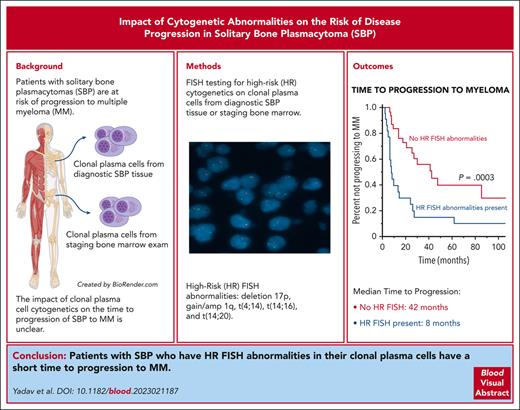
Key Points
Patients with SBP who have high-risk cytogenetic abnormalities by FISH in their clonal plasma cells have a short TTP to MM.
If there are insufficient clonal plasma cells in the bone marrow, FISH assessment can be performed on the diagnostic SBP tissue.
Abstract
Most patients with solitary bone plasmacytomas (SBP) progress to multiple myeloma (MM) after definitive radiation therapy as their primary treatment. Whether the presence of high-risk (HR) cytogenetic abnormalities by fluorescence in situ hybridization (FISH) in the clonal plasma cells, obtained either directly from the diagnostic SBP tissue or the corresponding bone marrow examination at the time of diagnosis, is associated with a shorter time to progression (TTP) to MM is unknown. This study evaluated all patients diagnosed with SBP at the Mayo Clinic from January 2012 to July 2022. The presence of del(17p), t(14;16), t(4;14), or +1q (gain or amplification) by FISH in clonal plasma cells was defined as HR. A total of 114 patients were included in this cohort, and baseline FISH was available for 55 patients (48%), of which 22 were classified as HR (40%). The median TTP to MM for patients with SBP and HR FISH was 8 months (95% confidence interval [CI], 6.3-26) compared with 42 months (95% CI, 25-not reached [NR]) in patients with SBP without HR FISH (P < .001). In a multivariate analysis, only HR FISH was a significant predictor for shorter TTP to MM, independent of minimal marrow involvement and an abnormal serum free light chain ratio at diagnosis. Deletion (17p) and gain 1q abnormalities were the most common FISH abnormalities responsible for the short TTP to MM. Thus, assessing for HR FISH abnormalities in clonal plasma cells derived from either the diagnostic SBP tissue or the staging bone marrow examination of patients with newly diagnosed SBP is feasible and prognostic for a shorter TTP to MM.
Introduction
Solitary plasmacytoma is a single mass of clonal plasma cells that may or may not present with minimal marrow involvement (defined by <10% clonal plasma cells in bone marrow).1 It may arise from an extramedullary site, such as a solitary extramedullary plasmacytoma, or from within a bone, such as a solitary bone plasmacytoma (SBP).2,3 Other than the isolated bone lesion that may or may not be symptomatic, patients with SBP do not have any clinical symptoms such as anemia, hypercalcemia, or renal insufficiency attributable to the presence of multiple myeloma (MM). The current standard of care for SBP with or without minimal marrow involvement is definitive fractionated radiation therapy of 40 to 50 Gy administered to the plasmacytoma followed by close observation.4,5 Surgery with complete or partial resection of the SBP is performed in certain instances before radiation therapy for diagnostic purposes or for the immediate relief of cord compression and stabilization of bone lesions. Despite excellent local control with radiation therapy, with or without the addition of surgery, ∼60% to 84% of patients will eventually progress to MM over the next 10 years.2,6-10 Previous studies assessing risk factors for shorter time to progression (TTP) of SBP to MM identified the presence of minimal marrow involvement either morphologically11 or by flow cytometry,12,13 the presence of an abnormal serum free light chain ratio,14 and the presence of high-grade angiogenesis within the plasmacytoma15 as risk factors for shorter TTP of SBP to MM. Furthermore, the persistence of an M-spike after radiation treatment was also identified as a risk factor for progression.9,10,16,17 A few studies assessed the cytogenetics by interphase fluorescence in situ hybridization (FISH) in extramedullary plasmacytomas.18 They found them to resemble those typically found in MM, but no association with the risk of progression to MM was observed.18,19 However, no studies have evaluated the baseline cytogenetic abnormalities detected by FISH in SBP and their impact on the risk of progression to MM. Assessing the FISH status in all patients with SBP has been challenging because of the limited number of clonal plasma cells available in the aspirate of the staging bone marrow examination, which is the conventional method for performing FISH analyses. With the successful establishment of procedures to perform FISH analyses on formalin-fixed paraffin-embedded tissue, it is possible to perform this assessment on residual clinical tissue samples obtained from the diagnostic biopsy of the SBP. Thus, this study aimed to assess the clonal plasma cell-associated cytogenetic abnormalities in patients with newly diagnosed SBP and their association with the risk of progression to MM.
Methods
Establishment of study cohort
The Mayo Clinic electronic medical record system was queried to identify all patients with an International Classification of Diseases diagnosis code “plasmacytoma” evaluated at the Mayo Clinic comprehensive cancer center from 1 January 2012 to 31 December 2022. The study was conducted with institutional review board approval and by the principles of the Declaration of Helsinki. Patients diagnosed before 2012 were excluded as advanced cross-sectional imaging was not routinely performed as part of the staging diagnostic tests. All patients were required to have had a biopsy-proven plasmacytoma arising from a bone, advanced cross-sectional imaging (positron emission tomography/computed tomography [CT], whole body–CT Skeletal Survey, or whole-body magnetic resonance imaging) demonstrating the absence of any additional lytic or 18F-fluorodeoxyglucose avid bone lesions or plasmacytomas, a posterior iliac crest bone marrow biopsy and aspirate containing <10% clonal plasma cells, and no clinical evidence of other CRAB symptoms (anemia, elevated creatinine, and hypercalcemia), which would fulfill the diagnosis for MM as per the International Myeloma Working Group criteria.1 We excluded patients with concurrent light chain amyloidosis, POEMS (polyneuropathy, organomegaly, endocrinopathy, monoclonal protein, skin changes) syndrome, ≥10% plasma cells on bone marrow biopsy, and those who fulfilled the criteria for smoldering or active MM.
Data including the various patient and disease-specific variables at the time of diagnosis of the SBP were obtained via review of the electronic medical health records and examined for prognostic significance. These included age, bone marrow plasma cell percentage, presence of high-risk (HR) cytogenetics by FISH, serum M spike, urine M spike, hemoglobin, creatinine, serum free light chains (sFLCs), and lactate dehydrogenase. In addition, time to progression (TTP) to MM was calculated from the date of diagnosis of SBP to the date of diagnosis of MM, with patients not progressing censored at the date of the last follow-up.
FISH assessments of clonal plasma cells
Cytogenetic assessments of clonal plasma cells via FISH were used to risk-stratify patients as having either HR or non-HR cytogenetics as per the Mayo Stratification of Myeloma and Risk-Adapted Therapy (mSMART) guidelines. The presence of del(17p), t(14;16), t(4;14), and t(14;20) +1q (gain or amplification) on FISH was defined as HR. At the Mayo Clinic, the FISH assay has been validated to be performed on clonal plasma cells within the diagnostic SBP paraffin-embedded tissue or on clonal plasma cells derived from the bone marrow aspirate at the time of disease staging. For FISH assessments in the bone marrow during the period of this study, both cytoplasmic immunoglobulin (cIg) and cell sorting to “isolate” the plasma cells before FISH staining were used. For cIg-FISH, preanalysis to assess the adequacy of plasma cell content of samples was performed with flow cytometry using an antibody panel containing anti-CD19, anti-CD38, anti-CD138, anti-CD45, anticytoplasmic κ (kappa), and anticytoplasmic λ (lambda). Samples with >0.1% plasma cells were deemed satisfactory for cIg-FISH analysis. After the hybridization of slides and after hybridization wash steps, plasma cells were stained with fluorescein isothiocyanate-conjugated antibodies directed against the κ and λ light chains. Only light chain–positive cells were targeted for scoring during the FISH analysis. For the cell sorting–based isolation of plasma cells (fluorescence-activated cell sorter–FISH), bone marrow cells (∼20 × 106) were subjected to 5 minutes of ammonium-chloride-potassium buffer for erythroid cell lysis. This was followed by 2 wash steps in phosphate-buffered saline (PBS) (lyse-wash procedure), and the cell pellet was resuspended in 3% bovine serum albumin/PBS. A total of 10 × 106 cells were then incubated for 15 minutes with the following antibodies: anti-CD19-BV510 (clone SJ25C1; BD Biosciences), anti–CD38-APC (clone REA671; Miltenyi Biotec), anti-CD45 Percp-Cy5.5 (clone HI30; BD Biosciences), anti-CD56-PE-Cy7 (clone NCAM16.2; BD Biosciences), anti-CD138-BV421 (clone MI15; BD Biosciences), and anti-CD319-PE (clone REA150; Miltenyi Biotec). The specimen was centrifuged and resuspended in 1.5 mL of PBS. Sorting was performed on BD fluorescence-activated cell sorter Melody (BD Biosciences, San Jose, CA). Sorting streams were defined for each case separately, using gates to include CD138+, CD19+, CD38-bright, CD56+, and/or CD45– plasma cells. A purity of at least 95% was achieved and verified by Kaluza software (Beckman Coulter Life Sciences, Indianapolis, IN). A minimum of 1000 sorted plasma cells collected in methanol/acetic acid was required to proceed with the FISH analysis. The sorted specimen was then processed for FISH analysis.
To perform the FISH assay on the diagnostic SBP tissue in this study, formalin-fixed paraffin-embedded tissue was cut at 5 microns and mounted on positively charged glass slides. The selection of tissue and identification of target areas on the hematoxylin and eosin–stained slides were performed by a trained hematopathologist (D.J.). Using the hematoxylin and eosin-stained slide as a reference, target areas were etched with a diamond-tipped etcher on the back of the unstained slide to be assayed. Each probe set was then hybridized to the appropriate target areas.
For FISH performed on the bone marrow or the SBP tissue, a minimum of 50 nuclei were analyzed per probe set. Probe sites with <15 interphase nuclei were deemed insufficient for analysis. FISH analysis was performed by 2 qualified clinical cytogenetic technologists and interpreted by a board-certified clinical cytogeneticist. The level of detection required to identify abnormalities for individual probe sets was as follows: a minimum of 3 of 50 cells (6%) displaying fusion signals in the setting of dual fusion probes, a minimum of 5 of 50 cells (10%) with disrupted separated signals in the setting of break-apart probes, a minimum 5 of 50 cells (10%) for tetraploid clones, or a minimum of 10 of 50 cells (20%) for enumeration probes such as TP53 or 1q.
Statistical analysis
Descriptive statistics (mean, median, and range) were used to describe the study population. Statistical analysis was performed using the SAS biostatistical software JMP 16.0.1 (SAS Institute Inc, Cary, NC). Differences between subgroups were compared using the χ2 test or t test. A Kaplan-Meier analysis was used to analyze and create the TTP curves, and the log-rank test was used to compare these curves. Finally, a multivariable analysis was performed using the Cox proportions hazards model to assess the influence of various prognostic factors on TTP found to be significant in univariate analyses.
Results
Baseline characteristics of patients with SBP
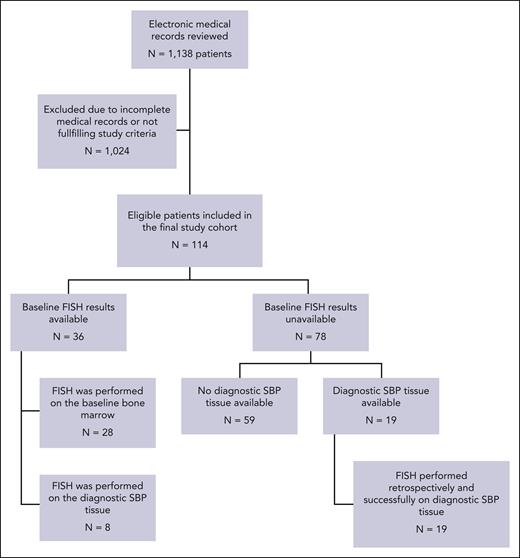
On review of the Mayo Clinic electronic medical record system, 1138 patients were identified as having an International Classification of Diseases code containing the term “plasmacytoma” in their diagnosis list. After review of their electronic health records, 114 patients fulfilled the study criteria and were included in the final study cohort (Figure 1). The baseline clinical and pathologic characteristics of this cohort are described in supplemental Table 1; available on the Blood website. The median age was 60 years (range, 28-83), and 20% of the cohort was aged ≥75 years. Most patients (85%) were white, and 66% of this cohort were male. Most patients (n = 100; 89%) had a positron emission tomography–CT performed at the time of diagnosis for staging purposes. The maximum standard uptake value (SUVmax) was available for 87 patients. The median SUVmax was 6.5 (interquartile range, 4.3-10.0), and 22 patients had a SBP with a SUVmax >10. All patients in the cohort received radiation therapy as part of their primary treatment for SBP. The radiation dose was known for 97 patients, with the median amount being 45 Gy (interquartile range, 39.8-50). Furthermore, 24 patients (21%) underwent partial or complete surgical resection of the SBP for diagnostic and palliative purposes before receiving radiation.
Consort diagram for the patients with SBP identified and included in this study.
Consort diagram for the patients with SBP identified and included in this study.
A total of 55 patients (48%) in this cohort had detectable clonal plasma cells (comprising <10% of bone marrow cellularity) in their staging bone marrow examination either by morphology of the bone marrow core sample or flow cytometry assessment of the bone marrow aspirate sample and hence were classified as SBP with minimal marrow involvement. However, next-generation flow (NGF) cytometry testing with a minimum sensitivity of (10−5) that is typically used for minimal residual disease evaluation was available only after 2018 and has since been routinely used on bone marrow samples for the assessment of clonal plasma cells. The actual median sensitivity of the NGF cytometry assay was 2.4 × 10–6.20 Flow cytometry assessments of bone marrow samples for clonal plasma cells before 2018 had a minimum sensitivity of 10–4, with the actual median sensitivity of 4.4 × 10–5.20 As a result, 27 patients (24%) in this cohort had their bone marrows assessed for minimal marrow involvement by NGF cytometry, of which 15 (56%) had detectable clonal plasma cells. Information on baseline cytogenetics by FISH was available for 36 patients, including 22 from the clonal plasma cells obtained from their bone marrow examination and 14 from the diagnostic biopsy of their SBP. Of these 36 patients, 15 had HR FISH (43%) and 21 did not have HR FISH (57%). Of the patients without baseline cytogenetics information, 19 patients had sufficient archived paraffin-embedded tissue from the diagnostic biopsy of their SBP available for use. An assessment for the presence of del(17p), t(14;16), t(4;14), t(14;20), and +1q (gain or amplification) by FISH was performed on these archived SBP samples. Of these 19 patient samples, 7 (37%) had HR FISH (del 17p: 4 patients; Gain 1q: 3 patients). Notably, out of the 19 patients in this subcohort, 11 patients went on to develop MM. Of these 11 patients who progressed, 2 patients had del 17p abnormalities detected in their diagnostic SBP tissue, which was also detectable in their staging bone marrow exams performed at the time of disease progression. After combining the initial 36 patients who had baseline cytogenetics by FISH already available with the additional 19 patients who had FISH testing performed retrospectively on their diagnostic SBP samples, there were a total of 55 patients (48%) in this entire cohort of patients who had baseline cytogenetics by FISH (28 performed on the plasma cells derived from the bone marrow and 27 performed on the diagnostic plasmacytoma). The clinical and pathologic characteristics of patients by FISH results are summarized in Table 1. More patients with HR FISH had minimal marrow involvement (n = 20; 91%) compared with patients without HR FISH (n = 21; 64%) (P = .029).
Baseline clinical and pathologic characteristics of all 55 patients with SBP who had cytogenetics by FISH available
| Characteristic . | High risk (n = 22; 40%) n (%) . | Standard risk (n = 33; 60%), n (%) . | P value . |
|---|---|---|---|
| Median age, y (range) | 62 (28-78) | 62 (29-83) | .72 |
| Male | 16 (73) | 22 (67) | 1.00 |
| White | 17 (77) | 31 (91) | .127 |
| Serum monoclonal protein detectable∗ | 21/21 (100) | 32/32 (100) | 1.00 |
| M spike (g/dL), median (range) | 0.75 (0.4-1.9) | 1 (0.4-1.8) | .436 |
| Abnormal FLC ratio† | 14 (64) | 18 (55) | .373 |
| Maximal SBP diameter of 5 cm or greater | 8/11 (73) | 8/24 (33) | .035 |
| Minimal marrow involvement present‡ | 20 (91) | 21 (64) | .029 |
| High-risk cytogenetic subtype§ | |||
| Del (17p) | 11 (50) | — | |
| Gain (1q) | 13 (59) | — | |
| t(4;14) | 2 (9) | — | |
| t(14;16) | 1 (5) | — | |
| t(14;20) | 0 | — |
| Characteristic . | High risk (n = 22; 40%) n (%) . | Standard risk (n = 33; 60%), n (%) . | P value . |
|---|---|---|---|
| Median age, y (range) | 62 (28-78) | 62 (29-83) | .72 |
| Male | 16 (73) | 22 (67) | 1.00 |
| White | 17 (77) | 31 (91) | .127 |
| Serum monoclonal protein detectable∗ | 21/21 (100) | 32/32 (100) | 1.00 |
| M spike (g/dL), median (range) | 0.75 (0.4-1.9) | 1 (0.4-1.8) | .436 |
| Abnormal FLC ratio† | 14 (64) | 18 (55) | .373 |
| Maximal SBP diameter of 5 cm or greater | 8/11 (73) | 8/24 (33) | .035 |
| Minimal marrow involvement present‡ | 20 (91) | 21 (64) | .029 |
| High-risk cytogenetic subtype§ | |||
| Del (17p) | 11 (50) | — | |
| Gain (1q) | 13 (59) | — | |
| t(4;14) | 2 (9) | — | |
| t(14;16) | 1 (5) | — | |
| t(14;20) | 0 | — |
The boldface P values are statistically significant at P < .05.
