In this issue of Blood, Bendapudi et al1 propose that inherited defects in complement proteins and associated receptors predispose to the maladaptive hyperinflammation seen in infectious purpura fulminans (PF), a disease characterized by severe sepsis with coagulopathy.2
The authors use whole-exome germ line sequencing to analyze whether a set of 27 complement genes is associated with PF. Uncovering much-needed new pathophysiological clues could enable better therapy for patients with PF in the future, but finding new and meaningful connections from a haystack full of molecular possibilities is not trivial.
Using a recently described rarity variant trend test (RVTT) method,3 the authors assess the combined effect of multiple complement gene variants predicted to result in an altered phenotype. Teasing out the aggregate effect is of importance because the rare variants in question do not have sufficient statistical power to be individually associated with purpurea fulminans.
One major finding is that the number of complement system variants per patient associates with PF. Variants are identified in both soluble complement plasma proteins and complement receptors (CRs). The authors particularly focus their work on the integrin family of CRs. These are frequently expressed in the myeloid subsets of leukocytes and comprise heterodimers with a unique α subunit and a shared β subunit: CR3 (αMβ2, CD11b/CD18) and CR4 (αXβ2, CD11c/CD18).
The soluble complement proteins are responsible for generating the main effector functions of the cascade. CR3 and CR4 recognize cargo (eg, microbial particles) marked with complement opsonins, thereby facilitating opsonophagocytosis. Low-frequency gene variants in both complement subgroups (soluble effector proteins and CRs of the integrin family) independently associate with PF. Using RVTT, Bendapudi et al reveal a likely genetic link between maladaptive hyperinflammation in PF and the complement system. However, the authors go one step further in their analysis and provide insightful functional aspects of a selected set of identified complement protein variants.
The PF-associated mutation E69K in the plasma protein factor D exhibits significantly reduced complement activity. But how might decreased complement activity lead to hyperinflammation in infectious PF?
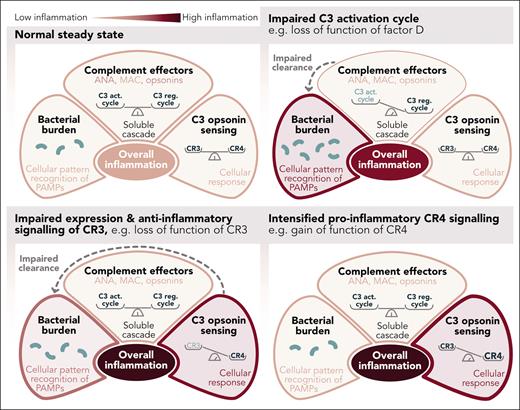
It may simply be a question of balance. It is generally accepted that the generation of complement effector functions is controlled by a complex balance between the C3 activation and C3 regulatory cycles (see figure).4 The complement cascade remains within its proximal and more noninflammatory pathways when the balance is tipped toward the C3 regulatory cycle. A bias toward the C3 activation cycle paves entry into the terminal, lytic, and inflammatory pathway. However, it is not just an imbalance in the complement system that contributes to hyperinflammation in PF. Loss-of-function mutations in complement effector proteins such as factor D may reduce the cascade’s chances of progressing to its terminal pathway, effectively limiting complement-mediated inflammation. However, savings in complement-mediated inflammation may lead to increased inflammatory signals, for example, mediated via Toll-like receptors, as the reduced complement effector functions lead to an impairment in the removal of bacteria.
Inflammatory signaling considering effects by soluble complement and CRs of the integrin family, in addition to sensing pathogen-associated molecular patterns (PAMPs) via pattern recognition receptors. ANA, complement anaphylatoxins C3a and C5a; C3 act. cycle, C3 activation cycle; C3 reg. cycle, C3 regulation cycle; MAC, complement membrane attack complex. Professional illustration by Somersault18:24.
Inflammatory signaling considering effects by soluble complement and CRs of the integrin family, in addition to sensing pathogen-associated molecular patterns (PAMPs) via pattern recognition receptors. ANA, complement anaphylatoxins C3a and C5a; C3 act. cycle, C3 activation cycle; C3 reg. cycle, C3 regulation cycle; MAC, complement membrane attack complex. Professional illustration by Somersault18:24.
Yet, bacterial clearance is only one variable in the equation of sepsis-associated inflammation. The signals induced by C3-opsonin-mediated opsonophagocytosis must also be considered. Consequently, Bendapudi et al assess the functional phenotypes of the CR3 and CR4 mutants using a kidney epithelial cell system. Tumor necrosis factor α–mediated activation of an NF-κB reporter in presence and absence of iC3b, the bona fide ligand of CR3 and CR4, serves as a surrogate for inflammatory signaling. This assay determines CR3 and CR4 signals on a homogenous genetic background, excluding other potentially confounding genetic variables. A key limitation that the authors acknowledge is that kidney epithelial cells do not recapitulate the cytokine production profiles of immune cells that are relevant to the pathophysiology of PF. This limits the extent to which these interesting molecular findings can be extrapolated to the clinical situation. Future studies could fill this gap.
Nevertheless, the NF-κB reporter assay shows how the PF-associated variants impair a second critical but often underestimated complement balance: the balance between suppressing and promoting inflammatory signals in response to ligation of CR3 and CR4, respectively.5-7
All CR3 variants examined in this study resulted in reduced surface expression and consequently less suppression of NF-κB activity (compared with wild-type CR3). This was observed for variants in the αM and β2 subunits of CR3. The identical variants in the β2 subunit were also investigated in the context of CR4, in addition to changes in the CR4-specific αX subunit. All CR4 variants resulted in increased NF-κB activity with mostly unchanged CR4 expression levels. What is particularly striking is that the identical single amino acid exchanges in the common β2 subunit impose opposite effects in CR3 and CR4: loss of function vs gain of function, respectively.
Taken together, CR3 variants lead to a loss of anti-inflammatory function, and CR4 variants lead to an increase in proinflammatory signals, fueling hyperinflammation. It is likely that the identified variants only acquire pathophysiological significance upon a second hit, for example, upon exposure to pathogens. Such a scenario is remarkably similar to the multiple-hit hypothesis described for the complement disease atypical hemolytic syndrome.8
What questions need to be answered next? The present study analyzes 27 complement genes, but the wider complement network includes more than 50 proteins or receptors, and it would be interesting to see whether there may also be a connection with other complement genes, for example, the lectin pathway. Could other C3 opsonins that bind to CR3 or CR4 also play a role in PF? Would bacterial cargo opsonized by iC3b affect the signaling pattern of the CRs studied? It would also be of interest to assess the composite effects of multiple variants in individual patients to determine their combined complotype.9 More importantly, can the new findings be used to develop anti-inflammatory drugs by stimulating CR3 signaling or inhibiting CR4 signaling? Seeking such therapies again resembles the search for a needle in a haystack. Thanks to the study by Bendapudi et al, we now know which haystack to search. We also know the thread that can be attached to such a “therapeutic needle.” We don't yet know its shape or size, but the chances of finding it have increased.
Conflict-of-interest disclosure: C.Q.S. is an inventor of patents and has patent applications that describe the use of complement inhibitors for therapeutic applications; received research funding from pharmaceutical companies; and received honoraria for speaking at symposia organized by Alexion Pharmaceuticals and Swedish Orphan Biovitrum.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal