Key Points
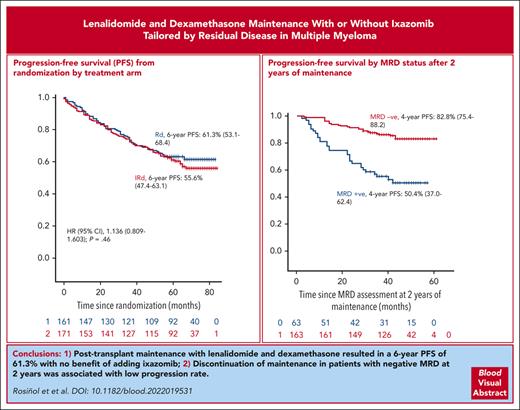
Postransplant maintenance with lenalidomide and dexamethasone resulted in a 6-year PFS of 61.3%, with no benefit of adding ixazomib.
Discontinuation of maintenance in patients with negative MRD (2 × 10−6) at 2 years was associated with a low progression rate.
Abstract
From November 2014 to May 2017, 332 patients homogeneously treated with bortezomib, lenalidomide, and dexamethasone (VRD) induction, autologous stem cell transplant, and VRD consolidation were randomly assigned to receive maintenance therapy with lenalidomide and dexamethasone (RD; 161 patients) vs RD plus ixazomib (IRD; 171 patients). RD consisted of lenalidomide 15 mg/d from days 1 to 21 plus dexamethasone 20 mg/d on days 1 to 4 and 9 to 12 at 4-week intervals, whereas in the IRD arm, oral ixazomib at a dose of 4 mg on days 1, 8, and 15 was added. Therapy for patients with negative measurable residual disease (MRD) after 24 cycles was discontinued, whereas those who tested positive for MRD remained on maintenance with RD for 36 more cycles. After a median follow-up of 69 months from the initiation of maintenance, the progression-free survival (PFS) was similar in both arms, with a 6-year PFS rate of 61.3% and 55.6% for RD and IRD, respectively (hazard ratio, 1.136; 95% confidence interval, 0.809-1.603). After 2 years of maintenance, treatment was discontinued in 163 patients with negative MRD, whereas 63 patients with positive MRD continued with RD therapy. Maintenance discontinuation in patients tested negative for MRD resulted in a low progression rate (17.2% at 4 years), even in patients with high-risk features. In summary, our results show the efficacy of RD maintenance and support the safety of maintenance therapy discontinuation in patients with negative MRD at 2 years. This trial was registered at www.clinicaltrials.gov as #NCT02406144 and at EudraCT as 2014-00055410.
Introduction
High-dose melphalan followed by autologous stem cell transplant (ASCT) remains the standard of care for younger patients with newly diagnosed multiple myeloma (MM). Posttransplant maintenance therapy in order to delay or prevent disease progression has been explored with controversial results. The immunomodulatory drug lenalidomide has been investigated in 4 randomized trials,1-4 showing a highly significant prolongation of progression-free survival (PFS; 18-27 months benefit), and a meta-analysis of 3 of them demonstrated a significant overall survival (OS) benefit.5 These results lead to the approval of lenalidomide for post-ASCT maintenance in myeloma by the regulatory agencies. It has been reported that in patients with relapsed/refractory myeloma failing to respond to lenalidomide, the addition of dexamethasone results in a substantial increase in the response rate.6,7 The synergy between lenalidomide and dexamethasone is further supported by in vitro studies showing that the addition of dexamethasone enhances the antiproliferative effect of lenalidomide.8,9 With this background, dexamethasone was added to the 2 arms of our trial. The proteasome inhibitor bortezomib as a posttransplant maintenance was investigated in a HOVON study with positive results.10 We also showed in a PETHEMA/GEM study that bortezomib plus thalidomide resulted in a significantly longer PFS than single-agent thalidomide or alfa2b-interferon (α2b-IFN).11 Ixazomib, an oral proteasome inhibitor, was also found to significantly prolong the PFS, with a 6-month benefit, when compared with placebo in the post-ASCT maintenance setting.12 We report, here, the results of maintenance therapy with lenalidomide and dexamethasone (RD) vs lenalidomide and dexamethasone plus ixazomib (IRD) as posttransplant maintenance therapy in patients previously included in the GEM2012 study.13
Methods
Eligibility
Patients aged ≤65 years with newly diagnosed MM who were included in the GEM12MENOS65 trial14 and were at least in minimal response entered this maintenance study. All patients received induction with bortezomib, lenalidomide, dexamethasone-Grupo Español de Mieloma (VRD-GEM), consisting of subcutaneous bortezomib, on days 1, 4, 8, and 11 of each cycle; lenalidomide 25 mg/d on days 1 to 21; and dexamethasone 40 mg on days 1 to 4 and 9 to 12 at 4-week intervals for 6 cycles. Patients received high-dose therapy with melphalan 200 mg/m2 (MEL-200) vs IV busulfan at 9.6 mg/kg plus melphalan 140 mg/m2 (BuMel), followed by consolidation with 2 additional cycles of VRD-GEM.13,14 The study was approved by the Spanish National Health Agency and the local institutional ethics committees and was conducted in accordance with the Declaration of Helsinki. All patients gave written informed consent. This trial is registered at clinicaltrials.gov (NCT02406144) and EudraCT (2014-00055410)
Study design and end points
Patients were randomly assigned to receive RD vs IRD. To ensure the balance of RD and IRD arms with respect to the ASCT conditioning regimens (Mel-200 vs BuMel) used in the previous GEM12MENOS65 trial, patients were randomly assigned at diagnosis with an open-label 2 × 2 factorial design and 1:1:1:1 allocation ratio. RD consisted of lenalidomide 15 mg daily for 21 days with 1-week rest at 4-week intervals plus oral dexamethasone 20 mg on days 1 to 4 and 9 to 12 of each cycle for 24 cycles. IRD consisted of the same RD regimen plus oral ixazomib at 4 mg/d on days 1, 8, and 15 also for 24 cycles. Measurable residual disease (MRD) was annually assessed after starting maintenance therapy. In patients who had an MRD-negative result after 24 cycles, the maintenance therapy was discontinued, whereas those who had an MRD-positive result remained on maintenance therapy with lenalidomide 15 mg/d for 21 days and dexamethasone 20 mg on days 1 to 4 at 4-week intervals for 36 more cycles. The primary end point was PFS, and the secondary end points were MRD-negative rate, OS, and safety.
Dose adjustments
Grading of adverse events was as per the National Cancer Institute Common Terminology Criteria for Adverse Events (version 4.03). On day 1 of each cycle, the patient should have had a neutrophil count ≥ 1 × 109/L, a platelet count ≥ 75 × 109/L, and all nonhematological toxicities returned to grade ≤1 or to the baseline level of the patient. If recovery from hematological or nonhematological toxicity was not observed after a 4-week delay, the drug suspected to be directly related to the toxicity was withheld, and the patient was able to continue in the trial with the remaining drugs. According to the toxicity, the dose adjustment levels for lenalidomide were 15 mg, 10 mg, and 5 mg; for dexamethasone, 20 mg, 10 mg, and 5 mg; and for ixazomib, 4 mg, 3 mg, and 2.3 mg.
Response and MRD assessments
Response and progression were assessed according to the International Myeloma Working Group criteria.15 Samples for MRD assessment were collected regardless of response status before starting maintenance and annually thereafter. MRD assessments were performed in the 3 referral PETHEMA/GEM laboratories, and data were centralized for MRD analyses. MRD was assessed using next-generation flow cytometry, following the EuroFlow standard operation procedures.16 In accordance with its limit of detection (ie, 2 × 10−6), patient statuses were considered as negative or positive if MRD levels were <2 × 10−6 or ≥2 × 10−6, respectively.
Fluorescence in situ hybridization studies
Bone marrow plasma cells were isolated with anti-CD138–coated magnetic beads using the AutoMACs automated separation system (Miltenyi Biotec, Auburn, CA). Interphase fluorescence in situ hybridization analysis was performed with specific probes (Abbott Molecular/Vysis, Des Plaines, IL) for 17p deletions and immunoglobulin heavy chain translocations including t(11;14), t (4;14), and t(14;16), as previously described.17 All cytogenetic studies were centrally performed at the 3 PETHEMA/GEM referral laboratories. The cutoff level for considering a positive result was set at 10%.
Statistical analysis
The primary objective was the achievement of 1-year PFS prolongation by the addition of ixazomib to RD. The planned number of patients to be included in the GEM12MENOS65 was 460. Assuming a patient loss of 33.5% (based on our previous post-ASCT maintenance trial),11 316 patients would enter the maintenance trial. This sample size would be enough to observe the expected difference in PFS with a statistical power of 80% using 1-sided likelihood ratio test. Finally, 332 patients entered the maintenance phase. Efficacy analyses were based on an intention-to-treat basis. PFS was calculated from the time of initiation of maintenance therapy to the date of progression or death from any cause. Survival curves were plotted according to the method of Kaplan and Meier18 and statistically compared by means of the log-rank test.19 Statistical analyses were conducted using SAS version 9.4 (SAS Institute Inc).
Results
Baseline characteristics
Between 24 November 2014 and 18 May 2017, 332 patients were included in the study. One hundred sixty-one were allocated to the RD arm and 171 to the IRD arm. The characteristics of the patients at the time of diagnosis including the M-protein type, international staging system (ISS) status, proportion of patients with high-risk cytogenetics, presence of soft-tissues plasmacytomas, and the depth of response at the time of maintenance initiation were well balanced between the 2 arms (Table 1). Regarding cytogenetics, t(4;14) was found in 16% of the patients (RD, 13.9%; IRD, 18.1%), t(14;16) in 5.7% (RD, 6%; IRD, 4.8%), and 17p deletion in 7.4% (RD, 8.2%; IRD, 6.7%). Deletion of 1p was found in 8% of the patients (RD, 10.6%; IRD, 5.5%). Furthermore, 1q gains was found in 39.7% of the patients (RD, 40%; IRD, 39%). Double hit, including chromosome 1 abnormalities, were present in 22.7% of the patients (RD and IRD, 11.3% each). Plasmacytomas were present at diagnosis in 67 patients (64 paraskeletal and 3 extramedullary).
Baseline characteristics according to treatment arm
| . | Overall N = 332 . | RD n = 161 . | IRD n = 171 . |
|---|---|---|---|
| Age, median (range), y | 58 (32-67) | 58 (36-67) | 59 (32-67) |
| Male sex, n (%) | 181 (54%) | 94 (58%) | 87 (51%) |
| ISS (%) | |||
| I | 136 (40.9%) | 69 (42.8%) | 67 (39.1%) |
| II | 116 (34.9%) | 49 (30.459%) | 67 (39.1%) |
| III | 75 (22.5%) | 39 (24.259%) | 36 (21%) |
| High-risk cytogenetics∗, n (%) | 64 (22.5%) | 33 (24.6%) | 31 (20.6%) |
| Plasmacytomas†, n (%) | 67 (20.1%) | 30 (18.6%) | 37 (21.6%) |
| Depth of response | |||
| sCR/CR, n (%) | 231 (69.5%) | 105 (65%) | 126 (73%) |
| Negative MRD‡, n (%) | 184 (55.4%) | 82 (50.9%) | 101 (59.6%) |
| . | Overall N = 332 . | RD n = 161 . | IRD n = 171 . |
|---|---|---|---|
| Age, median (range), y | 58 (32-67) | 58 (36-67) | 59 (32-67) |
| Male sex, n (%) | 181 (54%) | 94 (58%) | 87 (51%) |
| ISS (%) | |||
| I | 136 (40.9%) | 69 (42.8%) | 67 (39.1%) |
| II | 116 (34.9%) | 49 (30.459%) | 67 (39.1%) |
| III | 75 (22.5%) | 39 (24.259%) | 36 (21%) |
| High-risk cytogenetics∗, n (%) | 64 (22.5%) | 33 (24.6%) | 31 (20.6%) |
| Plasmacytomas†, n (%) | 67 (20.1%) | 30 (18.6%) | 37 (21.6%) |
| Depth of response | |||
| sCR/CR, n (%) | 231 (69.5%) | 105 (65%) | 126 (73%) |
| Negative MRD‡, n (%) | 184 (55.4%) | 82 (50.9%) | 101 (59.6%) |
CR, complete response; sCR, stringent complete response.
t(4;14), t(16;16), and del 17p.
Paraskeletal and extramedullary.
In the overall series.
Response upgrade during maintenance therapy
At the time of initiation of maintenance, all patients were at least in partial response (PR). Overall, a response improvement during maintenance was observed in 27% of the patients. The number of patients whose quality of response improved after a median of 12.1 months in the RD arm was 49 (30.4%). The number of patients whose response upgraded after a median of 10.2 months in the IRD arm was 42 (24.5%). The complete response rate increased from 65% to 84.4% (19.4%) with RD maintenance and from 73% to 89.4% (16.4%) with IRD.
At the time of initiation of maintenance, 184 patients (55.4%) tested negative for MRD and 148 (44.6%) tested positive for MRD. The upgrade in MRD status from positive to negative in the first 2 years of maintenance in patients with samples for MRD measurements is shown in Table 2. After 1 year of maintenance, 70.2% and 74.8% of patients in RD and IRD arms, resoectively, had undetectable MRD. After 2 years, 71.8% and 72.4% in RD and IRD arms, respectively, had undetectable MRD. Thus, at 2 years of maintenance, the MRD negativity increased from 50.9% to 71.8% (20.9%) in the RD arm and from 59.3% to 72.4% (12.8%) in the IRD arm (Table 2).
Upgrade of MRD in the first 2 years of maintenance (in patients with available samples for MRD analysis)
| . | At screening . | ||
|---|---|---|---|
| Overall series N = 332 . | RD n = 161 . | IRD n = 171 . | |
| MRD negative | 184 (55.4%) | 82 (50.9%) | 102 (59.6%) |
| At 1 y of maintenance | |||
| Overall series | RD | IRD | |
| N = 252 | n = 121 | n = 131 | |
| MRD negative | 192 (72.6%) | 85 (70.2%) | 98 (74.8%) |
| At 2 y of maintenance | |||
| Overall series | RD | IRD | |
| N = 226 | n = 110 | n = 116 | |
| MRD negative | 163 (72.1%) | 79 (71.8%) | 84 (72.4%) |
| Upgrade of MRD negative at 2 y of maintenance | |||
| Overall series | RD | IRD | |
| 16.7% | 20.9% | 12.8% | |
| . | At screening . | ||
|---|---|---|---|
| Overall series N = 332 . | RD n = 161 . | IRD n = 171 . | |
| MRD negative | 184 (55.4%) | 82 (50.9%) | 102 (59.6%) |
| At 1 y of maintenance | |||
| Overall series | RD | IRD | |
| N = 252 | n = 121 | n = 131 | |
| MRD negative | 192 (72.6%) | 85 (70.2%) | 98 (74.8%) |
| At 2 y of maintenance | |||
| Overall series | RD | IRD | |
| N = 226 | n = 110 | n = 116 | |
| MRD negative | 163 (72.1%) | 79 (71.8%) | 84 (72.4%) |
| Upgrade of MRD negative at 2 y of maintenance | |||
| Overall series | RD | IRD | |
| 16.7% | 20.9% | 12.8% | |
Survival outcomes
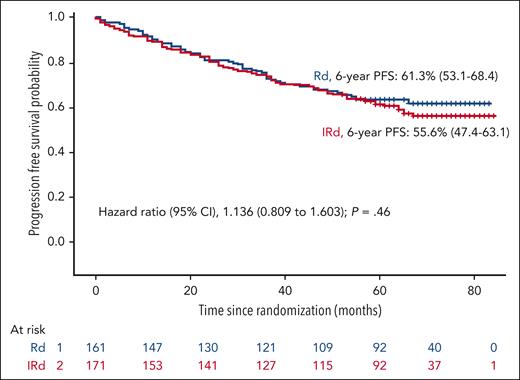
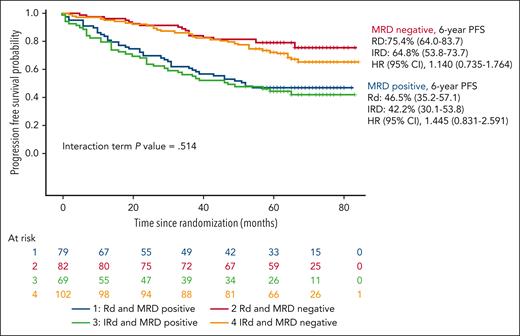
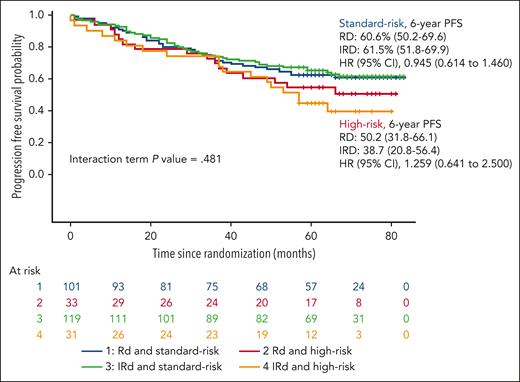
After a median follow-up of 69 months from the initiation of maintenance, the PFS was similar in both arms, the median was not reached in either arm, with a hazard ratio (HR) 1.136 (95% confidence interval [CI], 0.809-1.603; P = .460), and the 6-year PFS rate was 61.3% and 55.6% for the RD and the IRD arms, respectively (Figure 1). The PFS in patients who tested MRD negative at screening for maintenance was also similar in both arms with medians not reached and a PFS rate at 6 years of 75.4% and 64.8% for the RD and the IRD arms, respectively (Figure 2). For patients who tested positive for MRD at baseline, the median PFSs were 52 months for RD and 47 months for IRD, with a 6-year PFS rate of 46.5% and 42.2% for RD and IRD, respectively (Figure 2). Patients with standard cytogenetic risk had a PFS rates at 6 years of 60.6% and 61.5% for RD and IRD arms, respectively (HR, 0.9745; 95% CI, 0.614-1.460; Figure 3). Patients with high-risk cytogenetics also had similar PFS irrespective of the treatment arm (6-year PFSs of 50.2% with RD and 38.7% with IRD; HR, 1.259; 95% CI, 0.641-2.500; Figure 3). No significant differences in the PFS between RD and IRD were observed in patients with t(4;14), t(14,16), del 17p, 1q gains, double hit, revised-ISS (R-ISS) 1, R-ISS 2, and R-ISS 3. The OS was superimposable in both arms. The outcome of patients who required ixazomib dose reductions was similar to that of those who received the planned doses of ixazomib.
PFS according to MRD status at randomization based on the treatment arm.
Overall, 106 patients discontinued therapy before cycle 24 because of progressive disease (n = 48), toxicity (n = 27), withdrawal of consent (n = 14), investigator’s decision (n = 11), and death (n = 6). After 24 cycles of maintenance therapy, treatment was discontinued in 163 patients who had negative MRD results, whereas 63 patients who had positive MRD results continued maintenance with RD for 3 more years. The PFS was significantly longer in favor of patients with negative MRD results compared with those with positive MRD results despite the treatment discontinuation, with a PFS rate at 4 years of 82.8% vs 50.4%, respectively (HR, 0.253; 95% CI, 0.149-0.431; P < .0001; Figure 4). Only 25 patients who tested negative for MRD at 2 years relapsed beyond 2 years of maintenance discontinuation. Of interest, among these 25 patients, only 7 had high-risk cytogenetics, including 4 patients who had isolated t(4;14) and 3 patients who had 17p deletions plus t(4;14) and/or chromosome 1 abnormalities.
PFS based on the MRD status after 2 years of maintenance in the overall series.
PFS based on the MRD status after 2 years of maintenance in the overall series.
Impact of MRD kinetics on survival
As summarized in Table 3, 53.9% of the patients had sustained negative MRD status (RD, 49%; IRD, 58.6%); 7% had an MRD status that converted from negative to positive (RD, 6.3%; IRD, 7.7%); 18.1% had an MRD status that converted from positive to negative (RD, 22.7%; IRD, 13.7%); and 20.7% remained with positive MRD status (RD, 21.8%; IRD, 19.8%).
Kinetics of MRD (from randomization to 2 years of maintenance)
| MRD at randomization/at 2 y . | Overall series N = 226∗ . | Rd n = 110∗ . | IRd n = 116∗ . |
|---|---|---|---|
| Neg/neg | 122 (53.9%) | 54 (49%) | 68 (58.6%) |
| Neg/pos | 16 (7%) | 7 (6.3%) | 9 (7.7%) |
| Pos/neg | 41 (18.1%) | 25 (22.7%) | 16 (13.7%) |
| Pos/pos | 47 (20.7%) | 24 (21.8%) | 23 (19.8%) |
| MRD at randomization/at 2 y . | Overall series N = 226∗ . | Rd n = 110∗ . | IRd n = 116∗ . |
|---|---|---|---|
| Neg/neg | 122 (53.9%) | 54 (49%) | 68 (58.6%) |
| Neg/pos | 16 (7%) | 7 (6.3%) | 9 (7.7%) |
| Pos/neg | 41 (18.1%) | 25 (22.7%) | 16 (13.7%) |
| Pos/pos | 47 (20.7%) | 24 (21.8%) | 23 (19.8%) |
Neg, negative; pos, positive.
