In this issue of Blood, Yadav et al provide the first evidence for the prognostic role of myeloma high-risk chromosomal abnormalities in solitary bone plasmacytoma (SBP).1
Currently, SBP is considered a low-risk disease condition, and radiation therapy is curative for roughly one-half of the patients. However, Yadav et al convincingly show that this paradigm does not hold true anymore. Despite radiation therapy, patients presenting with at least 1 of the high-risk markers +1q and del(17p) have a median time to progression to symptomatic myeloma of only 8 months, demonstrating that there is a group of patients who need systemic treatment right away to prevent further end organ damage. Thus, high-risk cytogenetics is a potential biomarker for clinical decision making in SBP. It is fascinating that once again the molecular makeup of clonal plasma cells dictates the course of the disease. This is in line with the recent finding that patients with poor-prognosis myeloma can present with high-risk disease markers in focal lesions only, highlighting that high-risk disease drives prognosis, even if distributed heterogeneously in the bone marrow.2
The low and locally restricted tumor growth in SBP is a major challenge for routine diagnostics, which are mainly based on tumor samples derived from the iliac crest. To compensate for this, the authors performed fluorescence in situ hybridization (FISH) on the diagnostic paraffin-embedded tissue from the SBP in patients without sufficient systemic infiltration. The authors doubled the number of evaluable patients by using this approach. However, for approximately one-half of the study cohort, no tissue was available for FISH. In the future, a potential solution could be a noninvasive imaging-based strategy to uncover high-risk genetic abnormalities in the entire bone marrow cavity, a field of science recently labeled “radiogenomics.” Indeed, in this study, patients with high-risk cytogenetics were more likely to have large plasmacytomas with a maximal diameter ≥5 cm (8 of 11 high-risk patients compared with 8 of 24 standard-risk patients). These results mirror our own observation in systemic multiple myeloma (MM) in which spatial heterogeneity was linked to large focal lesions.2,3 Although lesion size is a prognostic factor in myeloma, larger patient cohorts are required to evaluate whether the same holds true for SBP. Other promising techniques include detection of circulating tumor cells or tumor DNA,4 but to the best of our knowledge, there are no data yet to test whether patients with SBP at high risk of early progression can be identified with these 2 methods.
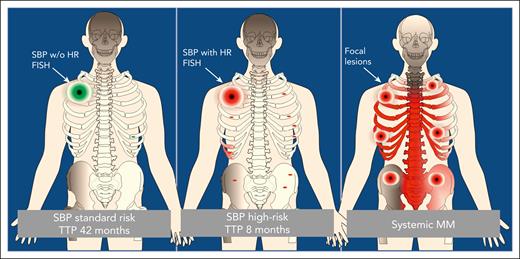
Besides its clear clinical impact, the study by Yadav et al also raises new questions regarding the pathogenesis of plasma cell dyscrasias. In current models, the evolution of symptomatic myeloma from its precursors monoclonal gammopathy of undetermined significance and smoldering myeloma is driven by the accumulation of driver aberrations and a rapid dissemination of aggressive subclones in the bone marrow. Yet a single lesion with a minimal or even absent diffuse infiltration defines SBP. Although focal lesions in MM are usually superimposed on diffuse infiltration patterns, both entities rather support a model that includes regional evolution. In SPB it seems that some clones can grow locally but have not gained the ability to disseminate, whereas high-risk clones disseminate early, causing systemic MM in the short run (see figure). This hypothesis is supported by the current study in which the authors reported the same cytogenetics between initial plasmacytomas and systemic progression, suggesting a phylogenetic relationship. We have seen a similar relationship between focal lesions at diagnosis and relapse in systemic MM.5 The idea of myeloma as a metastatic disease is not new,6 and it seems that subclones can differ in their potential to invade distant bone marrow sites. However, the exact molecular mechanisms underlying focal tumor growth and dissemination remain unclear. Here, SBP could serve as a promising model disease.
Dissemination of clonal plasma cells in solitary bone plasmacytoma and MM. In MM, focal lesions are usually superimposed on diffuse infiltration patterns, whereas in SBP the potential for invasion and dissemination at distant bone marrow sites and, as a consequence, the time to progression (TTP) are associated with high-risk (HR) cytogenetics.
Dissemination of clonal plasma cells in solitary bone plasmacytoma and MM. In MM, focal lesions are usually superimposed on diffuse infiltration patterns, whereas in SBP the potential for invasion and dissemination at distant bone marrow sites and, as a consequence, the time to progression (TTP) are associated with high-risk (HR) cytogenetics.
Another question for future studies will be whether local radiation therapy can still be considered a curative approach for patients with SBP without high-risk cytogenetics. Most of them still progress to myeloma after years of observation, suggesting that even in patients without a detectable minimal diffuse infiltration, clonal plasma cells have already disseminated but have very low proliferation rates. Last but not least, there are no data yet that indicate there is a long-term negative impact of radiation in patients with SBP, but ionizing radiation certainly has mutagenic potential.7
Together, the data reported by Yadav et al could impact clinical decision making in SBP. We hope that future studies will successfully address remaining open questions, including a solution for patients with insufficient diagnostic material and evaluating the role of high-risk cytogenetics in the potential to invade distant bone marrow sites.
Conflict-of-interest disclosure: The authors declare no competing financial interests.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal