIN−CD34+CD38+CD90−CD123−CD45RA−).
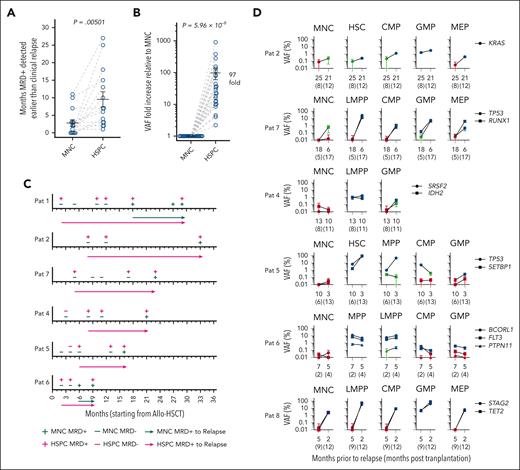
Early prediction of relapse through assessment of clonal involvement of distinct stem and progenitor cells. (A) Number of months by which mutational MRD-positivity in BM MNCs and HSPCs preceded diagnosis of relapse. Individual values (circles) for all patients in relapse and mean (± SEM) are shown. The paired MNC and HSPC sample for each patient are connected by gray dashed lines. P value; the Wilcoxon paired test. (B) Fold changes in %VAF in HSPCs relative to BM MNCs in patients who later relapsed, for all remission BM samples in which MNCs and/or HSPCs were MRD-positive (15 patients, 25 remission time points indicated by blue circles). VAF of mutations with the highest VAF within the highest clonally involved HSPC population was compared with the VAF of MNCs in the same BM remission sample. For mutations in which MNC VAFs were below the LOD, the LOD value was used instead of the actual VAF. The paired MNC and HSPC samples for each patient are connected by gray dashed lines. Mean (± SEM) values are also shown. P value; a binomial test. (C) Kinetic assessment of MRD in purified HSPCs after allo-HSCT, as assessed by ddPCR analysis for patient-specific mutations in patients in whom MRD positivity of HSPCs preceded MRD positivity of BM MNCs for at least 2 consecutive remission time points. Arrowheads indicate time of diagnosis of clinical relapse from the time of transplantation (0), and the length of the arrows indicates months from detection of molecular MRD to time of clinical diagnosis of relapse. MNC MRD+; at least 1 mutation confidently detected in BM MNCs by ddPCR; MNC MRD−, no mutation confidently detected in BM MNCs by ddPCR; HSPC MRD+, at least 1 mutation confidently detected in ≥1 stem and progenitor cell populations by ddPCR; HSPC MRD−, no mutation confidently detected by ddPCR in any investigated stem and progenitor cell populations (see “Methods” and supplemental Methods). (D) ddPCR-based VAFs for patient-specific mutations in BM MNCs and purified HSPCs in consecutive remission samples for patients who later relapsed. Error bars represent the 95% confidence interval of VAFs calculated according to Poisson distribution as further specified in “Methods.” Blue color indicates highly confident clonal involvement, red indicates negative, and green inconclusive, as described in supplemental Methods. Only HSPC populations with highly confident clonal involvement for at least 1 time point are shown. Raw data can be found in source data file 1.
Early prediction of relapse through assessment of clonal involvement of distinct stem and progenitor cells. (A) Number of months by which mutational MRD-positivity in BM MNCs and HSPCs preceded diagnosis of relapse. Individual values (circles) for all patients in relapse and mean (± SEM) are shown. The paired MNC and HSPC sample for each patient are connected by gray dashed lines. P value; the Wilcoxon paired test. (B) Fold changes in %VAF in HSPCs relative to BM MNCs in patients who later relapsed, for all remission BM samples in which MNCs and/or HSPCs were MRD-positive (15 patients, 25 remission time points indicated by blue circles). VAF of mutations with the highest VAF within the highest clonally involved HSPC population was compared with the VAF of MNCs in the same BM remission sample. For mutations in which MNC VAFs were below the LOD, the LOD value was used instead of the actual VAF. The paired MNC and HSPC samples for each patient are connected by gray dashed lines. Mean (± SEM) values are also shown. P value; a binomial test. (C) Kinetic assessment of MRD in purified HSPCs after allo-HSCT, as assessed by ddPCR analysis for patient-specific mutations in patients in whom MRD positivity of HSPCs preceded MRD positivity of BM MNCs for at least 2 consecutive remission time points. Arrowheads indicate time of diagnosis of clinical relapse from the time of transplantation (0), and the length of the arrows indicates months from detection of molecular MRD to time of clinical diagnosis of relapse. MNC MRD+; at least 1 mutation confidently detected in BM MNCs by ddPCR; MNC MRD−, no mutation confidently detected in BM MNCs by ddPCR; HSPC MRD+, at least 1 mutation confidently detected in ≥1 stem and progenitor cell populations by ddPCR; HSPC MRD−, no mutation confidently detected by ddPCR in any investigated stem and progenitor cell populations (see “Methods” and supplemental Methods). (D) ddPCR-based VAFs for patient-specific mutations in BM MNCs and purified HSPCs in consecutive remission samples for patients who later relapsed. Error bars represent the 95% confidence interval of VAFs calculated according to Poisson distribution as further specified in “Methods.” Blue color indicates highly confident clonal involvement, red indicates negative, and green inconclusive, as described in supplemental Methods. Only HSPC populations with highly confident clonal involvement for at least 1 time point are shown. Raw data can be found in source data file 1.
HSCs, multipotent progenitors, lymphoid-primed multipotent progenitors, and granulocyte-monocyte progenitors were most frequently clonally involved and showed highest VAFs, typically increasing in consecutive remission samples (Figure 2C; Figure 3D, source data file 1), and high VAF of HSPCs correlated with shorter time to relapse (supplemental Figure 5C). HSPCs most clonally involved at CR were also highly clonally involved at diagnosis/pretransplantation (supplemental Figure 3; supplemental Table 11, source data file 1). MRD-positivity was often confirmed in >1 HSPC subset, although frequently with considerable VAF differences. When multiple mutations were assessed within the same patient, most showed similar MRD differences between HSPCs and MNCs (Figures 2C and 3D, source data file 1). In the 11 patients who relapsed for whom we also had diagnostic/pretransplantation samples, the extensive increase in HSPC clonal involvement was observed in the HSPC subset most expanded before transplantation relative to that of age-matched controls (Figure 2C-D; supplemental Table 11, source data file 1). Because 6 HSPC populations were investigated, this finding was highly significant (P = 6.106 × 10−7; a binomial test).
Targeted sequencing, widely applied for mutational MRD assessment,22 showed similar results to that of ddPCR analysis on MRD-positive remission HSPCs from 5 analyzed patients (patients 2-5 and 7; supplemental Figure 5D-E, source data file 2).
Lack of HSPC clonal involvement in patients in continuous-CR
The consistent selective persistence of MRD-positive HSPCs preceding clinical relapse, implicated a causative relationship between MRD-positive HSPCs and relapses. We therefore performed the same HSPC mutational screening in 13 patients who remained in continuous-CR, 66 to 101 months after allo-HSCT (patients 17-29; supplemental Tables 1-3), when risk for relapse is very low. HSPCs were purified from multiple remission samples, including at least 1 early (≤6 months) and 1 late (≥20 months) time point after allo-HSCT (Figure 4A; supplemental Figure 6; supplemental Table 10, source data file 1). In contrast to patients who relapsed, in only 1 case (patient 27, 3 months after transplantation, the earliest time point investigated) did any mutation show clonal HSPC involvement, and this became and remained mutation-negative in multiple subsequent remission samples.
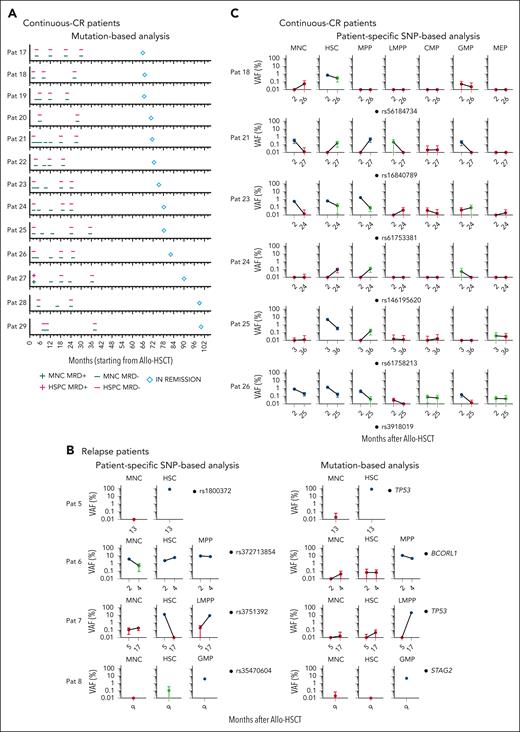
Lack of evidence for clonal involvement of HSPCs in patients in long-term continuous-CR after HSCT. (A) Kinetic assessment of MRD by ddPCR for patient-specific mutations identified at diagnosis, in BM MNCs and HSPCs in patients in continuous-CR (≥66 months); diamond-shaped symbol indicates time point of last clinical assessment without evidence of relapse after allo-HSCT, from time of transplantation (0). For complete ddPCR data for each patient and time point see supplemental Figure 6. MNC MRD+, at least 1 mutation confidently detected in BM MNCs by ddPCR; MNC MRD−; no mutation confidently detected in BM MNCs by ddPCR; HSPC MRD+, at least 1 mutation confidently detected in ≥1 stem and progenitor cell populations by ddPCR; HSPC MRD−, no mutation confidently detected by ddPCR in any investigated stem and progenitor cell populations (see “Methods”). (B) ddPCR-based VAF analysis of CR samples from 4 patients who relapsed for patient-specific SNPs (left; see supplemental Methods for selection of SNPs) as compared with mutational VAF (right) in BM MNCs, HSCs, and the most clonally involved HSPC population (in patient 5, HSCs were most clonally involved). (C) Patient-specific SNP analysis in all HSPC populations from CR samples from 6 patients in continuous-CR, at the earliest and latest available time point after allo-HSCT at which remission BM was analyzed, and for which mutational MRD was negative in all cell populations and cases. Error bars represent the 95% confidence interval of VAFs calculated according to Poisson distribution as further specified in “Methods.” Blue and red indicate confidently positive and negative data, respectively, and green inconclusive, as defined in the supplemental Methods. Raw data can be found in source data file 1 (mutational HSPC ddPCR) and source data file 3 (SNP ddPCR).
Lack of evidence for clonal involvement of HSPCs in patients in long-term continuous-CR after HSCT. (A) Kinetic assessment of MRD by ddPCR for patient-specific mutations identified at diagnosis, in BM MNCs and HSPCs in patients in continuous-CR (≥66 months); diamond-shaped symbol indicates time point of last clinical assessment without evidence of relapse after allo-HSCT, from time of transplantation (0). For complete ddPCR data for each patient and time point see supplemental Figure 6. MNC MRD+, at least 1 mutation confidently detected in BM MNCs by ddPCR; MNC MRD−; no mutation confidently detected in BM MNCs by ddPCR; HSPC MRD+, at least 1 mutation confidently detected in ≥1 stem and progenitor cell populations by ddPCR; HSPC MRD−, no mutation confidently detected by ddPCR in any investigated stem and progenitor cell populations (see “Methods”). (B) ddPCR-based VAF analysis of CR samples from 4 patients who relapsed for patient-specific SNPs (left; see supplemental Methods for selection of SNPs) as compared with mutational VAF (right) in BM MNCs, HSCs, and the most clonally involved HSPC population (in patient 5, HSCs were most clonally involved). (C) Patient-specific SNP analysis in all HSPC populations from CR samples from 6 patients in continuous-CR, at the earliest and latest available time point after allo-HSCT at which remission BM was analyzed, and for which mutational MRD was negative in all cell populations and cases. Error bars represent the 95% confidence interval of VAFs calculated according to Poisson distribution as further specified in “Methods.” Blue and red indicate confidently positive and negative data, respectively, and green inconclusive, as defined in the supplemental Methods. Raw data can be found in source data file 1 (mutational HSPC ddPCR) and source data file 3 (SNP ddPCR).
Assessment of HSPC MRD with patient-specific SNPs
For most patients included in this study we had available historical routine clinical recipient CD34+ chimerism data analyzed at the same time points after transplantation as for the ddPCR analysis of MNCs and HSPCs (supplemental Figure 7A-B).39 In the 12 continuous-CR cases for which we were able to compare these data sets, multiple consecutive CR samples were mutational MRD-negative in MNCs and HSPCs, whereas chimerism analysis was compatible with low-level recipient-derived cells (mean ± SEM: 0.92% ± 0.12%; supplemental Figure 7A). In 8 of 9 investigated relapse cases, >10% recipient chimerism was observed at the time of clinical diagnosis of relapse, but this was preceded by mutation-positive HSPCs by a mean of 13 months (supplemental Figure 7B). Notably, in most cases, when HSPCs became reliably mutational MRD-positive, recipient chimerism was comparable with the continuous-CR cases, underscoring its lower MRD specificity.
We also assessed the ability of patient-specific SNPs to specifically detect MRD in HSPCs in 4 patients who relapsed (patients 5-8) and 6 patients in continuous-CR (patients 18, 21, and 23-26). In all 4 analyzed patients who relapsed (patients 5-8), mutational MRD-positive HSPCs showed comparable VAFs using probes for patient-specific SNPs. However, in 2 patients (patients 6 and 7) mutational MRD-negative HSCs were highly SNP-positive, and for 1 patient (patient 6) in consecutive CR samples, probably reflecting persistence of normal HSCs (Figure 4B, source data files 1 and 3). In agreement, in continuous-CR BM samples that were consistently mutational MRD-negative, patient-specific SNPs were, in all cases, reliably positive in MNCs and/or HSPCs, even 2 to 3 years after transplantation (Figure 4C, source data file 3).
FCM MRD analysis
An alternative MRD approach is to apply FCM to detect malignant cells with aberrant antigen expression,13,35 but the sensitivity and specificity compared with mutational MRD analysis in MDS after transplantation remains unclear and, accordingly, there are no general ELN recommendations for FCM in monitoring MDS except using MRD assessment for AML.33,35 We therefore compared mutational and FCM-based MRD analysis, using panels assessing leukemia-associated immunophenotype and different-from-normal phenotypes.13,33-36,40 We first analyzed CR samples for coexpression of CD123 and CD45RA, within the Lin−CD34+CD38− compartment, for identification of leukemic stem cells.34 Lin−CD34+CD38−CD123+CD45RA+ were present at higher frequencies after transplantation than in normal steady-state BM, although with no significant differences between mutation-positive and mutation-negative CR samples from patients who later relapsed, or mutation-negative continuous-CR samples (supplemental Figure 8). Using 2 ELN-recommended panels for MRD analysis,36,40 predefined aberrant phenotypes were, as expected, rare in normal steady-state BM but equally rare in pretransplantation (highly mutation-positive) and posttransplantation (mutation-negative) BM from patients in continuous-CR (Figure 5A-D), demonstrating that these panels lack the specificity and sensitivity required for MRD screening after allo-HSCT. However, aberrant phenotypes were distinctly present in multiple (nontransplanted) high-risk MDS/AML BM samples included as controls (Figure 5A-D; supplemental Table 1), although not for all aberrant phenotypes investigated, in agreement with other studies.11,41-43
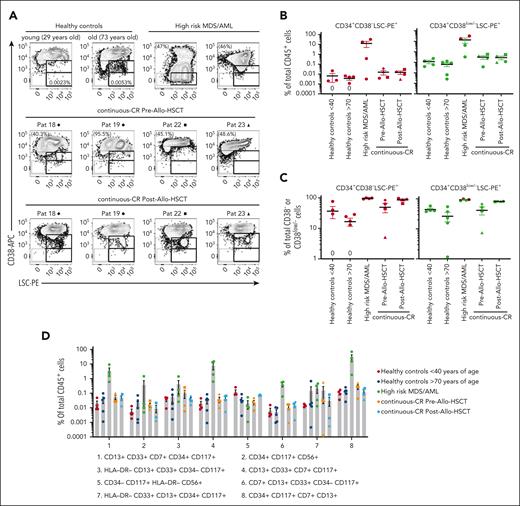
FCM–based leukemic stem cell and aberrant antigen expression analysis. (A) FCM profiles of CD34+-gated BM cells showing expression of CD38 (CD38-APC) vs a combination of cell surface antigens (CD7, CD11b, CD22, CD56, CD366, and CD371; LSC-PE) reported as aberrantly expressed on CD34+CD38low/− LSCs. LSC-PE expression was, as indicated, investigated in a gate set strictly on CD38− cells (lower CD38 gate based on erythrocytes that are CD38−), as well as on the 10% lowest CD38-expressing CD34+ cells for samples in which <10% of CD34+ cells were CD38−. Representative data from 2 healthy donors (left: young, aged <40 years; right: old, aged >70 years), 2 patients with high-risk MDS/AML, and pre– and post–allo-HSCT samples from 4 patients (also analyzed by ddPCR; see Figure 4A) achieving continuous-CR after allo-HSCT. Percentages shown in FCM plots for the 2 patients with high-risk MDS/AML (left, patient 32; right, patient 33), and the continous-CR pre–allo-HSCT samples indicate the VAF for the most clonal recurrent mutation. For all continuous-CR posttransplantation samples the mutational VAF was below detection level. Percentages for the representative healthy control BM samples show CD34+CD38−LSC-PE+ cells out of total cells. (B) Mean (± SEM) percentage CD34+CD38− LSC-PE+ cells (left, red) and CD34+CD38low/−LSC-PE+ cells (right, green) of total CD45+ BM cells. (C) Mean (± SEM) percentage LSC-PE+ cells in CD34+CD38− (left, red) and CD34+CD38low/− (right, green) gates, of total CD34+CD38−/CD34+CD38low/− BM cells. Equal symbols for the patients who achieved continuous-CR indicate sequential samples taken before and after allo-HSCT for the same patient. “0” indicates a sample showing no LSC+ events within the set gates. For 2 patients with high-risk MDS/AML, >10% of the CD34+ cells were CD38− and were therefore not analyzed based on a separate CD38low/− gate (red symbols in right B and C panels). (D) The same BM samples analyzed in panels A to C were also analyzed for other aberrant antigen expression patterns reported in myeloid malignancies. Each dot represents an individual BM sample. Results are presented as mean (± SEM) percentages of total CD45+ BM cells coexpressing indicated (populations 1-8) combinations of antigens. Equal symbols for the patients in continuous-CR indicate sequential samples taken before and after allo-HSCT for the same patient. Blast counts for the patients with high-risk MDS/AML (patients 30-33) were 62.5%, 10%, 43.5%, and 11%, respectively (supplemental Table 1).
FCM–based leukemic stem cell and aberrant antigen expression analysis. (A) FCM profiles of CD34+-gated BM cells showing expression of CD38 (CD38-APC) vs a combination of cell surface antigens (CD7, CD11b, CD22, CD56, CD366, and CD371; LSC-PE) reported as aberrantly expressed on CD34+CD38low/− LSCs. LSC-PE expression was, as indicated, investigated in a gate set strictly on CD38− cells (lower CD38 gate based on erythrocytes that are CD38−), as well as on the 10% lowest CD38-expressing CD34+ cells for samples in which <10% of CD34+ cells were CD38−. Representative data from 2 healthy donors (left: young, aged <40 years; right: old, aged >70 years), 2 patients with high-risk MDS/AML, and pre– and post–allo-HSCT samples from 4 patients (also analyzed by ddPCR; see Figure 4A) achieving continuous-CR after allo-HSCT. Percentages shown in FCM plots for the 2 patients with high-risk MDS/AML (left, patient 32; right, patient 33), and the continous-CR pre–allo-HSCT samples indicate the VAF for the most clonal recurrent mutation. For all continuous-CR posttransplantation samples the mutational VAF was below detection level. Percentages for the representative healthy control BM samples show CD34+CD38−LSC-PE+ cells out of total cells. (B) Mean (± SEM) percentage CD34+CD38− LSC-PE+ cells (left, red) and CD34+CD38low/−LSC-PE+ cells (right, green) of total CD45+ BM cells. (C) Mean (± SEM) percentage LSC-PE+ cells in CD34+CD38− (left, red) and CD34+CD38low/− (right, green) gates, of total CD34+CD38−/CD34+CD38low/− BM cells. Equal symbols for the patients who achieved continuous-CR indicate sequential samples taken before and after allo-HSCT for the same patient. “0” indicates a sample showing no LSC+ events within the set gates. For 2 patients with high-risk MDS/AML, >10% of the CD34+ cells were CD38− and were therefore not analyzed based on a separate CD38low/− gate (red symbols in right B and C panels). (D) The same BM samples analyzed in panels A to C were also analyzed for other aberrant antigen expression patterns reported in myeloid malignancies. Each dot represents an individual BM sample. Results are presented as mean (± SEM) percentages of total CD45+ BM cells coexpressing indicated (populations 1-8) combinations of antigens. Equal symbols for the patients in continuous-CR indicate sequential samples taken before and after allo-HSCT for the same patient. Blast counts for the patients with high-risk MDS/AML (patients 30-33) were 62.5%, 10%, 43.5%, and 11%, respectively (supplemental Table 1).
Assessment of mutational MRD in CD34+ BM cells
Recent studies showed enhanced MRD detection using CD34+ cells.23 Because HSPCs in MDS and related myeloid malignancies typically express CD34 and represent a small fraction of BM cells, we assessed mutation MRD in CD34+ cells in 14 patients who relapsed (patients 2-15) investigated for mutational MRD in HSPCs (supplemental Table 8). In HSPC MRD-positive samples, total CD34+ cells were more clonally involved than MNCs (mean, 16-fold; P = 1.91 × 10−6). However, in all 21 investigated samples, clonal involvement was higher in the most clonally involved HSPC than total CD34+ cells (mean, 8-fold; P = 9.54 × 10−7). In 10 patients who relapsed (patients 2-3, 6, and 8-14) we also experimentally validated clonal involvement of bead-enriched CD34+ BM cells vs MNCs and HSPCs from CR samples, and CD34-enriched cells were more clonally involved than MNCs but always less than purified HSPCs (Figure 6A-D, source data file 4).
Mutational MRD screening of BM CD34+ cells. (A-D) ddPCR-based screening for clonal involvement of patient-specific mutations (for which HSPC VAF was >1%) in CD34-enriched cells from remission BM. (A) Five patients in whom BM MNCs were MRD-negative but purified HSPCs MRD-positive and (B) 5 patients in whom BM MNCs were MRD-positive but at lower level than in HSPCs. Blue and red indicate confidently positive and negative data, respectively, and green inconclusive as defined in the supplemental Methods. The dashed lines in CD34+ graphs (A-B) represent the predicted VAF (of the mutation with highest VAF) in CD34+ cells based on calculations from FCM and VAF data from HSPCs as described in supplemental Table 8. (C) VAF (%) for mutation with the highest VAF at indicated time points for all patients in panels A-B. The paired BM MNC, CD34+ cells and HSPC for each patient (blue circles) are connected by gray dashed lines. Mean (± SEM) values are also shown. P values were calculated using the Wilcoxon paired test. (D) Fold changes in VAF in BM CD34+ cells and HSPCs relative to MNCs (for all patients in panels A-B), in each patient using the mutation with the highest VAF. For mutations with VAFs below the LOD, the LOD value was used instead of the actual VAF. The VAFs for paired BM MNC, CD34+ cells and HSPCs for each patient (blue circles) are connected by gray dashed lines. Mean (± SEM) values are also shown. P values were calculated using a binomial test. Raw data can be found in source data file 1 (mutational HSPC ddPCR) and source data file 4 (mutational ddPCR in CD34+ fraction).
Mutational MRD screening of BM CD34+ cells. (A-D) ddPCR-based screening for clonal involvement of patient-specific mutations (for which HSPC VAF was >1%) in CD34-enriched cells from remission BM. (A) Five patients in whom BM MNCs were MRD-negative but purified HSPCs MRD-positive and (B) 5 patients in whom BM MNCs were MRD-positive but at lower level than in HSPCs. Blue and red indicate confidently positive and negative data, respectively, and green inconclusive as defined in the supplemental Methods. The dashed lines in CD34+ graphs (A-B) represent the predicted VAF (of the mutation with highest VAF) in CD34+ cells based on calculations from FCM and VAF data from HSPCs as described in supplemental Table 8. (C) VAF (%) for mutation with the highest VAF at indicated time points for all patients in panels A-B. The paired BM MNC, CD34+ cells and HSPC for each patient (blue circles) are connected by gray dashed lines. Mean (± SEM) values are also shown. P values were calculated using the Wilcoxon paired test. (D) Fold changes in VAF in BM CD34+ cells and HSPCs relative to MNCs (for all patients in panels A-B), in each patient using the mutation with the highest VAF. For mutations with VAFs below the LOD, the LOD value was used instead of the actual VAF. The VAFs for paired BM MNC, CD34+ cells and HSPCs for each patient (blue circles) are connected by gray dashed lines. Mean (± SEM) values are also shown. P values were calculated using a binomial test. Raw data can be found in source data file 1 (mutational HSPC ddPCR) and source data file 4 (mutational ddPCR in CD34+ fraction).
Discussion
Although new relapse treatments are being explored,44,45 initiation of such treatments has been severely delayed because of lack of highly sensitive methods predicting an impending relapse at an early stage after transplantation. In previous studies of MNCs after allo-HSCT, 20% of mutational MRD-negative cases later relapsed.22 In our study, of the 25 remission samples that were MRD-positive in MNCs and/or HSPCs, all were MRD-positive in HSPCs whereas only 8 were MRD-positive in MNCs, and the VAF was always higher (mean, 97-fold) in HSPCs. Although CD34+ cells showed increased MRD sensitivity compared with MNCs, MRD sensitivity was always higher in HSPCs. Importantly, in all patients who relapsed, when HSPCs became MRD-positive they remained positive, with increasing VAF in subsequent remission samples, and a high VAF correlated with a shorter time to relapse.
Although a heterogenous group of patients were investigated, including different MDS subgroups, chronic myelomonocytic leukemia and AML-MRC, enhanced mutational MRD detection in HSPCs vs MNCs was observed in all relapse cases and in each patient in every BM sample investigated at CR, regardless of their World Health Organization–based diagnosis, prognostic scoring system score, pretransplantation treatment and conditioning, and mutational profiles, suggesting that these findings should be widely applicable to MDS and related myeloid malignancies.
As expected, there was a strong correlation between poor prognosis genomic abnormalities, MRD-positivity, and relapse. However, 4 of the patients who relapsed did not have high-risk genomic abnormalities, but also in these cases, HSPCs were found to be MRD-positive at CR. Of the patients who remained in CR for >5 years after transplantation, 2 patients had high-risk genomic alterations before transplantation, but also in these cases MRD was consistently MRD-negative as for the patients in continuous-CR without high-risk genetic changes, with exception of the first CR sample of Patient 27. Therefore, our data support that HSPC MRD-positivity has relapse-predicting value independently of high-risk genomic alterations.
However, MRD-positive results after transplantation in patients who later do not relapse are not uncommon,11,22 representing a significant limitation when deciding whether, how, and when to start preemptive relapse treatment based on a reliable MRD-positive finding while patients remain in clinical CR after transplantation, and also for assessment of effects of such treatments. Whether or not an MRD-positive finding in HSPCs might predict a relapse not only earlier but potentially also more reliably than in whole BM will require prospective studies of much larger cohorts of patients, because in 12 of 13 patients in this study remaining in continuous-CR ≥66 months after allo-HSCT, no clonal involvement was observed in MNCs nor HSPCs in multiple sequential BM samples after transplantation. In the only continuous-CR case in which MNCs were MRD-positive at the first time point after transplantation (patient 27), HSPCs were also MRD-positive, and subsequently both MNCs and HSPCs became and remained consistently MRD-negative. Incorporation of the specific diagnosis, presence or absence of high-risk genomic lesions, other risk factors, pretransplantation treatment and conditioning, as well as other clinical variables are likely to enhance the predictive value of an MRD-positive finding regarding whether and when a relapse is likely to occur. Because of the limited number of heterogenous patients combined with CR samples investigated from different patients having been collected at different time points posttransplantation we could not assess the relationship between MRD-positive HSPCs, other variables, and the occurrence and timing of relapse. Therefore, further and larger prospective studies assessing these and other variables will be required toward a more robust and predictive model for early interventions based on an MRD-positive finding, to be able to intervene at an early stage and also to avoid overtreatment of patients who might not relapse. In the meantime, the best individualized relapse-predictive value of a mutational MRD-positive finding might be a rising VAF in a subsequent CR sample.
The vast majority of patients with MDS undergoing allo-HSCT have clonal driver mutations.5,22 For the 2 patients that did not (patients 1 and 17), we identified clonal mutations by WGS that were tracked by ddPCR with similar sensitivity as recurrent mutations. However, MRD assessment using patient-specific SNPs showed that SNP-positive HSPCs might reflect persistence of patient-derived normal HSPCs rather than MDS HSPCs. Although previous studies showed that allo-HSCT eliminates recipient-derived clonal hematopoiesis,46 isolated detection of mutations in DTA, the most common clonal hematopoiesis mutations, is not correlated with increased relapses.25,47,48 Therefore, in DTA relapse cases, we confirmed MRD positivity with at least 1 additional recurrent mutation. In 1 continuous-CR case (patient 26) with only an identified DNMT3A mutation, the mutation was no longer reliably detected in MNCs or HSPCs in multiple posttransplantation CR BM samples.
We also used established FCM-based panels for MRD assessment after transplantation. Although confirming their usefulness in diagnostic assessment of high-risk MDS/AML, these panels lacked the specificity and sensitivity required for MRD analysis at CR, in agreement with previous studies failing to identify leukemia-associated immunophenotype for MRD assessment in MDS.42,43
The distinct patterns of HSPC clonal involvement in remission BM after allo-HSCT, correlate with known MDS stem cell identities. Whereas HSC involvement appears almost mandatory in early and intermediate MDS,28 more advanced MDS stages subjected to allo-HSCT frequently have suppressed HSCs and expanded progenitor compartments38 similar to AML.49 Our findings support that, in patients who later relapse, HSPCs selectively escape toxic preconditioning and immune targeting after allo-HSCT. The identification herein of rare relapse-initiating cells after transplantation will facilitate identification of mechanisms by which these cells might escape pretransplantation conditioning, posttransplantation immune targeting, as well as discovery of novel therapeutic targets and targeting of these cells upon early diagnosis of an impending relapse.
Mutational-MRD assessment of HSPCs will require FCM-based cell sorting. The HSPC panel herein applied is well established and less advanced than many FCM panels routinely used for diagnostic purposes at hospitals performing allo-HSCT. Moreover, FCM-based cell sorting takes advantage of the same reagents and expertise as FCM analysis and is already being applied for diagnostic purposes.50,51
Our studies provide a platform for high sensitivity mutational-MRD screening of relapse-initiating MDS stem cells, and early prediction of impending relapses, representing the first step toward initiation and assessment of very early interventional relapse treatments.
The current MRD platform applying combined FCM sorting and mutational screening of relapse-initiating stem cells in MDS and related myeloid malignancies is likely to be relevant also for other cancers, because CR followed by late relapses is observed after cancer therapies also in other hematological malignancies and solid tumors, in which therapy-resistant cancer stem cells have been identified.52
Acknowledgments
Sequencing was performed by the SNP&SEQ Technology Platform in Uppsala, part of the National Genomics Infrastructure Sweden and Science for Life Laboratory, and was supported by the Swedish Research Council and the Knut and Alice Wallenberg Foundation. The authors are grateful to Warren Kretzschmar for providing expert input on statistical analysis; Dan-Mikael Hauzenberger for providing clinical data on chimerism analysis performed at the Department of Clinical Immunology, Karolinska University Hospital, and the nursing staff and physicians at the Departments of Hematology and Cellular Therapy and Allogeneic Stem Cell Transplantation, Karolinska University Hospital; and the patients for their participation in this study.
This study was supported by grants to S.E.W.J. from the Knut and Alice Wallenberg Foundation (KAW 2016.0105), the Tobias Foundation (4-1122/2014), the Torsten Söderberg Foundation, the Center for Innovative Medicine at Karolinska Institutet (613/06), The Swedish Research Council (538-2013-8995), Bloodwise (17017), and The Medical Research Council (MC_UU_12009/5); to E.H.-L. from the Swedish Cancer Society-Cancerfonden (170397), The Swedish Research Council (521-2013-3577 and 2017-00476), and Stockholm Cancer Society-Cancerförening (151103); to P.S.W. from The Swedish Research Council (2015-03561), and the Knut and Alice Wallenberg Foundation (Academy Fellowship Award, 2015.0195); and to M.D. from Åke Olsson hematology research (2018-00179). Core services including technical support for cell sorting was provided by the MedH Flow Cytometry Core Facility (Karolinska Institutet), supported by infrastructure and core facility grant from the Karolinska Institutet (20170655).
Authorship
Contribution: M.D. designed, performed, and analyzed all experiments and wrote the manuscript; T.M.-B. contributed on the flow cytometry (FCM) sorting and droplet digital polymerase chain reaction (ddPCR) experiments; M.T. provided input on clinical data collection, analysis and presentation, and on the writing of the manuscript; S.M. contributed on the FCM sorting and ddPCR experiments; M.L. contributed to the FCM-based measurable residual disease (MRD) analysis, and provided input in writing the manuscript; K.H. contributed to the FCM-based MRD analysis and ddPCR experiments; M.K. contributed to the targeted mutational screening of bone marrow (BM) samples; G.W. and M.J. coordinated clinical data collection and processing, and freezing and registration of BM samples in the biobank; S.V. provided raw real-time PCR or short tandem repeat (STR) PCR data; P.L. planned the stem cell transplantations and provided input on the writing of the manuscript; S.L. provided input on clinical data collection and analysis; T.Y. contributed to the targeted mutational and single nucleotide polymorphism screening of hematopoietic stem and progenitor cell samples, whole-genome sequencing experiments and analysis, and provided input on the writing of the manuscript; E.H.-L. contributed to design of the study, led and coordinated the collection and initial mutational screening of BM samples, analysis, and presentation of clinical data, and provided input on the writing of the manuscript; P.S.W. contributed to the design and analysis of all experiments, and provided input on the writing of the manuscript; S.E.W.J. conceptualized the study, designed and analyzed experiments, and wrote the manuscript; and all authors read and approved the final manuscript.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Sten Eirik W. Jacobsen, Karolinska Institute, Department of Medicine Huddinge, Center for Hematology and Regenerative Medicine, Karolinska University Hospital Huddinge, 141 86 Stockholm, Sweden; email: sten.eirik.jacobsen@ki.se.
References
Author notes
M.T., S.M., and M.L. contributed equally to this study.
Targeted DNA sequencing data (with the exception of 2 patients [patients 24 and 29] for whom only the DNA-mutation analysis output was available and selected mutations confirmed by ddPCR) used for identification of DNA mutations as well as for comparison of mutation detection by next-generation sequencing (NGS) to ddPCR, and the somatic mutation list identified through whole-genome sequencing have been deposited on the Swedish National Data Service’s research data repository with restricted access in accordance with the European Union General Data Protection Regulation (DOI: https://doi.org/10.48723/0k7w-2k05).
Additional data reported in this study are available upon request from the corresponding author, Sten Eirik W. Jacobsen (sten.eirik.jacobsen@ki.se).
The online version of this article contains a data supplement.
There is a Blood Commentary on this article in this issue.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.



This feature is available to Subscribers Only
Sign In or Create an Account Close Modal