Patients with available samples for MRD measurement at 2 years.
Patients who had an MRD status that converted from positive to negative MRD had similar PFS compared with patients with sustained negative MRD status from baseline. In contrast, patients who had an MRD status that converted from negative to positive had worse prognosis. The PFS according to the kinetics of MRD is shown in Figure 5.
PFS according to the kinetics of MRD from ramdomization to 2 years of maintenance in the overall series.
PFS according to the kinetics of MRD from ramdomization to 2 years of maintenance in the overall series.
The PFS rates at 4 years of maintenance discontinuation among 119 patients who had MRD-negative status at baseline and at 2 years (2 years-sustained), 31 patients who had positive status at baseline and negative at 1 and 2 years (1 year-sustained), and 8 patients who had positive status at baseline and at 1 year but negative status at 2 years were similar, at 86%, 83%, and 87%, respectively. There were no differences according to the treatment arm. Patients with persistent positive MRD status or those whose condition converted from negative to positive after 1 year of maintenance had similar outcomes (PFS at 3 years, 63% and 60%, respectively), whereas patients who tested negative at baseline and 1 year but whose converted to positive at 2 years had a median PFS of only 10 months. There were no differences between the 2 arms.
Safety
Grade 3 and 4 toxicities as well as dose reductions and discontinuations according to each treatment arm are shown in Table 4. Concerning grade 3 to 4 hematological toxicity, there was no significant difference between RD and IRD in terms of neutropenia, whereas the incidence of thrombocytopenia was significantly higher with IRD (16.3% vs 7.4%; P = .011). The incidence of cutaneous toxicity (1.8% with RD vs 4% with IRD), fatigue (6.8% vs 6.4%), and deep vein thrombosis (1.8% vs 2.9%) was similar in both arms, whereas the incidence of grade 3 to 4 gastrointestinal toxicity was significantly higher with IRD (15.7% vs 2.4%; P < .0001). Grade 3 to 4 infections were similar in both arms. Grades 3 and 4 dexamethasone-related toxicity consisted of irritability or anxiety (8%), insomnia (6%), fatigue (4%), Cushing syndrome symptoms (3%), myopathy (2%), cataract (2%), and edema or hyperglycemia (1% each). In the IRD arm, the need for dose reductions were as follows: ixazomib, 29.2%; lenalidomide, 29.2%; and dexamethasone, 20.4%. In the RD arm, 20.4% and 23.6% required dose reductions of lenalidomide and dexamethasone, respectively. In the IRD arm, 10.5% of the patients required ixazomib discontinuation, 4.6% required dexamethasone discontinuation, and only 2 required lenalidomide discontinuation, whereas in the RD arm, 2 patients discontinued receiving lenalidomide and 8% discontinued dexamethasone. The cumulative doses of each drug were similar in both arms. Thus, in the RD arm, patients received 79% and 72.5% of the planned doses of lenalidomide and dexamethasone, respectively, compared with 74% and 73% in the IRD arm. Regarding ixazomib, patients received 71% of the planned doses. The cumulative vs planned doses of each drug are shown is Table 5. Twenty-seven patients (8.1%) developed a second primary malignancy from the time of randomization, with no differences between the 2 maintenance arms (RD, 14 patients; IRD, 13 patients; Table 6).
Grade 3 and 4 toxicities, dose reductions, and drug discontinuations in each treatment arm
| . | RD . | IRD . | P value . |
|---|---|---|---|
| n = 161 . | n = 171 . | ||
| Toxicity | |||
| Hematological | |||
| Neutropenia | 64 (39.7%) | 64 (37.4%) | NS |
| Thrombocytopenia | 12 (7.4%) | 28 (16.3%) | .011 |
| Nonhematological | |||
| Infections | 34 (21%) | 49 (28%) | NS |
| Skin | 3 (1.8%) | 7 (4%) | NS |
| Gastrointestinal | 4 (2.4%) | 27 (15.7%) | < .0001 |
| Fatigue | 11 (6.8%) | 11 (6.4%) | NS |
| Deep vein thrombosis | 3 (1.8%) | 5 (2.9%) | NS |
| Dose reductions | |||
| Ixazomib | - | 50 (29.2%) | - |
| Lenalidomide | 33 (20.4%) | 50 (29.2%) | .06 |
| Dexamethasone | 38 (23.6%) | 35 (20.4%) | NS |
| Drug discontinuations | |||
| Ixazomib | - | 18 (10.5%) | - |
| Lenalidomide | 2 (1.2%) | 2 (1.1%) | NS |
| Dexamethasone | 14 (8.6%) | 8 (4.6%) | NS |
| . | RD . | IRD . | P value . |
|---|---|---|---|
| n = 161 . | n = 171 . | ||
| Toxicity | |||
| Hematological | |||
| Neutropenia | 64 (39.7%) | 64 (37.4%) | NS |
| Thrombocytopenia | 12 (7.4%) | 28 (16.3%) | .011 |
| Nonhematological | |||
| Infections | 34 (21%) | 49 (28%) | NS |
| Skin | 3 (1.8%) | 7 (4%) | NS |
| Gastrointestinal | 4 (2.4%) | 27 (15.7%) | < .0001 |
| Fatigue | 11 (6.8%) | 11 (6.4%) | NS |
| Deep vein thrombosis | 3 (1.8%) | 5 (2.9%) | NS |
| Dose reductions | |||
| Ixazomib | - | 50 (29.2%) | - |
| Lenalidomide | 33 (20.4%) | 50 (29.2%) | .06 |
| Dexamethasone | 38 (23.6%) | 35 (20.4%) | NS |
| Drug discontinuations | |||
| Ixazomib | - | 18 (10.5%) | - |
| Lenalidomide | 2 (1.2%) | 2 (1.1%) | NS |
| Dexamethasone | 14 (8.6%) | 8 (4.6%) | NS |
NS, not significant.
Cumulative vs planned doses of lenalidomide, dexamethasone, and ixazomib in each treatment arm
| . | RD . | IRD . | ||
|---|---|---|---|---|
| Cumulative dose, mg (mean ± SD) . | Mean cumulative dose, % . | Cumulative dose, mg (mean ± SD) . | Mean cumulative dose, % . | |
| Lenalidomide | 6002 ± 2220 | 79 | 5629 ± 2388 | 74 |
| Dexamethasone | 2784 ± 1201 | 72.5 | 2820 ± 1242 | 73 |
| Ixazomib | NA | NA | 205 ± 95 | 71 |
| . | RD . | IRD . | ||
|---|---|---|---|---|
| Cumulative dose, mg (mean ± SD) . | Mean cumulative dose, % . | Cumulative dose, mg (mean ± SD) . | Mean cumulative dose, % . | |
| Lenalidomide | 6002 ± 2220 | 79 | 5629 ± 2388 | 74 |
| Dexamethasone | 2784 ± 1201 | 72.5 | 2820 ± 1242 | 73 |
| Ixazomib | NA | NA | 205 ± 95 | 71 |
NA, not applicable; SD, standard deviation.
Second primary malignancies from randomization
| Type of malignancy . | All patients N = 332 . |
|---|---|
| Hematological cancers | 2 |
| Solid tumors | 25 |
| Nonmelanoma skin cancer | 6 |
| Gastrointestinal | 5 |
| Prostate | 5 |
| CNS | 3 |
| Lung | 2 |
| Others | 4 |
| Total∗ | 27 (8.1%) |
| Type of malignancy . | All patients N = 332 . |
|---|---|
| Hematological cancers | 2 |
| Solid tumors | 25 |
| Nonmelanoma skin cancer | 6 |
| Gastrointestinal | 5 |
| Prostate | 5 |
| CNS | 3 |
| Lung | 2 |
| Others | 4 |
| Total∗ | 27 (8.1%) |
CNS, central nervous system.
RD: 14 patients; IRD: 13 patients.
Discussion
Maintenance therapy is critical in order to delay or even prevent disease progression after ASCT in patients with MM. Glucocorticoids, α2b-IFN, thalidomide, and proteasome inhibitors have been investigated over the last decades in a number of clinical trials, but none of these drugs has been approved by the regulatory agencies.10-12,20-28 Lenalidomide resulted in a significant PFS and OS prolongation as maintenance therapy after ASCT in patients with MM1-5 and was approved to be used as a single agent until disease progression after ASCT.
In our study, the control arm consisted of daily lenalidomide for 21 days in a 28-day schedule in combination with dexamethasone. Studies in patients with relapsed/refractory myeloma showed that single-agent lenalidomide had limited efficacy, with a PR rate ranging from 17% to 29%,6,7,29 whereas the addition of dexamethasone for patients who failed to respond or whose condition progressed with lenalidomide resulted in an additional 29% of at least PR.7 In vitro studies showed that dexamethasone enhanced the antiproliferative effect of lenalidomide by inducing tumor suppression gene expression, cell cycle arrest, caspases activation, and apoptosis.8,9 The high efficacy of prolonged administration of lenalidomide in association with dexamethasone has been demonstrated in the pivotal studies in the relapsed/refractory setting30,31 and in the front-line therapy in older patients.32,33 With this rational, dexamethasone was associated with lenalidomide in the 2 arms of this trial. Our planned dose of lenalidomide at 15 mg on days 1 to 21 of each 28-day cycle is similar to the target dosing in the pivotal maintenance trials.1,2 The dose of dexamethasone, equivalent to 40 mg weekly, is similar to that used in the continuous lenalidomide-based therapy in the FIRST32 and MAIA33 trials for older patients.
The HOVON-65/GMMG-HD4 trial showed that the proteasome inhibitor (PI) bortezomib administered during induction and maintenance resulted in a better PFS and OS than the thalidomide counterpart, particularly in patients with renal function impairment and patients with 17p deletion.10 In addition, we showed the superiority in terms of PFS of a 3-year treatment with the combination of bortezomib and thalidomide over α2b-IFN or thalidomide alone in the post-ASCT maintenance setting.11 However, the use of bortezomib as maintenance is limited by the practicalities of parenteral administraton and the risk of peripheral neuropathy. The administration of a 2-year fixed duration of ixazomib as posttransplant maintenance significantly improved the PFS when compared with placebo.12 Ixazomib is ideally suitable for maintenance, given its once-weekly oral dosing and low toxicity profile in terms of peripheral neuropathy. With the evidence that PIs have a synergistic effect with immunomodulatory drugs, this study was aimed at investigating whether lenalidomide and dexamethasone plus ixazomib could be superior to lenalidomide and dexamethasone in terms of PFS as post-ASCT maintenance therapy. Our results show that the PFS was almost identical in the 2 treatment arms, with medians not reached and a PFS rate of 61.3% and 55.6% for RD and IRD, respectively, at 6-years since maintenance initiation.
The addition of ixazomib to lenalidomide and dexamethasone did not result in a significant PFS benefit in any subgroup of patients (standard risk, high-risk cytogenetics, R-ISS, presence of plasmacytomas at diagnosis, or MRD status at baseline). Ixazomib dose reductions/discontinuations did not result in inferior survival outcomes in the IRD arm. Therefore, the toxicity does not seem to be an explanation for the lack of difference between the 2 arms. In contrast, the lack of clear efficacy of IRD vs RD in the older patients34 as well as the small benefit of ixazomib maintenance vs placebo in the post-ASCT setting (TOURMALINE -MM3)12 indicate the limited value of adding ixazomib at this dose and schedule. In addition, in the TOURMALINE MM312 study, there was no effect of ixazomib maintenance in patients who received induction therapy with a PI and an immunomodulatory drug, similar to that observed in this trial.
In our study, to our knowledge, the PFS is the longest reported so far and compares favorably with the median PFS of 52.8 months reported in the meta-analysis5 and the 57 months observed in the Myeloma XI trial.4 Whether the longest PFS observed in our trial compared with single-agent lenalidomide maintenance programs is due to the more intensive pretransplant therapy with a high baseline MRD-negativity rate, the addition of dexamethasone, the starting dose of lenalidomide being 15 mg, or the fact that all our patients received a lenalidomide-based regimen before the initiation of maintenance is unclear. Because a single-agent lenalidomide arm is lacking in our study, we cannot be certain about the potential added value from the association of dexamethasone with lenalidomide.
In our trial, the maintenance duration was tailored according to the MRD status at 2 years, with discontinuation for patients who tested negative, whereas those who tested positive received lenalidomide and dexamethasone for 3 more years, irrespective of the treatment arm. Patients who tested negative for MRD at 2 years for whom maintenance was discontinued had a low progressive disease rate (17.2% at 4 years from discontinuation), whereas patients who tested positive for MRD at 2 years had a progressive disease rate of 50.4% at 4 years, despite the fact that they received maintenance for 3 additional years. Whether the continuation of lenalidomide is of benefit to patients who have received VRD as induction/consolidation, ASCT, and remain with an MRD-positive stats after 2 years of maintenance with lenalidomide and dexamethasone is unclear. The fact that continuation of lenalidomide does not avoid progression in ∼50% of our patients indicates that the effect is of limited value. In contrast, the results of the updated Myeloma XI trial indicates that long-term maintenance is beneficial to patients with MRD-positive status.35 Of interest, patients who had MRD-negative status and showed progression after discontinuation had initial characteristics similar to those of the overall population included in the study. Consequently, the MRD negative status at 2 years of maintenance overcame the poor prognostic features, and it was safe to discontinue maintenance therapy even in the population at high risk. None of the studies included in the lenalidomide meta-analysis or the Myeloma XI trial investigated predetermined maintenance duration.1-5 Although treatment until progression is a guideline recommendation, the optimal duration of maintenance therapy needs to be revisited. It has been reported that longer duration of lenalidomide maintenance results in an increased PFS. In this regard, the results of the recently reported DETERMINATION trial consisting of lenalidomide, bortezomib, and dexamethasone (RVD), ASCT, and lenalidomide maintenance until disease progression35 showed that PFS was 20.2 months longer than that observed in the parallel IFM 2009 trial with the same study design, except that in the latter study, maintenance with lenalidomide was limited to 1 year.36 To our knowledge, this study is the first trial tailoring maintenance duration based on the MRD status. It seems safe to stop maintenance in patients with undetectable MRD at 2 years, even in the population at high risk. The fact that >70% of our patients had MRD-negative status at 2 years of maintenance enforces the importance of considering a fixed maintenance vs indefinite, not only for the long-term potential concerns but also for patients who eventually relapse after discontinuing therapy will have the opportunity to be offered lenalidomide-based rescue regimens that, at the moment, are more effective than lenalidomide-free regimens. Patients who achieved MRD negative status in the second year had a similar outcome to those with MRD negativity from baseline, whereas patients who lost their MRD negative status during the second year of maintenance had a significantly shorter survival than those who with MRD positivity from baseline. The results of the updated MRC XI trial also support that the benefit of maintenance beyond 3 years in patients who have negative MRD status is questionable.37 On the other hand, the cost of long-term maintenance including other drugs that are not yet generic, such as carfilzomib38 or anti-CD38 monoclonal antibodies,39,40 under investigation in clinical trials should be considered.
Grade 3 to 4 toxicities were low in both arms with a significantly higher rate of thrombocytopenia and gastrointestinal toxicity in the IRD arm, which led to a need for ixazomib dose reduction in ∼30% of the patients, with no significant impact on dose reductions or treatment discontinuations of lenalidomide or dexamethasone when compared with the RD arm. The tolerance to an equivalent dose of 40 mg weekly of dexamethasone may be mainly explained through the patient population, aged <66 years at study entry in at least PR of their myeloma. The incidence of second primary malignancies in our study is 8.1%, similar to the 9.5% reported in the CALGB100104 study2 and lower than the 12.7% reported in the IFM-2005 to 02 study.1
In summary, maintenance therapy with lenalidomide and dexamethasone in patients treated with VRD-GEM induction, ASCT, and VRD-GEM consolidation resulted in a long PFS rate of 61.3% at 6 years from the start of maintenance. The addition of ixazomib to the RD regimen did not result in a PFS benefit in any subgroup of patients. Maintenance discontinuation in patients who tested negative for MRD at 2 years resulted in a low progression rate, even in patients with initial high-risk features; however, the impact of treatment discontinuation in patients with undetectable MRD at 2 years should be formally confirmed by prospective randomized studies. Our results support RD as a highly effective maintenance regimen and its use as comparator arm when investigating maintenance approaches.
Acknowledgments
The study was supported by unrestricted grants to the Programa Español de Tratamientos en Hematología by Bristol Myers Squibb-Celgene and Takeda and a Spanish grant from the Instituto de Salud Carlos III (PI20/00436).
Authorship
Contribution: L.R., M.-V.M., J.S.M., J.J.L., and J. Bladé designed the study; L.R., J.d.l.C., B.P., N.P., M.T.C., and J. Bladé analyzed the data; L.R. and J. Bladé wrote the paper; and all authors contributed to the study conduct, collection and interpretation of the data, reviewed the manuscript, approved the final version, and had full access to the study data.
Conflict-of-interest disclosure: L.R. receives honoraria for lectures from and serves on the advisory boards of Janssen, Bristol Myers Squibb-Celgene, Amgen, Takeda, Sanofi, and GlaxoSmithKline. A.O. receives honoraria for lectures from and serves on the advisory boards of Janssen, Bristol Myers Squibb-Celgene, Amgen, GlaxoSmithKline, Sanofi, and Pfizer; E.C.-C. receives honoraria for lectures from and serves on the advisory boards of Janssen and Bristol Myers Squibb-Celgene. J.M.-L. receives honoraria for lectures from and serves on the advisory boards of Janssen, Bristol Myers Squibb-Celgene, Takeda, Amgen, GlaxoSmithKline, AbbVie, Pfizer, Regeneron, Roche, Sanofi, and Oncopeptides. M.E.G. receives honoraria for lectures from and serves on the advisory boards of Janssen, Bristol Myers Squibb-Celgene, Takeda, and Amgen; and receives research funding from Janssen, Bristol Myers Squibb-Celgene, and Amgen. L.F.C. receives consultancy fee from Incyte, Janssen, Roche, Novartis, Bristol Myers Squibb-Celgene, Amgen, Pfizer, AbbVie, and GlaxoSmithKline; and research funding from Incyte, Janssen, Roche, Novartis, Bristol Myers Squibb-Celgene, Amgen, Pfizer, AbbVie, and GlaxoSmithKline; F.d.A. receives honoraria for lectures from and serves on the advisory boards of Janssen, Bristol Myers Squibb-Celgene, Amgen, GlaxoSmithKline, Sanofi, and Takeda. B.P. receives honoraria for lectures from and serves on the advisory boards of Adaptive, Amgen, Bristol Myers Squibb-Celgene, Gilead, GlaxoSmithKline, Janssen, Oncopeptides, Roche, Sanofi, and Takeda; receives unrestricted grants from BeiGene, Bristol Myers Squibb-Celgene, EngMab, GlaxoSmithKline, Roche, Sanofi, and Takeda; and receives consultancy fee from Bristol Myers Squibb-Celgene, Janssen, and Sanofi. M.-V.M. receives honoraria for lectures from and serves on the advisory boards of Janssen, Celgene, Takeda, Amgen, GlaxoSmithKline, AbbVie, Pfizer, Regeneron, Roche, Sanofi, and Oncopeptides. J.S.M. receives honoraria and consulting services fee from and serves on the advisory boards of AbbVie, Amgen, Bristol Myers Squibb, Celgene, GlaxoSmithKline, Haemalogix, Janssen, Karyopharm, Merck Sharp & Dohme, Novartis, Takeda, Regeneron, Roche, Sanofi, SecuraBio, and Clínica Universitaria de Navarra. J. Bladé receives honoraria for lectures from and serves on the advisory boards of Janssen, Bristol Myers Squibb-Celgene, Amgen, Takeda, and Oncopeptides. The remaining authors declare no competing financial interests.
Correspondence: Laura Rosiñol, Hospital Clínic de Barcelona, Insitut d'Investigacions Biomèdiques August Pi i Sunyer, Universitat de Barcelona, Villarroel, 170, 08036 Barcelona, Spain; e-mail: lrosinol@clinic.cat.
References
Author notes
The Spanish Myeloma Group is open to the possibility of sharing the data used in this study for research projects as long as they do not interfere with the present or future objectives of the clinical trial. The interest and feasibility of any clinical or biological research proposal based on the data from this study must be approved by the board of directors of the Spanish Myeloma Group. In such a case, the data, deposited in REDCap, will be presented in anonymized CSV format. This availability is subject to the laws and provisions in force that regulate the development of clinical trials both in Spain and in the European Union.
Data are available on request from the corresponding author, Laura Rosiñol (lrosinol@clinic.cat).
There is a Blood Commentary on this article in this issue.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.



This feature is available to Subscribers Only
Sign In or Create an Account Close Modal