Detectable by either immunofixation or mass-fixation
Abnormal ratio defined as <0.26 or >1.65
Clonal plasma cells were considered positive if monoclonal plasma cells were detected by flow cytometry or immunohistochemistry on the bone marrow biopsy core or aspirate.
Some patients have multiple different high-risk cytogenetic subtypes and are counted in each category.
TTP of patients with SBP to MM
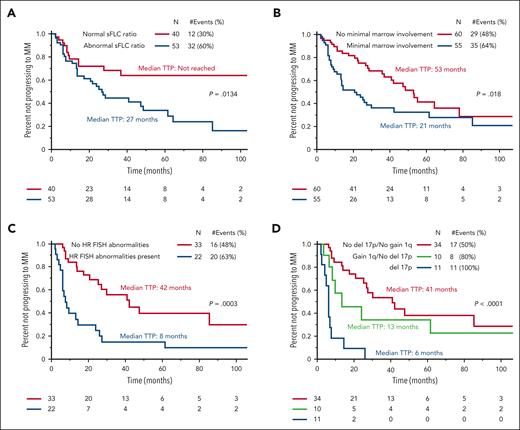
Of the entire cohort, 63 patients (55%) progressed to MM with a median TTP of 37 months (95% confidence interval [CI], 26-55). Details on the clinical symptoms at the time of progression to MM were available for 41 patients (65%), of whom almost all (n = 39, 91%) presented with new lytic bone lesions. In addition, of the 93 patients with baseline sFLC levels available, 53 (57%) had abnormal sFLC ratios at the time of diagnosis. In patients with abnormal sFLC ratios, 31 (58%) progressed to MM compared with 12 (30%) without abnormal sFLC ratios. The median TTP to MM for patients with abnormal sFLC ratios was 27 months (95% CI, 14-49) compared with those not reached (95% CI, 28-not reached [NR]) without abnormal sFLC ratios (P = .013) (Figure 2A). Furthermore, 55 patients (48%) had minimal marrow involvement at the time of diagnosis, of which 35 (64%) progressed to MM compared with 28 (48%) without marrow involvement. The median TTP to MM for patients with minimal marrow involvement was 21 months (95% CI, 13-30) compared with 53 months (95% CI, 37-78) for those without minimal marrow involvement (P = .018) (Figure 2B). Among patients whose maximal SBP diameter was measurable (n = 65), there was no statistically significant impact of increasing maximal diameter of the SBP at diagnosis and TTP to MM (P = .250). When using a cutoff of 5 cm, the median TTP to MM for SBPs that were ≥5 cm in maximal diameter (n = 29) was 41 months (95% CI, 15-62) compared with 64 months (95% CI, 23-NR) in patients with SBPs <5 cm in maximal diameter (n = 36) (P = .333).
Time to progression (TTP) to MM. (A) Kaplan-Meier curves comparing the TTP to MM for patients with SBP based on the presence of a normal or abnormal sFLC ratio. (B) Kaplan-Meier curves comparing the TTP to MM for patients with SBP based on the presence or absence of minimal marrow involvement. (C) Kaplan-Meier curves comparing the TTP to MM for patients with SBP based on the presence or absence of HR FISH abnormalities. (D) Kaplan-Meier curves comparing the TTP to MM for patients with SBP based on the presence or absence of gain 1q and del 17p cytogenetic abnormalities.
Time to progression (TTP) to MM. (A) Kaplan-Meier curves comparing the TTP to MM for patients with SBP based on the presence of a normal or abnormal sFLC ratio. (B) Kaplan-Meier curves comparing the TTP to MM for patients with SBP based on the presence or absence of minimal marrow involvement. (C) Kaplan-Meier curves comparing the TTP to MM for patients with SBP based on the presence or absence of HR FISH abnormalities. (D) Kaplan-Meier curves comparing the TTP to MM for patients with SBP based on the presence or absence of gain 1q and del 17p cytogenetic abnormalities.
Impact of FISH status on TTP for patients with SBP to MM
Out of the 55 patients with FISH results, 22 patients (40%) had HR FISH and 33 patients (60%) had SR FISH. Furthermore, 35 of these 55 patients with FISH results had SBPs with measurable maximal diameters, and those patients with SBP with HR FISH were more likely to have the maximal diameter of their SBP be ≥5 cm (8/11 or 73%) compared with those without HR FISH (8/24 or 33%) (P = .035). After a median follow-up of 54 months, more patients with HR FISH (n = 20; 91%) progressed to MM than patients without HR FISH (n = 16; 49%) (P = .0012). The median TTP to MM for patients with HR FISH was 8 months (95% CI, 6.3-26) compared with 42 months (95% CI, 25-NR) for patients with SR FISH (P = .0003) (Figure 2C). Of the patients with HR FISH, 59% progressed within 12 months of diagnosis compared with 15% of patients without HR FISH (P < .001), and 73% of patients with HR FISH progressed within 24 months of diagnosis compared with 27% of patients without HR FISH (P < .001). Upon assessments of individual HR FISH abnormalities, patients with a deletion 17p cytogenetic abnormality detected in their clonal plasma cell population either in the marrow or the SBP had a median TTP to MM of 6 months compared with 13 months for those patients with an isolated gain 1q detected without a concurrent deletion 17p abnormality and 41 months for those without a deletion 17p or gain 1q abnormality (P < .001) (Figure 2D). The impact of other HR translocations was not observed because only 2 patients had a t(4;14), 1 patient had a t(14;16), and no patients had a t(14;20). supplemental Figure 1 demonstrates the proportion of patients with and without HR FISH in their clonal plasma cells based on the presence or absence of either minimal marrow involvement or an abnormal sFLC ratio.
Finally, in a multivariate model including HR FISH, abnormal sFLC ratio at diagnosis, and minimal marrow involvement, only HR FISH was a significant predictor for shorter TTP to MM (Table 2). Given that availability of baseline cytogenetics by FISH is at times reliant on sufficient numbers of clonal plasma cells in the bone marrow aspirate, the impact of HR FISH based on the presence of minimal marrow involvement was assessed. Among patients with SBP and minimal marrow involvement whose FISH status was known (n = 41), the median TTP to MM for patients with HR FISH (n = 20) was 8 months (95% CI, 6-14) compared with 85 months (95% CI, 14-NR) in patients without HR FISH (n = 21) (P = .0006) (supplemental Figure 2A). When assessing only the patients with SBP who had their FISH cytogenetics performed on the diagnostic SBP tissue (n = 27), the median TTP to MM for patients with HR FISH was 14 months (95% CI, 2-26) compared with 42 months (95% CI, 21-NR) in patients without HR FISH (P = .035) (supplemental Figure 2B). Similarly, when assessing only the patients with SBP who had their FISH cytogenetics performed on the staging bone marrow sample (n = 28), the median TTP to MM for patients with HR FISH was 7 months (95% CI, 3-10) compared with 30 months (95% CI, 9-NR) for patients without HR FISH (P = .0024) (supplemental Figure 2C).
Univariable and multivariable analysis of factors at diagnosis of SBP that predict for shorter TTP to MM
| Variable . | TTP to MM . | |||
|---|---|---|---|---|
| Univariable . | Multivariable∗ . | |||
| Hazard ratio . | P value . | Hazard ratio . | P value . | |
| Minimal marrow involvement | 1.81 (1.10-2.99) | .020 | 0.72 (0.23-2.22) | .570 |
| Abnormal sFLC ratio | 2.26 (1.16-4.39) | .011 | 1.48 (0.63-3.52) | .370 |
| Presence of HR FISH cytogenetics | 3.23 (1.66-6.27) | .0006 | 5.88 (2.47-14.0) | <.001 |
| Variable . | TTP to MM . | |||
|---|---|---|---|---|
| Univariable . | Multivariable∗ . | |||
| Hazard ratio . | P value . | Hazard ratio . | P value . | |
| Minimal marrow involvement | 1.81 (1.10-2.99) | .020 | 0.72 (0.23-2.22) | .570 |
| Abnormal sFLC ratio | 2.26 (1.16-4.39) | .011 | 1.48 (0.63-3.52) | .370 |
| Presence of HR FISH cytogenetics | 3.23 (1.66-6.27) | .0006 | 5.88 (2.47-14.0) | <.001 |
The boldface P values are statistically significant at P < .05.
Multivariate model included 49 patients with 31 events.
Discussion
SBP is a relatively rare plasma cell malignancy with a greater than 60% to 80% risk of progression to MM over the next 10 years from diagnosis.3,5 Localized therapy with definitive radiation with or without the need for surgical resection followed by observation remains the current standard of care for patients with SBP, irrespective of the status of minimal marrow involvement. Several studies have demonstrated minimal marrow involvement as a possible risk factor for progression to MM. However, a limitation in some of these studies was the absence of advanced cross-sectional imaging, which could have missed the presence of other early lytic lesions that would have led to patients being categorized as MM.11 In this study, only patients who strictly fulfilled the International Myeloma Working Group definition of SBP with or without minimal marrow involvement were included in the final analysis, such that every patient had advanced cross-sectional imaging assessing their bones for additional lytic bone lesions. Nevertheless, in concordance with previously published data, about half of our patients progressed to MM after a median follow-up of 53 months, and the median TTP for those with minimal marrow involvement was similar to those of previously reported studies.12,13 Furthermore, the presence of an abnormal sFLC ratio at diagnosis, which has previously been implicated as a major risk factor for shorter TTP to MM,14 was also observed to be a risk factor in this study. The size of the SBP being ≥10 cm was found to be a risk factor for shorter TTP to MM in 1 study.17 In contrast, this study did not observe size as determined by the maximal diameter of the SBP as a risk factor for shorter TTP to MM when assessed as a continuous variable. There were only 5 patients whose maximal diameter of their SBPs was ≥10 cm, and thus a maximal diameter size of 5 cm was used to dichotomize the cohort. There was a numerically shorter TTP to MM in patients whose maximal diameter of the SBP was ≥5 cm compared with those with <5 cm (41 vs 64 months), but this was not statistically significant. However, SBPs with a maximal diameter of ≥5 cm were more likely to have HR FISH than SBPs with a maximal diameter <5 cm. It is well known that patients with MM with HR FISH abnormalities have a shorter duration of response to systemic therapy and worse overall survival than those without HR FISH.21 Furthermore, patients with smoldering MM with certain HR FISH abnormalities have a shorter TTP to MM.22-24 However, the impact of HR FISH abnormalities on the risk and TTP to MM has not been reported in SBP. Apart from being a rare entity, 1 potential reason for the lack of this information is that most patients with SBP do not have an adequate quantity of clonal plasma cells in their staging bone marrow aspirate sample to perform FISH studies reliably, and most do not go on to have FISH assessments performed on the diagnostic samples of their SBP. In this study, by performing FISH assessments on the SBP diagnostic tissue, an additional 27 patients could be provided with their baseline cytogenetic risk status. Furthermore, on multivariate analysis, HR FISH was a significant and independent factor for shorter TTP to MM, irrespective of minimal marrow involvement or an abnormal sFLC ratio at diagnosis. Specifically, the presence of deletion 17p cytogenetic abnormality in clonal plasma cells, either from the bone marrow or the diagnostic SBP tissue, was associated with the shortest median TTP to MM of 6 months. Although different studies evaluating the prognostic significance of gain 1q abnormalities in MM have produced divergent results,25-27 in this study, a gain 1q abnormality in SBP was associated with a significantly shorter TTP to MM.
The observations in this study, if validated in additional studies, raise 3 major implications for clinical practice. First, from a disease biology perspective, the study strongly demonstrates the role of HR FISH in being a major determinant in the progression of SBP to MM. It could also possibly reflect that patients with SBP who have HR FISH abnormalities either in the diagnostic SBP tissue or in the staging bone marrow are already “en route” to developing MM. One hypothesis could be that because MM is a heterogeneous disease with multiple competing subpopulations of clones, it is possible that a subpopulation harboring a HR cytogenetic abnormality like deletion 17p or gain 1q has a greater propensity to proliferate and progress into a symptomatic SBP in its neighboring anatomic vicinity. Furthermore, these same HR subpopulations could also be more likely to have already undergone hematogenous dispersion to other anatomical regions of the bone marrow, eventually developing more lytic bone lesions in those areas signifying their progression to active MM, as demonstrated in this study. This phenomenon could explain the higher incidence of minimal marrow involvement in patients with SBP with HR FISH in their clonal plasma cells. Seminal work by Rasche et al evaluated the spatiotemporal evolution of MM from baseline to relapse refractory and demonstrated a similar role of focal lesions serving as a nidus for tumor evolution and disease relapse.28 Second, from a clinical practice standpoint, it would suggest that hematology and oncology clinicians should work closely with their cytogeneticist and hematopathologist colleagues to request that FISH assessment for HR cytogenetic abnormalities be performed on the diagnostic tissue sample of a SBP in the event that there was not an adequate quantity of clonal plasma cells in the corresponding staging bone marrow aspirate to perform FISH. Lastly, because of the high risk of progression of >70% within 2 years of diagnosis, observation alone after definitive radiation therapy may not be an optimal management strategy in patients with SBP who have HR FISH abnormalities in any of their clonal plasma cells. With this respect, the use of adjuvant chemotherapy in managing plasmacytomas has shown mixed results.29-31 Ascione et al in their recently published retrospective analysis of 77 patients, showed adjuvant systemic therapy benefited patients at high risk of progression.29 However, none of these studies isolated the impact of adjuvant systemic therapy based on cytogenetic status. Thus, there is insufficient prospective data assessing the role of adjuvant systemic therapy in SBP with HR FISH after radiation therapy. The US Cooperative Clinical Trial groups led by the ALLIANCE group conducted a randomized trial of adjuvant systemic treatment after definitive radiation in patients with solitary plasmacytoma of the bone with HR features (NCT02516423). Patients were randomized to zoledronate alone or lenalidomide, ixazomib, and dexamethasone with zoledronate for 6 months after definitive radiation. The trial was closed prematurely in 2018 owing to poor accrual. There are currently 2 active trials looking at adjuvant chemotherapy in SBP. One trial randomizes patients with SBP between lenalidomide and dexamethasone for up to 9 cycles vs observation after definitive radiation (NCT02544308). Another pilot trial will evaluate patients with SBP with minimal marrow involvement to lenalidomide with oral azacytidine (CC-486) for 6 cycles after definitive radiation therapy (NCT04174196).
Major limitations in our study include that it was retrospective in design from a single institution, and although this is one of the largest cohorts of SBP studied to date, not all patients had baseline FISH cytogenetics available and an even smaller proportion of patients had their staging bone marrows evaluated by NGF cytometry techniques for minimal marrow involvement. Thus, the next important step that needs to occur is a collaborative effort by multiple centers to either combine data sets of adequately staged patients with SBP who also have FISH status at diagnosis or consider performing FISH assessments retrospectively on clinical residual SBP tissue to determine if the presence of HR FISH holds similar prognostic value as demonstrated in this study. Nevertheless, to our knowledge, this study is the first to shed light on the clinical value of cytogenetic assessments by FISH in patients with SBP. The presence of HR FISH significantly increases the risk of progression to MM. Thus, it supports the performance of FISH assessments on the diagnostic SBP tissue samples in those patients whose bone marrow aspirates do not have sufficient numbers of clonal plasma cells to perform the same FISH assessment. In the future, from a biological perspective, studies evaluating the genomic profiles of clonal plasma cells in the SBP from the diagnostic tissue and their subsequent paired clonal plasma cells from the bone marrow at the time of their progression to MM will be important to determine if there is a “spontaneous evolution” or “static” model of progression.32 Furthermore, prospective data and clinical trials are needed to address if patients with SBP who have HR FISH could benefit from incorporating systemic adjuvant therapy after definitive radiation therapy to reduce their high risk of rapid progression to MM.
Acknowledgments
Research reported in this publication was supported by Mayo Clinic Hematological Malignancies Program and in part by grants from National Institutes of Health, National Cancer Institute under award number R01CA254961 and P50CA186781. This research is also supported in part by the Marion Schwartz Foundation for Multiple Myeloma.
The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
Authorship
Contribution: U.Y. and W.I.G. designed the research plan, analyzed the data, and wrote the manuscript; and S.K.K., L.B.B., A.D., P.G., R.K., D.J., F.K.B., D.D., M.Q.L., R.F., P.L.B., S.A., V.R., R.P., T.S., S.R.H., P.K., N.L., J.C., M.B., E.M., R.W., T.V.K., R.S.G., Y.L., A.S., S.C.L., W.G.B., R.A.K., M.A.G., and S.V.R. critically reviewed and edited the final version of this manuscript.
Conflict-of-interest disclosure: R.F. reports consulting for AbbVie, Adaptive Biotechnologies, AMGEN, AstraZeneca, Bayer, Binding Site, Bristol Myers Squibb (Celgene), Millenium Takeda, Janssen, Juno, Kite, Merck, Pfizer, Pharmacyclics, Regeneron, and Sanofi; is a member of scientific advisory boards for Adaptive Biotechnologies, Caris Life Sciences, and ONCOtracker; is a member of board of directors for Antengene (for profit) and AZBio (not for profit); and has a patent for fluorescence in situ hybridization in multiple myeloma receiving ∼$2000 per year. The remaining authors declare no competing financial interests.
Correspondence: Wilson I. Gonsalves, Division of Hematology, Mayo Clinic, 200 First St SW, Rochester, MN 55905; e-mail: gonsalves.wilson@mayo.edu.
References
Author notes
Data are available on request from the corresponding author, Wilson I. Gonsalves (gonsalves.wilson@mayo.edu).
The online version of this article contains a data supplement.
There is a Blood Commentary on this article in this issue.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal