In this issue of Blood, Dimitriou and colleagues report that mutational screening for malignant hematopoietic stem and progenitor cells (HSPCs) that persist after allogenic hematopoietic stem cell transplantation (allo-HSCT) consistently improved the sensitivity of measurable residual disease (MRD) detection, thus allowing an earlier prediction of relapse in patients with myeloid malignancies.1
Myelodysplastic syndromes (MDSs) arise from a rare population of disease-initiating hematopoietic stem cells (HSCs). MDS HSCs, which can persist and expand during conventional therapy, are the major effectors of disease progression and relapse.2 Aside from allo-HSCT, no curative treatments for MDS have emerged over the past decade,3 which underscores an urgent need to improve preventive strategies, including current approaches for the early detection of relapse. In particular, the lack of highly sensitive methods capable of predicting relapse in the early posttransplant phase has significantly inhibited the clinical implementation of new exploratory treatments for patients with MDS whose disease relapses after allo-HSCT. The detection of MRD during complete remission (CR) is an important predictor of survival in patients with acute myeloid leukemia (AML).4 However, MRD assessment is not yet a routine part of the management of patients with MDS.
MDSs are propagated by the expansion of HSC clones carrying preexisting or newly acquired recurrent mutations. Given that the progression of MDS to AML and the relapse of MDS after allo-HSCT are mainly caused by clones harboring persistent founder mutations,5 targeted screening for these recurrent disease-initiating mutations could facilitate the earlier detection of MDS relapse after allo-HSCT. Indeed, in a retrospective study, the detection of oncogenic mutations in bone marrow (BM) after allo-HSCT was associated with a higher risk of disease progression.6 However, the same study showed that 20% of patients without detectable MRD after allo-HSCT ultimately had disease progression and that 30% of patients with detectable MRD after allo-HSCT did not have disease progression. These results challenge the sensitivity of our approaches to detecting MRD, thus underlining current limitations in predicting relapse after allo-HSCT. Other technologies commonly used to assess MRD in MDS BM cells, such as flow cytometry for aberrant cell surface antigens, have not been extensively validated to detect MRD in MDS after allo-HSCT.7 Current efforts to improve MRD assessment in MDS are focused on detecting MRD in the CD34+ HSPC compartment, which drives MDS progression and relapse.8
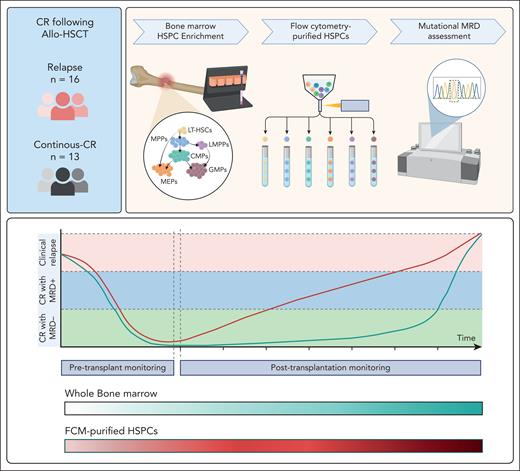
Dimitriou et al compared the sensitivity of MRD detection using flow cytometry–purified HSPCs with that of MRD detection using unfractionated mononuclear cells. The authors analyzed 25 samples from 15 patients who initially had CR after allo-HSCT and then had disease relapse. Among these samples, MRD was observed in all flow cytometry–sorted HSPCs but only 9 of the 16 available BM samples. MRD detection in HSPCs consistently preceded that in mononuclear cells across multiple sequential samples, with an average lead time of 10 months, which translated into a 97-fold higher sensitivity of MRD detection. Interestingly, HSCs, multipotent progenitors, lymphoid-primed multipotent progenitors, and granulocyte-monocyte progenitors were more frequently clonally involved than other BM populations and had the highest variant allele frequencies (VAFs) among the different HSPC populations analyzed. Sequential samples collected during CR typically showed increasing clonal burden, with higher VAFs in HSPCs, which were correlated with shorter time to relapse (see figure).
MRD detection in flow cytometry (FCM)-purified HSPCs enriched from the BM of patients in CR after allo-HSCT precedes that in whole BM cells and more reliably predicts disease relapse. CMP, cytidine 5′-monophosphate; GMP, granulocyte monocyte progenitors; LMPP, lymphomyeloid primed progenitors; LT-HSC, long-term hematopoietic stem cells; MEP, megakaryocyte-erythroid progenitors; MPP, multipotent progenitors. The figure was created with BioRender.com.
MRD detection in flow cytometry (FCM)-purified HSPCs enriched from the BM of patients in CR after allo-HSCT precedes that in whole BM cells and more reliably predicts disease relapse. CMP, cytidine 5′-monophosphate; GMP, granulocyte monocyte progenitors; LMPP, lymphomyeloid primed progenitors; LT-HSC, long-term hematopoietic stem cells; MEP, megakaryocyte-erythroid progenitors; MPP, multipotent progenitors. The figure was created with BioRender.com.
However, owing to the limited number of samples used in their study, the authors could not evaluate the implication of detecting MRD in HSPCs from patients whose disease did not progress after allo-HSCT. The detection of MRD after allo-HSCT in patients whose disease ultimately does not relapse is common, which limits our ability to determine whether and when preemptive treatments for relapse should be initiated in patients who become MRD+ after allo-HSCT but still have CR. Therefore, prospective studies involving a larger cohort of patients than that analyzed by Dimitriou et al are urgently needed to evaluate whether MRD detection in HSPCs predicts disease relapse more reliably than MRD detection in unfractionated BM cells. Taking into consideration the type of genomically defined MDS-specific subtypes, the presence or absence of higher-risk genomic alterations, the type of allo-HSCT treatment, and other clinical variables may also improve our capability to assess the risk of progression.
Interestingly, the authors showed that in patients who had CR after allo-HSCT, distinct patterns of HSPC clonality were correlated with the MDS cellular architecture. Indeed, whereas HSC clonality was predominant in lower- and intermediate-risk MDS, more differentiated progenitor cell clonality was predominant in higher-risk MDS.9 Thus, further characterization of these relapse-driving HSPCs could elucidate how these cells selectively evade pretransplant conditioning regimens and posttransplant graft-versus-leukemia effects and lead to the discovery of novel therapeutic approaches to prevent recurrence after allo-HSCT.
Our understanding of the clinical implications of detecting MRD in MDS continues to evolve as new technologies, such as single-cell sequencing, are introduced.10 However, formal guidelines for the clinical application of these technologies to detect MRD in patients with MDS have not yet been defined. Larger prospective studies are needed to assess the impact of different approaches for MRD detection toward the development of a more robust and reliable predictive model for early intervention. Although intervening in the early stages of disease recurrence after allo-HSCT is important, overtreating patients who may not later experience relapse should be avoided to decrease the risk of secondary therapy-related cancers.
Conflict-of-interest disclosure: The authors declare no competing financial interests.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal