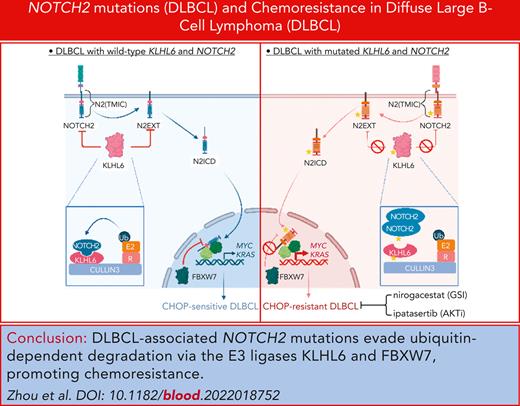
Key Points
DLBCL-associated NOTCH2 mutations evade ubiquitin-dependent degradation via the E3 ligases KLHL6 and FBXW7 and promote chemoresistance.
Inhibition of γ-secretase and AKT with nirogacestat and ipatasertib synergistically promotes CHOP-resistant DLBCL destruction.
Abstract
Diffuse large B-cell lymphoma (DLBCL) is the most common subtype of non-Hodgkin lymphoma. Up to 40% of patients with DLBCL display refractory disease or relapse after standard chemotherapy treatment (rituximab, cyclophosphamide, doxorubicin, vincristine, and prednisone [R-CHOP]), leading to significant morbidity and mortality. The molecular mechanisms of chemoresistance in DLBCL remain incompletely understood. Using a cullin–really interesting new gene (RING) ligase-based CRISPR-Cas9 library, we identify that inactivation of the E3 ubiquitin ligase KLHL6 promotes DLBCL chemoresistance. Furthermore, proteomic approaches helped identify KLHL6 as a novel master regulator of plasma membrane–associated NOTCH2 via proteasome-dependent degradation. In CHOP-resistant DLBCL tumors, mutations of NOTCH2 result in a protein that escapes the mechanism of ubiquitin-dependent proteolysis, leading to protein stabilization and activation of the oncogenic RAS signaling pathway. Targeting CHOP-resistant DLBCL tumors with the phase 3 clinical trial molecules nirogacestat, a selective γ-secretase inhibitor, and ipatasertib, a pan-AKT inhibitor, synergistically promotes DLBCL destruction. These findings establish the rationale for therapeutic strategies aimed at targeting the oncogenic pathway activated in KLHL6- or NOTCH2-mutated DLBCL.
Introduction
Diffuse large B-cell lymphoma (DLBCL) is an aggressive lymphoma characterized by the proliferation of mature B cells, featuring genetic heterogeneity.1 In the United States, DLBCL is the most common subtype of lymphoma accounting for ∼32 000 new cases annually2 and pursues an aggressive course leading to death in up to 40% or 50% of the cases.
Based on gene expression, DLBCL is divided into following 2 major subtypes: the activated B-cell–like and the germinal center B-cell–like (GCB) lymphomas.3,4 More recently, 2 DLBCL classifications have been proposed based on mutation cooccurrence.5,6 Schmitz et al identified the MCD (cooccurrence of MYD88[L265P] and CD79B mutations), BN2 (cooccurrence of B-cell lymphoma 6 [BCL6] fusions and NOTCH2 mutations), N1 (NOTCH1 mutations), and EZB (cooccurrence of enhancer of zeste homolog 2 [EZH2] mutations and BCL2 translocations). Chapuy et al identified 5 clusters based on mutations of NOTCH2 signaling pathway (C1); inactivation of TP53 and copy loss of CDKN2A (C2); alterations in BCL2, KMT2D, CREBBP, and EZH2 (C3); mutations in immune molecules (CD83, CD58, and CD70), B-cell receptor (BCR)/phosphoinositide-3-kinase (PI3K) signaling (RHOA, GNA13, and SGK1), NF-κB (CARD11, NFKBIE, and NFKBIA), and RAS/Janus kinase (JAK)/STAT pathway (BRAF and STAT3) (C4); and mutations of CD79B, MYD88, and TBL1XR1(C5).
For the past 20 years, patients with DLBCL have been treated with a chemotherapy regimen based on a combination of rituximab, cyclophosphamide, doxorubicin, vincristine, and prednisone (R-CHOP).7-9 Unfortunately, up to 40% of patients display refractory disease or short-term relapse.10 The mechanisms of drug resistance can vary and depend on either genetic mutations or tumor microenvironment changes leading to drug resistance. At this stage, several clinical trials are underway to assess the response in R-CHOP relapsed/refractory DLBCLs (rrDLBCLs), including trials using drugs that specifically target Bruton tyrosine kinase, spleen tyrosine kinase, EZH2, PI3K, mammalian target of rapamycin, JAK, and BCL protein.11
The clinical success of a proteasome inhibitor (bortezomib) and the E3 ubiquitin ligase glues, such as immunomodulatory imide drugs, sulfonimides, and proteolysis-targeting chimera, stimulated great interest in the therapeutic opportunities of targeting protein-ubiquitylation and degradation in cancer.12-19 Protein degradation is the result of covalent attachment of chains of the small protein ubiquitin, which allows recruitment to the proteasome for proteolysis. Ubiquitylation is catalyzed in a stepwise enzymatic cascade in which ubiquitin is activated by an E1 enzyme, transferred to an E2 ubiquitin-conjugating enzyme, and then transferred to a substrate specified by an E3 ubiquitin ligase adapter protein. Cullin–really interesting new gene (RING) ligases (CRLs) are one of the largest family of E3 ubiquitin ligases.20,21
In this study, we conducted a CRL-based CRISPR screening and identified the KLHL6 gene as the master regulator of CHOP resistance in DLBCL. We also identified NOTCH2 as a bona fide target of KLHL6, providing mechanistic insights on the role of NOTCH2 stabilization in promoting CHOP resistance. Our research advances the understanding of the relevant oncogenic pathways in DLBCL resistance to CHOP and paves an avenue for personalized therapy for DLBCL.
Methods
Cell culture
U2932, TMD8, HLY1, SUDHL6, SUDHL10, OCI-LY1, OCI-LY7, VAL, and FARAGE cells were maintained in RPMI1640 media (Gibco) containing 10% fetal bovine serum (FBS; Hyclone) and 100 U/mL penicillin, 100 μg/ml streptomycin (Gibco). OCI-LY10 cells were cultured in Iscove modified Dulbecco media (Gibco) containing 10% FBS and 100 U/ml penicillin, 100 μg/ml streptomycin. HEK293T cells were maintained in Dulbecco’s modified Eagle media (Corning) containing 10% bovine serum (Gibco) and 100 U/mL penicillin, 100 μg/ml streptomycin. OP9-delta–like NOTCH ligand1 (DLL1) cells were maintained in minimum essential medium α (Gibco) containing 20% FBS and 100 U/mL penicillin, 100 μg/ml streptomycin.
Immunoprecipitation, biotin AP, and immunoblot
For anti-FLAG immunoprecipitation and immunoblot, protocols were previously described.22 For biotin affinity purification (AP), cells were washed with ice-cold phosphate-buffered saline (PBS), suspended in 500 μL of PBS, and incubated with 50 μL of 10 mM Sulfo-NHS-SS-biotin (Thermo Fisher, A39258) at room temperature for 30 minutes. After washing with ice-cold PBS, the cells were lysed with NP-40 buffer, and the lysates were incubated with streptavidin-coated magnetic beads (Thermo Fisher, 88816) at 4°C overnight. After washing with NP-40 buffer, the streptavidin-coated magnetic beads were mixed with Laemmli buffer and boiled at 95°C for 5 minutes. Paraffin-embedded DLBCL biopsies were obtained from patients in accordance with the clinical practices of the hospital of the University of Pennsylvania, using an Institutional Review Board–approved protocol (852722). All the biopsies were from patients with rrDLBCL not otherwise specified (NOS). The following primary antibodies were used: anti-KLHL6 (Abcam, ab182163), anti-NOTCH2 (Cell Signaling, 5732S), anti-NOTCH1 (Cell Signaling, 3608S), anticleaved NOTCH1 (Cell Signaling, 4147S), anti-Roquin2 (Bethyl, A305-149A), anti-NCOR1 (Cell Signaling, 5948S), anti-HDAC3 (Santa Cruz, sc-11417), anti-ALMS1 (Bethyl, A301-815A), anti-TAB1 (Bethyl, A302-166A), anti-FLAG (Sigma, F7425), anti-HA (Cell Signaling, 3724S), anti-FBXW7 (Bethyl, A301-721A), anti-RBP-J (Cell Signaling, 5313T), anti-CULLIN3 (Bethyl, A301-109A), anti-tubulin (Developmental Studies Hybridoma Bank, 12G10), and anti-vinculin (Santa Cruz, sc-73614). The following horseradish peroxidase (HRP)–linked secondary antibodies were used: antirabbit immunoglobulin G HRP (Cell Signaling, 7074S) and antimouse immunoglobulin G HRP (GE HealthCare, NA931V).
Xenotransplantation experiments
All work related to animals was performed in accordance with the ethical guidelines and protocols approved by the institutional animal care and use committee of the University of Pennsylvania. NSG mice were purchased from The Jackson Laboratory (005557). Eight-to-12-week-old NSG mice received subcutaneous flank injections of 1 × 107 U2932 cells expressing single guide RNAs (sgRNAs) or NOTCH2 constructs. Tumor volume was calculated via caliper measurement. When the average tumor volumes reached 100 mm3, mice were treated with cyclophosphamide (30 mg/kg; Selleckchem, S2057), doxorubicin (2.48 mg/kg; Selleckchem, S1208), and vincristine (0.38 mg/kg; Selleckchem, S1241), which were administered IV on day 1. Prednisone (0.2 mg/kg; Sigma, P6254) was given via oral gavage daily for 5 days. Nirogacestat (100 mg/kg; Selleckchem, S8018) and ipatasertib (50 mg/kg; MedChemExpress, HY-15186) were given via oral gavage daily for 7 days and 5 days, respectively. When the tumor volume reached 1000 mm3, mice were euthanized. Survival was calculated using Kaplan-Meier survival curve method, and differences in survival were calculated with the log-rank (Mantel-Cox) test (GraphPad Prism).
CRISPR screen, mass spectrometry, flow cytometry, drug sensitivity assay, RNA sequencing, and chromatin immunoprecipitation followed by sequencing
Protocols are provided in the supplemental Methods, which is available on the Blood website.
Statistical analyses
All graphs show mean values, with error bars signifying the standard deviation. Sample sizes and reproducibility for each figure are shown in the figure legends. Combination index was calculated with CompuSyn software. The one-way or two-way analyses of variance, the Fisher exact test, and the two-tailed unpaired Student t test were performed and are indicated in the figure legends.
Results
KLHL6 inactivation promotes resistance to CHOP therapy in DLBCL cells
To identify genes in the ubiquitin-proteasome system that enable resistance to doxorubicin, which is thought to be a critical drug of CHOP therapy,23 we performed a CRISPR/Cas9–based knockout (KO) screen targeting CRL family in the human DLBCL cell line U2932-Cas9 (Figure 1A), whose editing efficiency was confirmed (supplemental Figure 1A). Deep sequencing demonstrated significant enrichment of sgRNAs targeting 17 CRL receptors in the doxorubicin-treated cells (Figure 1B). Because no published data are available on the potential function of these genes in resistance toward doxorubicin, we prioritized our investigation on KLHL6, a gene displaying a high frequency of mutations in DLBCL5,6,24 (Figure 1C).
KLHL6 promotes chemosensitivity of DLBCL cells. (A) Schematic representation of the CRISPR screen using the CRL sgRNA library in U2932-Cas9 cells. (B) Volcano plot representing the CRISPR score and P value. The red circles represent the genes whose sgRNAs were significantly enriched in doxorubicin-treated cells (two-tailed Student t test; P ≤ .01). (C) Genes selected in panel B were evaluated for the percentages of genetic alterations in DLBCL (Duke Cancer Institute, n = 1001; Dana-Farber Cancer Institute, n = 135; Broad Institute, n = 58; The Cancer Genome Atlas PanCancer Atlas, n = 48; The Cancer Genome Atlas Firehose Legacy, n = 48; BC Cancer Canada’s Michael Smith Genome Sciences Centre, n = 53). (D) 1 × 107 OCI-LY10 KLHL6+/+ and KLHL6–/– cells were xenografted in the flanks of NSG mice. After the average tumor volumes reached 100 mm3, mice were treated with cyclophosphamide (30 mg/kg), doxorubicin (2.48 mg/kg), and vincristine (0.38 mg/kg) IV on day 1, and prednisone (0.2 mg/kg) was given via oral gavage daily for 5 days. The experimental end point was reached when the tumor volume reached 1000 mm3. Each line represents 1 tumor in 1 mouse (n = 5 per group, two-way analysis of variance [ANOVA]). (E) Kaplan-Meier survival curves of OCI-LY10 KLHL6+/+ and KLHL6–/– xenografts as shown in panel D (n = 5 per group; the Mantel-Cox test). DMSO, dimethyl sulfoxide.
KLHL6 promotes chemosensitivity of DLBCL cells. (A) Schematic representation of the CRISPR screen using the CRL sgRNA library in U2932-Cas9 cells. (B) Volcano plot representing the CRISPR score and P value. The red circles represent the genes whose sgRNAs were significantly enriched in doxorubicin-treated cells (two-tailed Student t test; P ≤ .01). (C) Genes selected in panel B were evaluated for the percentages of genetic alterations in DLBCL (Duke Cancer Institute, n = 1001; Dana-Farber Cancer Institute, n = 135; Broad Institute, n = 58; The Cancer Genome Atlas PanCancer Atlas, n = 48; The Cancer Genome Atlas Firehose Legacy, n = 48; BC Cancer Canada’s Michael Smith Genome Sciences Centre, n = 53). (D) 1 × 107 OCI-LY10 KLHL6+/+ and KLHL6–/– cells were xenografted in the flanks of NSG mice. After the average tumor volumes reached 100 mm3, mice were treated with cyclophosphamide (30 mg/kg), doxorubicin (2.48 mg/kg), and vincristine (0.38 mg/kg) IV on day 1, and prednisone (0.2 mg/kg) was given via oral gavage daily for 5 days. The experimental end point was reached when the tumor volume reached 1000 mm3. Each line represents 1 tumor in 1 mouse (n = 5 per group, two-way analysis of variance [ANOVA]). (E) Kaplan-Meier survival curves of OCI-LY10 KLHL6+/+ and KLHL6–/– xenografts as shown in panel D (n = 5 per group; the Mantel-Cox test). DMSO, dimethyl sulfoxide.
We generated 2 activated B-cell–like cell lines (U2932, OCI-LY10) and 2 germinal center B-cell–like cell lines (SUDHL6 and SUDHL10) ablating KLHL6, using CRISPR/Cas9–based genome editing (supplemental Figure 1B). Loss of KLHL6 with 2 independent sgRNAs resulted in a significant rightward shift of the toxicity curves, indicating desensitization toward doxorubicin. Half-maximal inhibitory concentration (IC50) quantification revealed a desensitization up to threefold, as compared with control sgRNA (gCTRL)-expressing cells (supplemental Figure 1C). Importantly, loss of KLHL6 did not compromise DLBCL response toward rituximab (supplemental Figure 1D), a monoclonal antibody against CD20 with therapeutic activity in DLBCL.7
Next, we assessed the role of KLHL6 in DLBCL chemosensitivity in vivo. OCI-LY10-Cas9 KLHL6–/– and KLHL6+/+ cells were xenografted in NSG mice before treatment with a regimen of CHOP. CHOP-treatment resulted in rapid tumor shrinkage; however, tumors relapsed over time, resulting in the death of mice bearing KLHL6+/+ cells around day 90 (Figure 1D). Notably, KLHL6–/– tumors relapsed earlier, resulting in the death of the colony by day 70 (Figure 1D). Kaplan-Meier survival analysis confirmed that the overall survival of mice bearing KLHL6–/– tumors was significantly reduced (Figure 1E). Importantly, RC3H2 (Roquin2), a previously identified KLHL6-target,25 was not involved in the doxorubicin sensitivity of DLBCL because Roquin2 KO DLBCL cells displayed a sensitivity toward doxorubicin similar to that of the control (supplemental Figure 1E-F).
Altogether, these data identify KLHL6 as a novel gene regulating CHOP-sensitivity in DLBCL cells and provide evidence that an uncharacterized target of KLHL6 may be involved in this phenotype.
Unbiased proteomic approaches identify NOTCH2 as a specific KLHL6 interactor
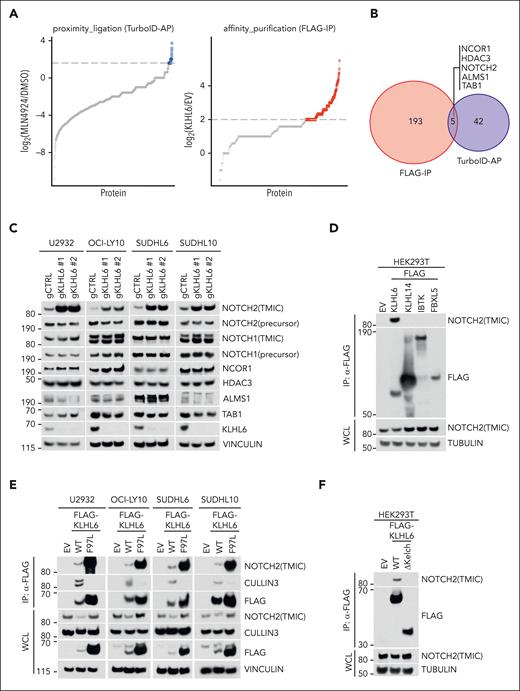
To identify the targets of KLHL6, we conducted an unbiased proteomic screen based on biotin proximity ligation using cells stably expressing TurboID-KLHL6; hits were selected based on enrichment upon MLN4924 treatment (Figure 2A; supplemental Table 1). In parallel, a FLAG-immunoprecipitation based proteomic screen using cells stably expressing FLAG-KLHL6 identified bona fide interactors (Figure 2A; supplemental Table 1). The overlap of both datasets revealed 5 proteins (NCOR1, HDAC3, NOTCH2, ALMS1, and TAB1; Figure 2A-B).
NOTCH2 is a specific interactor of KLHL6. (A, left) HLY1-TurboID-KLHL6 was treated with DMSO or 5 μM MLN4924 for 6 hours; biotin was added at 5 μM for 6 hours. The graph is a scatter plot showing the log2 ratio between the unique peptides identified via mass spectrometry upon biotin AP in the MLN4924 and DMSO condition. The blue circles represent the KLHL6 interactors enriched by at least threefold. (A, right) Scatter plot showing the log2 ratio between the unique peptides identified via mass spectrometry in the FLAG immunoprecipitation (FLAG-IP) of KLHL6 and the empty vector (EV).25 The red circles represent the KLHL6 interactors enriched by at least fourfold. (B) Venn diagram showing the overlap of the KLHL6 interactors that were identified in panel A. (C) Immunoblot analysis for the indicated proteins from the whole cell lysate (WCL) of U2932, OCI-LY10, SUDHL6, and SUDHL10 cells stably expressing Cas9 and 2 sgRNAs against KLHL6 and control sgRNA, (D) Immunoblot analysis for the indicated proteins from the WCL and FLAG-immunoprecipitated (FLAG-IP) samples of HEK293T cells overexpressing the indicated FLAG-tagged BTB proteins and F-box protein. The cells were treated with MLN4924 for 6 hours before harvesting. (E) Immunoblot analysis for the indicated proteins from WCL and FLAG- IP samples of U2932, OCI-LY10, SUDHL6, and SUDHL10 cells stably expressing EV, FLAG-KLHL6 (WT) or FLAG-KLHL6 (F97L). (F) Immunoblot analysis for the indicated proteins from WCL and FLAG- IP samples of FLAG-taggedWT KLHL6 and KLHL6 mutant lacking the Kelch domain (ΔKelch). The cells were treated with MLN4924 for 6 hours before harvesting. Unless otherwise noted, immunoblots are representative of 3 independent experiments. gCTRL, control sgRNA.
NOTCH2 is a specific interactor of KLHL6. (A, left) HLY1-TurboID-KLHL6 was treated with DMSO or 5 μM MLN4924 for 6 hours; biotin was added at 5 μM for 6 hours. The graph is a scatter plot showing the log2 ratio between the unique peptides identified via mass spectrometry upon biotin AP in the MLN4924 and DMSO condition. The blue circles represent the KLHL6 interactors enriched by at least threefold. (A, right) Scatter plot showing the log2 ratio between the unique peptides identified via mass spectrometry in the FLAG immunoprecipitation (FLAG-IP) of KLHL6 and the empty vector (EV).25 The red circles represent the KLHL6 interactors enriched by at least fourfold. (B) Venn diagram showing the overlap of the KLHL6 interactors that were identified in panel A. (C) Immunoblot analysis for the indicated proteins from the whole cell lysate (WCL) of U2932, OCI-LY10, SUDHL6, and SUDHL10 cells stably expressing Cas9 and 2 sgRNAs against KLHL6 and control sgRNA, (D) Immunoblot analysis for the indicated proteins from the WCL and FLAG-immunoprecipitated (FLAG-IP) samples of HEK293T cells overexpressing the indicated FLAG-tagged BTB proteins and F-box protein. The cells were treated with MLN4924 for 6 hours before harvesting. (E) Immunoblot analysis for the indicated proteins from WCL and FLAG- IP samples of U2932, OCI-LY10, SUDHL6, and SUDHL10 cells stably expressing EV, FLAG-KLHL6 (WT) or FLAG-KLHL6 (F97L). (F) Immunoblot analysis for the indicated proteins from WCL and FLAG- IP samples of FLAG-taggedWT KLHL6 and KLHL6 mutant lacking the Kelch domain (ΔKelch). The cells were treated with MLN4924 for 6 hours before harvesting. Unless otherwise noted, immunoblots are representative of 3 independent experiments. gCTRL, control sgRNA.
NOTCH proteins are transmembrane receptors with 4 homologs in mammals (NOTCH1-4). In cells, they are processed in 3 distinct proteolytic processing steps26,27 (supplemental Figure 2A). Firstly, the newly synthesized NOTCH precursor is cleaved by a furin-like protease, at site 1 (S1) in the secretory pathway, resulting in a noncovalent heterodimerization domain (HD) interaction between the NOTCH extracellular and transmembrane intracellular (TMIC) domain.28 After ligand interaction, the S2 site in the HD is cleaved by the metalloproteinase ADAM10. Next, S2-cleaved NOTCH extracellular truncation domain is rapidly cleaved at S3 by γ-secretase complex to release the NOTCH intracellular domain (NICD).26,29-31
We validated the proteomic analysis via the immunoprecipitation of FLAG-KLHL6 in 4 DLBCL cell lines (supplemental Figure 2B); KLHL6 specifically interacted with endogenous TMIC NOTCH2, NCOR1, and HDAC3. However, NOTCH2 (TMIC) was the only protein upregulated in 4 DLBCL cell lines upon ablation of KLHL6 via CRISPR-mediated gene editing (Figure 2C). Binding between KLHL6 and NOTCH2 was specific because it was not detected with other CRLs tested (Figure 2D). Furthermore, a KLHL6 BTB-domain mutant, incapable of binding to cullin-3, remained associated to NOTCH2 in the 4 DLBCL cell line tested (Figure 2E), suggesting that NOTCH2 interacts with the C-terminal Kelch domain, which promotes substrate interaction.25 Accordingly, ablation of the Kelch domain completely abolished the interaction with NOTCH2 (Figure 2F).
Importantly, cell lysis using denaturing buffers results in loss of the noncovalent linkage of NOTCH heterodimer.30 As such, after cell lysis, most of the full-length NOTCH2 is represented by the NOTCH2 precursor in the secretory pathway that has not been cleaved by furin. This NOTCH2 precursor is not affected by KLHL6 loss, suggesting that KLHL6 cannot access NOTCH2 precursor (before S1 cleavage) during its maturation (Figure 2C).
To determine the role of NOTCH proteins in doxorubicin sensitivity, we first assessed NOTCH1-4 expression using the Cancer Cell Line Encyclopedia dataset32 (supplemental Figure 2C). NOTCH1 and NOTCH2 were highly expressed in DLBCL; as such, we used NOTCH1 or NOTCH2 KO DLBCL cells to assess doxorubicin sensitivity (supplemental Figure 2D-E). IC50 calculation upon doxorubicin treatment revealed a loss of NOTCH2 sensitized DLBCL cells by at least twofold (supplemental Figure 2E), whereas NOTCH1 loss had a nonsignificant effect. Importantly, ablation of NCOR1 or HDAC3, in 4 DLBCL cell lines, did not affect DLBCL sensitivity toward doxorubicin (supplemental Figure 2F-H).
Altogether, these data identified NOTCH2 (TMIC) as a specific interactor of KLHL6 and regulator of doxorubicin resistance in DLBCL.
KLHL6 targets plasma membrane NOTCH2 for protein degradation
Because an antibody detecting cleaved NOTCH2 intracellular domain (N2ICD) is not available, we assessed the impact of KLHL6 ablation on the levels of NOTCH2 extracellular truncation domain (N2EXT), which is generated upon NOTCH ligand stimulation and reflects the strength of NOTCH signaling. To better visualize N2EXT, we blocked the N2EXT cleavage with γ-secretase inhibitor (GSI)33 and strengthened NOTCH ligand stimulation by using OP9 stroma cells expressing DLL1.34,35 Cleaved NOTCH1 intracellular domain (N1ICD) was used as positive control because it increased upon DLL1 stimulation (supplemental Figure 3A). Importantly, under GSI treatment, both NOTCH2 (TMIC) and NOTCH2 (NOTCH extracellular truncation domain) were upregulated in KLHL6–/– cells, confirming that plasma membrane NOTCH2 is targeted by KLHL6 for protein degradation.
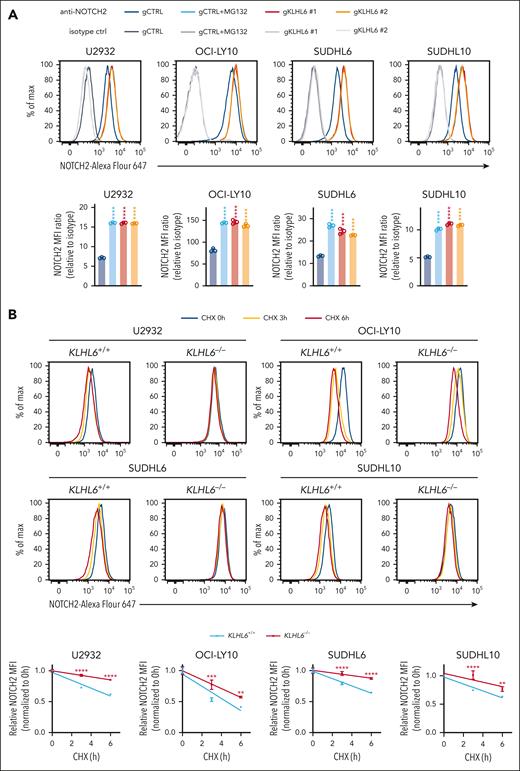
To measure the level of plasma membrane NOTCH2 in live cells, we validated 2 NOTCH2 antibodies via flow cytometry (supplemental Figure 3B-C). Next, we demonstrated that the levels (Figure 3A) and half-life (Figure 3B) of plasma membrane NOTCH2 increased in 4 DLBCL KLHL6 KO cells. Similarly, KLHL6–/– DLBCL cells displayed a prolonged half-life of NOTCH2 (TMIC), as assessed via immunoblot analysis (Figure 3C). In contrast, stable overexpression of KLHL6 (wild type [WT]) in 4 DLBCL cells induced downregulation of plasma membrane NOTCH2, whereas expression of KLHL6 (F97L) did not (Figure 3D). We also used a biochemical method based on plasma membrane biotin crosslinking of live cells coupled to streptavidin AP (supplemental Figure 3D) and demonstrated that NOTCH2 (TMIC) was upregulated when KLHL6 was ablated.
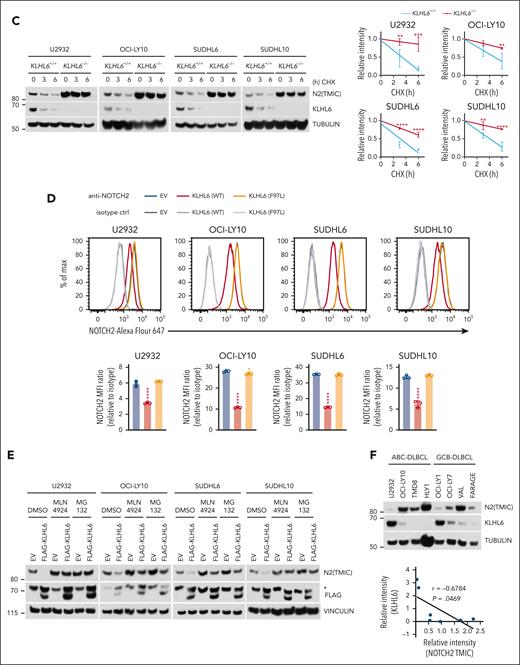
KLHL6 promotes degradation of plasma membrane NOTCH2. (A, top) Flow cytometry analysis of the plasma membrane NOTCH2 in U2932, OCI-LY10, SUDHL6, and SUDHL10 cells stably expressing the indicated sgRNAs against KLHL6 or control sgRNA. (A, bottom) The quantification of the mean fluorescent intensity (MFI) of the plasma membrane NOTCH2. MFI ratio (relative to isotype) was calculated (mean ± standard deviation [SD]; n = 3 independent experiments; one-way ANOVA; ∗∗∗∗P ≤ .0001). When indicated, cells were treated with 10 μM MG132 for 6 hours. (B) Same as that in panel A, but cells were treated with cycloheximide for the indicated time points. Quantification of the relative MFI is shown on the right plot (mean ± SD; n = 3 independent experiments; two-way ANOVA; ∗∗P ≤ .01; ∗∗∗∗P ≤ .0001). (C, left) Immunoblot analysis for the indicated proteins in U2932, OCI-LY10, SUDHL6, and SUDHL10 KLHL6+/+ or KLHL6–/– cells treated with cycloheximide (CHX) for the indicated time points. (C, right) quantification of NOTCH2 (TMIC) immunoblots. Relative intensity was plotted over time (mean ± SD; n = 3 independent experiments; two-way ANOVA; ∗∗P ≤ .01; ∗∗∗P ≤ .001; ∗∗∗∗P ≤ .0001). (D, top) Flow cytometry analysis of the plasma membrane NOTCH2 in U2932, OCI-LY10, SUDHL6, and SUDHL10 cells stably expressing the indicated complementary DNA (cDNA). (D, bottom) Quantification of the MFI ratio for the plasma membrane NOTCH2 (mean ± SD; n = 3 independent experiments; one-way ANOVA; ∗P ≤ .05; ∗∗∗∗P ≤ .0001). (E) Immunoblot analysis for the indicated proteins in U2932, OCI-LY10, SUDHL6, and SUDHL10 cells stably expressing FLAG-tagged KLHL6; when indicated, cells were treated with DMSO, 5 μM MLN4924, or 10 μM MG132 for 6 hours. Asterisk indicates nonspecific bands. (F, top) Immunoblot analysis for the indicated proteins in a panel of activated B-cell–like and germinal center B-cell–like DLBCL cell lines. (F, bottom) Quantification of NOTCH2 (TMIC) (x-axis) and KLHL6 (y-axis) protein levels in each DLBCL cell line. Unless otherwise noted, immunoblots are representative of 3 independent experiments. r, Pearson correlation coefficient.
KLHL6 promotes degradation of plasma membrane NOTCH2. (A, top) Flow cytometry analysis of the plasma membrane NOTCH2 in U2932, OCI-LY10, SUDHL6, and SUDHL10 cells stably expressing the indicated sgRNAs against KLHL6 or control sgRNA. (A, bottom) The quantification of the mean fluorescent intensity (MFI) of the plasma membrane NOTCH2. MFI ratio (relative to isotype) was calculated (mean ± standard deviation [SD]; n = 3 independent experiments; one-way ANOVA; ∗∗∗∗P ≤ .0001). When indicated, cells were treated with 10 μM MG132 for 6 hours. (B) Same as that in panel A, but cells were treated with cycloheximide for the indicated time points. Quantification of the relative MFI is shown on the right plot (mean ± SD; n = 3 independent experiments; two-way ANOVA; ∗∗P ≤ .01; ∗∗∗∗P ≤ .0001). (C, left) Immunoblot analysis for the indicated proteins in U2932, OCI-LY10, SUDHL6, and SUDHL10 KLHL6+/+ or KLHL6–/– cells treated with cycloheximide (CHX) for the indicated time points. (C, right) quantification of NOTCH2 (TMIC) immunoblots. Relative intensity was plotted over time (mean ± SD; n = 3 independent experiments; two-way ANOVA; ∗∗P ≤ .01; ∗∗∗P ≤ .001; ∗∗∗∗P ≤ .0001). (D, top) Flow cytometry analysis of the plasma membrane NOTCH2 in U2932, OCI-LY10, SUDHL6, and SUDHL10 cells stably expressing the indicated complementary DNA (cDNA). (D, bottom) Quantification of the MFI ratio for the plasma membrane NOTCH2 (mean ± SD; n = 3 independent experiments; one-way ANOVA; ∗P ≤ .05; ∗∗∗∗P ≤ .0001). (E) Immunoblot analysis for the indicated proteins in U2932, OCI-LY10, SUDHL6, and SUDHL10 cells stably expressing FLAG-tagged KLHL6; when indicated, cells were treated with DMSO, 5 μM MLN4924, or 10 μM MG132 for 6 hours. Asterisk indicates nonspecific bands. (F, top) Immunoblot analysis for the indicated proteins in a panel of activated B-cell–like and germinal center B-cell–like DLBCL cell lines. (F, bottom) Quantification of NOTCH2 (TMIC) (x-axis) and KLHL6 (y-axis) protein levels in each DLBCL cell line. Unless otherwise noted, immunoblots are representative of 3 independent experiments. r, Pearson correlation coefficient.
In agreement with these data, forced expression of KLHL6 in 4 DLBCL cells induced a downregulation of NOTCH2 (TMIC) in a manner that was dependent on MLN4924 or MG132 (Figure 3E), suggesting that KLHL6 promotes NOTCH2 (TMIC) degradation in a ubiquitin- and proteasome-dependent manner. Finally, the abundance of KLHL6 and NOTCH2 (TMIC) displayed a significantly inverse correlation in multiple DLBCL cell lines (Figure 3F).
Overall, the data demonstrate that plasma membrane NOTCH2 is targeted by KLHL6 for protein degradation.
NOTCH2 binds KLHL6 in a degron that is different from the FBXW7-degron
Cancer-associated mutations in the HD of NOTCH1 have been described in T-cell acute lymphoblastic leukemia, resulting in ligand-independent cleavage of NOTCH1.36,37 However, in DLBCL, most mutations in either NOTCH1 or NOTCH2 are localized in the C-terminal proline, glutamic acid, serine, threonine-rich (PEST) domain, impairing the E3 ligase FBXW7–mediated degradation of NICD.5,24,37 Importantly, PEST domain mutated NOTCHs are weak oncogenes38; hence cooperation with additional pathways to strengthen NOTCH activation is likely to occur in DLBCL.
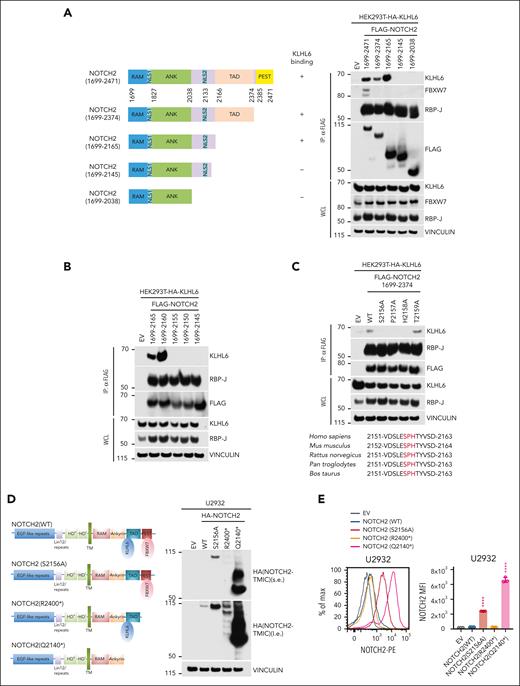
To identify the KLHL6-degron, we performed C-terminal truncation of NOTCH2 and tested the interaction with KLHL6 (Figure 4A). Our initial effort identified NOTCH2 amino acids from 2145 to 2165 (Figure 4A) and, with more refined deletions, a region between 2155 and 2160 as necessary for KLHL6 binding (Figure 4B). Alanine scanning mutagenesis showed that a conserved Ser2156-Pro2157-His2158 motif was required for interaction (Figure 4C). Our analysis showed that the binding motif of KLHL6 differs from that of the PEST domain, which is targeted by FBXW7.5,24 Importantly, patients with DLBCL display nonsense mutations resulting in truncated proteins ablating the FBXW7 degron alone (ΔdegFBXW7) or the KLHL6 degron plus FBXW7 degron (ΔdegKLHL6/ΔdegFBXW7)5,6,24,39-42 (supplemental Figure 4A).
NOTCH2 binds KLHL6 in a degron that is different from FBXW7-degron. (A, left) Schematic representation of WT or mutated NOTCH2 interacting (+) or not (−) with KLHL6 is shown. (A, right) Immunoblot analysis for the indicated proteins from the WCL or FLAG-IP samples from HEK293T cells stably expressing hemagglutinin (HA)-tagged KLHL6 and overexpressing the indicated FLAG-tagged WT or mutated NOTCH2. (B) Same as that in panel A, but the indicated NOTCH2 mutants are used. (C, top) Same as that in panel A, but the indicated NOTCH2 mutants are used. (C, bottom) Alignment of the NOTCH2 degron in different species. The conserved Ser-Pro-His degrons are shown in red. (D, left) Schematic representation of NOTCH2 (WT), NOTCH2 (S2156A), and DLBCL-associated NOTCH2 mutants, NOTCH2 (R2400∗) and NOTCH2 (Q2140∗). (D, right) Immunoblot analysis for the indicated proteins from the WCL in U2932 cells stably expressing HA-tagged NOTCH2 (WT), NOTCH2 (S2156A) or DLBCL-associated NOTCH2 mutants, NOTCH2 (R2400∗) and NOTCH2 (Q2140∗). (E, left) Flow cytometry analysis for the plasma membrane NOTCH2 in U2932 cells stably expressing HA-tagged NOTCH2 (WT), NOTCH2 (S2156A) or DLBCL-associated NOTCH2 mutants, NOTCH2 (R2400∗) and NOTCH2 (Q2140∗). (E, right) Quantification of the MFI of the plasma membrane NOTCH2 in U2932 cells (mean ± SD; n = 3 independent experiments; one-way ANOVA; ∗∗∗∗P ≤ .0001). Unless otherwise noted, immunoblots are representative of 3 independent experiments. ANK, ankyrin; RAM, RBP-J associated module; TAD, transactivation domain.
NOTCH2 binds KLHL6 in a degron that is different from FBXW7-degron. (A, left) Schematic representation of WT or mutated NOTCH2 interacting (+) or not (−) with KLHL6 is shown. (A, right) Immunoblot analysis for the indicated proteins from the WCL or FLAG-IP samples from HEK293T cells stably expressing hemagglutinin (HA)-tagged KLHL6 and overexpressing the indicated FLAG-tagged WT or mutated NOTCH2. (B) Same as that in panel A, but the indicated NOTCH2 mutants are used. (C, top) Same as that in panel A, but the indicated NOTCH2 mutants are used. (C, bottom) Alignment of the NOTCH2 degron in different species. The conserved Ser-Pro-His degrons are shown in red. (D, left) Schematic representation of NOTCH2 (WT), NOTCH2 (S2156A), and DLBCL-associated NOTCH2 mutants, NOTCH2 (R2400∗) and NOTCH2 (Q2140∗). (D, right) Immunoblot analysis for the indicated proteins from the WCL in U2932 cells stably expressing HA-tagged NOTCH2 (WT), NOTCH2 (S2156A) or DLBCL-associated NOTCH2 mutants, NOTCH2 (R2400∗) and NOTCH2 (Q2140∗). (E, left) Flow cytometry analysis for the plasma membrane NOTCH2 in U2932 cells stably expressing HA-tagged NOTCH2 (WT), NOTCH2 (S2156A) or DLBCL-associated NOTCH2 mutants, NOTCH2 (R2400∗) and NOTCH2 (Q2140∗). (E, right) Quantification of the MFI of the plasma membrane NOTCH2 in U2932 cells (mean ± SD; n = 3 independent experiments; one-way ANOVA; ∗∗∗∗P ≤ .0001). Unless otherwise noted, immunoblots are representative of 3 independent experiments. ANK, ankyrin; RAM, RBP-J associated module; TAD, transactivation domain.
Next, we expressed NOTCH2 (WT) and KLHL6 degron mutant NOTCH2 (S2156A) as well as DLBCL-associated mutants, NOTCH2 (R2400∗) (ΔdegFBXW7) and NOTCH2 (Q2140∗) (ΔdegKLHL6/ΔdegFBXW7), in U2932 cells (Figure 4D). Firstly, NOTCH2 (S2156A) and, to a lesser extent, NOTCH2 (R2400∗) displayed an upregulation of their TMIC levels as compared with NOTCH2 (WT). Strikingly, the double-degron NOTCH2 (Q2140∗) mutant displayed a greater increase in NOTCH2 levels than NOTCH2 (WT) or single degron mutants. To further assess whether plasma membrane NOTCH2 mutants were upregulated, we measured NOTCH2 levels via flow cytometry (Figure 4E). Accordingly, the double-degron NOTCH2 (Q2140∗) mutant expressing cells displayed a significant increase in cell surface NOTCH2 over NOTCH2 (WT) and single degron mutants expressing cells. Similar data were also observed by analyzing the half-life of these mutants via cycloheximide chase experiments (supplemental Figure 4B).
Altogether, our findings indicate that DLBCL-associated NOTCH2 mutants evade the protein degradation machinery to sustain their own protein levels.
KLHL6 promotes DLBCL sensitivity to CHOP by controlling NOTCH2 levels
Because intracellularly cleaved NOTCH translocates to the nucleus and assembles a transcriptional complex,26 we investigated the effect of KLHL6 ablation on the DLBCL transcriptome via RNA sequencing (RNA-seq; supplemental Figure 5A; supplemental Table 2). Gene set enrichment analysis (GSEA) revealed MYC, E2F, unfolded protein response, and oxphos signatures as the top and most significant upregulated signatures in the KLHL6–/–cells (supplemental Figure 5B-C). The RNA-seq analysis of U2932 KLHL6+/+ and KLHL6–/– cells upon coculturing with OP9-DLL1 cells showed a significant number of NOTCH-activated and NOTCH-repressed genes, as previously defined,33 to be further upregulated or repressed in KLHL6–/– cells, respectively (supplemental Figure 5D; supplemental Table 2).
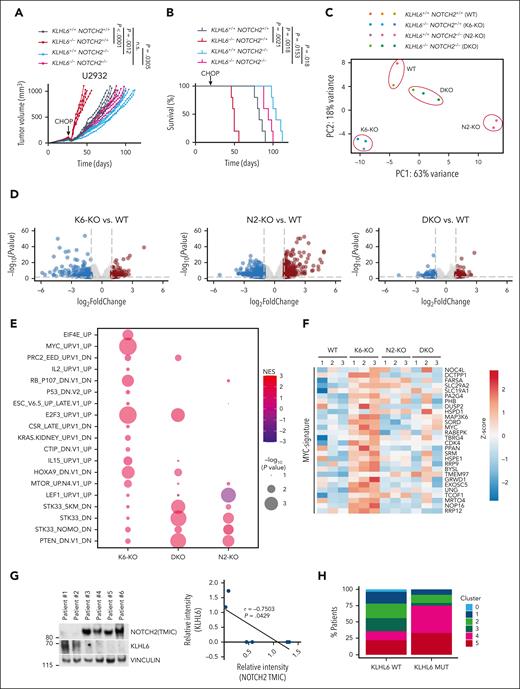
Based on these data, we assessed whether the CHOP-resistance phenotype observed in KLHL6 KO tumors was dependent upon NOTCH2. For this purpose, U2932 KLHL6+/+/NOTCH2+/+ (WT), KLHL6–/–/NOTCH2+/+ (K6-KO), KLHL6+/+/NOTCH2–/– (N2-KO), and KLHL6-/-/NOTCH2–/– (DKO) were xenotransplanted into the flanks of NSG mice, which were then treated with CHOP. As previously shown, K6-KO tumors relapsed earlier than WT tumors; however, concomitant ablation of NOTCH2 (DKO) rescued this phenotype (Figure 5A). Accordingly, overall survival of mice bearing DKO tumors was significantly prolonged as compared with those bearing K6-KO tumors (Figure 5B). Notably, N2-KO and DKO tumors displayed a significant difference, although the biological difference in tumor size and survival was minor as compared with the difference in tumor size and survival between the WT and DKO groups; these data would point a function of KLHL6 that is partially NOTCH2-independent. Immunoblot analysis of tumors at experimental end point helped confirm the tumor genotype and NOTCH2 (TMIC) upregulation in the K6-KO tumors (supplemental Figure 5E). Similar data were observed in vitro in which concomitant ablation of NOTCH2 and KLHL6 rescued doxorubicin desensitization upon single KLHL6 loss (supplemental Figure 5F).
KLHL6 promotes DLBCL sensitivity to CHOP by controlling NOTCH2 levels. (A) 1 × 107 U2932-Cas9 cells with the indicated genotypes were xenografted in the flanks of NSG mice. After the average tumor volumes reached 100 mm3, mice were treated with cyclophosphamide (30 mg/kg), doxorubicin (2.48 mg/kg), and vincristine (0.38 mg/kg) IV on day 1, and prednisone (0.2 mg/kg) was given via oral gavage daily for 5 days. Experimental end point was reached when the tumor volume reached 1000 mm3. Each line represents one tumor in one mouse (n = 5 per group; two-way ANOVA). (B) Kaplan-Meier survival analysis of mice shown in panel A (n = 5 per group; Mantel-Cox test). (C) Principal component analysis for the transcriptomes of tumors with the indicated genotypes. (D) Volcano plot representing the log2(fold change) over the -log10(P value) of the differentially expressed genes. The red circles represent the significantly upregulated genes, and the blue circles represent the significantly downregulated genes (n = 3; DeSeq2; P ≤ .01). (E) Dot plot showing the GSEA for the transcriptomes in (D). Size of the dots is a function of the P value, and the color represents the normalized enrichment score. (F) Heat map showing the relative expression of MYC-signature genes using the normalized counts from the transcriptome analysis in panel D. (G, left) Immunoblot analysis for the indicated proteins in a panel of primary DLBCL biopsies. (G, right) Quantification of NOTCH2 (TMIC) (x-axis) and KLHL6 (y-axis) protein levels in each DLBCL biopsy specimen. (H) Percentage of KLHL6-mutated vs KLHL6 WT patients with DLBCL grouped based on the clusters, as previously defined.6
KLHL6 promotes DLBCL sensitivity to CHOP by controlling NOTCH2 levels. (A) 1 × 107 U2932-Cas9 cells with the indicated genotypes were xenografted in the flanks of NSG mice. After the average tumor volumes reached 100 mm3, mice were treated with cyclophosphamide (30 mg/kg), doxorubicin (2.48 mg/kg), and vincristine (0.38 mg/kg) IV on day 1, and prednisone (0.2 mg/kg) was given via oral gavage daily for 5 days. Experimental end point was reached when the tumor volume reached 1000 mm3. Each line represents one tumor in one mouse (n = 5 per group; two-way ANOVA). (B) Kaplan-Meier survival analysis of mice shown in panel A (n = 5 per group; Mantel-Cox test). (C) Principal component analysis for the transcriptomes of tumors with the indicated genotypes. (D) Volcano plot representing the log2(fold change) over the -log10(P value) of the differentially expressed genes. The red circles represent the significantly upregulated genes, and the blue circles represent the significantly downregulated genes (n = 3; DeSeq2; P ≤ .01). (E) Dot plot showing the GSEA for the transcriptomes in (D). Size of the dots is a function of the P value, and the color represents the normalized enrichment score. (F) Heat map showing the relative expression of MYC-signature genes using the normalized counts from the transcriptome analysis in panel D. (G, left) Immunoblot analysis for the indicated proteins in a panel of primary DLBCL biopsies. (G, right) Quantification of NOTCH2 (TMIC) (x-axis) and KLHL6 (y-axis) protein levels in each DLBCL biopsy specimen. (H) Percentage of KLHL6-mutated vs KLHL6 WT patients with DLBCL grouped based on the clusters, as previously defined.6
To identify the transcriptional pathways downstream of the KLHL6-NOTCH2 axis, we performed RNA-seq from the tumors at the experimental end point. Principal component analysis revealed that WT and DKO tumors were more closely related, as opposed to K6-KO and N2-KO tumors (Figure 5C; supplemental Table 3), suggesting that the transcriptional changes induced by KLHL6 loss could be rescued via NOTCH2 ablation. Next, we analyzed the differentially expressed genes upon loss of KLHL6 alone or in combination with NOTCH2 ablation (Figure 5D). We used GSEA to identify pathways dysregulated because of KLHL6 loss and rescued because of concomitant NOTCH2 ablation (Figure 5E). This analysis revealed the most significant oncogenic signatures that were rescued in the DKO tumors were MYC- and KRAS-activated and p53-repressed genes (Figure 5E). Consistent with the MYC being a known NOTCH-target,33,43,44 heatmap analysis displayed the upregulation of MYC-signature genes in K6-KO tumors in a manner that was dependent on NOTCH2 (Figure 5F). Analysis of KLHL6 and NOTCH2 at protein levels revealed an inverse correlation in primary DLBCL biopsies (Figure 5G); accordingly, KLHL6 expression correlated negatively with a NOTCH-target gene signature score in patients with DLBCL (supplemental Figure 5G).
Notably, KLHL6 mutations were more likely to occur in DLBCLs of cluster 4 (C4)6 (Figure 5H), suggesting that inactivation of KLHL6 could cooperate with BCR/PI3K, NF-κB or RAS signaling. Further, patients carrying KLHL6 mutations or low messenger RNA levels displayed a significantly worse overall survival (supplemental Figure 5H and 5I, respectively). Notably, a similar trend was observed in the DLBCL C4 survival (supplemental Figure 5H), although the patient number was relatively low.
Overall, these data suggest that loss of KLHL6 promotes DLBCL relapse after CHOP in a NOTCH2-dependent manner.
DLBCL-associated NOTCH2 mutants promote resistance to CHOP therapy
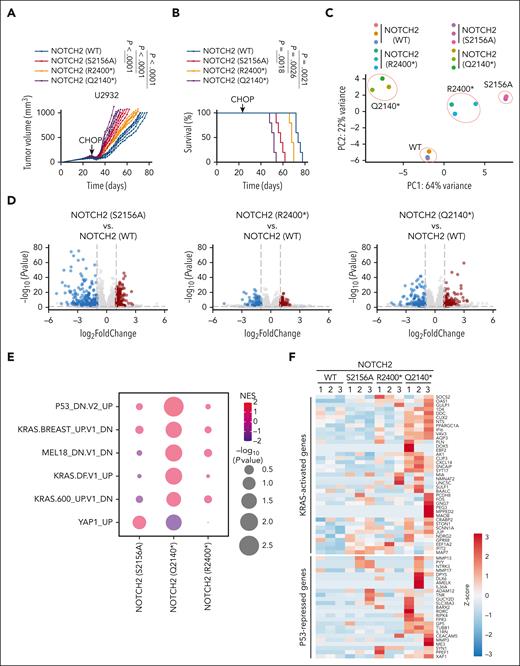
Next, we transplanted U2932-expressing NOTCH2 (WT) and degron mutants NOTCH2 (S2156A), NOTCH2 (R2400∗), and NOTCH2 (Q2140∗) into the flanks of NSG mice (Figure 6A). Analysis of tumor volume over time revealed that tumors expressing cancer-derived mutant NOTCH2 (Q2140∗) relapsed earlier than the tumors expressing single degron mutants NOTCH2 (S2156A) or NOTCH2 (R2400∗) as well as the tumors expressing NOTCH2 (WT). Accordingly, overall survival of mice bearing tumors expressing NOTCH2 (Q2140∗) was significantly reduced as compared with single degron mutants or NOTCH2 (WT)-expressing tumors (Figure 6B).
DLBCL-associated NOTCH2 mutants promote resistance to CHOP therapy. (A) 1 × 107 U2932 cells stably expressing the indicated NOTCH2 cDNA were xenografted in the flanks of NSG mice. After the average tumor volumes reached 100 mm3, mice were treated with cyclophosphamide (30 mg/kg), doxorubicin (2.48 mg/kg), and vincristine (0.38 mg/kg) IV on day 1, and prednisone (0.2 mg/kg) was given via oral gavage daily for 5 days. The experimental end point was reached when the tumor volume reached 1000 mm3. Each line represents one tumor in one mouse (n = 5 per group, two-way ANOVA). (B) Kaplan-Meier survival analysis of mice shown in panel A (n = 5 per group; Mantel-Cox test). (C) Principal component analysis for the transcriptomes of tumors with the indicated genotypes. (D) Volcano plot representing the log2 (fold change) over the -log10 (P value) of the differentially expressed genes. The red circles represent the significantly upregulated genes, and the blue circles represent the significantly downregulated genes (n = 3; DeSeq2; P ≤ .01). (E) Dot plot showing the GSEA for the transcriptomes in (D). The size of the dots is a function of the P value; the color represents the normalized enrichment score. (F) Heat map showing the relative expression of KRAS-activated and P53-repressed signature genes using the normalized counts from the transcriptome analysis in panel D.
DLBCL-associated NOTCH2 mutants promote resistance to CHOP therapy. (A) 1 × 107 U2932 cells stably expressing the indicated NOTCH2 cDNA were xenografted in the flanks of NSG mice. After the average tumor volumes reached 100 mm3, mice were treated with cyclophosphamide (30 mg/kg), doxorubicin (2.48 mg/kg), and vincristine (0.38 mg/kg) IV on day 1, and prednisone (0.2 mg/kg) was given via oral gavage daily for 5 days. The experimental end point was reached when the tumor volume reached 1000 mm3. Each line represents one tumor in one mouse (n = 5 per group, two-way ANOVA). (B) Kaplan-Meier survival analysis of mice shown in panel A (n = 5 per group; Mantel-Cox test). (C) Principal component analysis for the transcriptomes of tumors with the indicated genotypes. (D) Volcano plot representing the log2 (fold change) over the -log10 (P value) of the differentially expressed genes. The red circles represent the significantly upregulated genes, and the blue circles represent the significantly downregulated genes (n = 3; DeSeq2; P ≤ .01). (E) Dot plot showing the GSEA for the transcriptomes in (D). The size of the dots is a function of the P value; the color represents the normalized enrichment score. (F) Heat map showing the relative expression of KRAS-activated and P53-repressed signature genes using the normalized counts from the transcriptome analysis in panel D.
To define the transcriptional pathways controlled by the DLBCL-associated NOTCH2 mutants, we performed RNA-seq on the tumors shown in Figure 6A at the experimental end point. Principal component analysis grouped the tumors based on their genotypes (Figure 6C; supplemental Table 4). Analysis of differentially expressed genes of tumors expressing NOTCH2 mutants were compared with the differentially expressed genes of tumors expressing NOTCH2 (WT) (Figure 6D). To identify significant gene signature changes, GSEA was carried out, and KRAS and p53 signature were identified as the most significantly dysregulated signatures in tumors expressing NOTCH2 (Q2140∗) (Figure 6E). Specifically, KRAS-activated genes and p53-repressed genes were found to be upregulated upon expression of NOTCH2 (Q2140∗) (Figure 6F). Furthermore, chromatin immunoprecipitation followed by sequencing (supplemental Figure 6A) revealed an increased association of RBP-J with DNA when NOTCH2 (Q2140∗) was expressed, as compared with cells expressing either NOTCH2 (WT) or NOTCH2 (R2400∗) (supplemental Figure 6B-C). EnrichR analysis revealed that the increased RBP-J peaks in the cells expressing NOTCH2 (Q2140∗) were significantly clustered in the BCR pathway (supplemental Figure 6D). Notably, genome visualization tracks displayed an accumulation of RBP-J in proximity to the transcription start site and gene bodies of FYN, KRAS, and SOS1 as well as MYC, BCL6, and IRF4 (supplemental Figure 6E).
These data reveal that the loss of NOTCH2 degradation control via FBXW7 and KLHL6 promotes CHOP resistance, upregulates the expression of genes controlled by the RAS pathway, and drives RBP-J binding to the genes of the BCR pathway.
Clinical relevance and combined chemical inhibition of γ-secretase and AKT synergistically impair CHOP-resistant DLBCL viability.
Analysis of the mutational profile in DLBCL data set,45 revealed an increased frequency of KLHL6 and NOTCH2 mutations in cases of early failure to R-CHOP therapy, suggesting that these 2 mutational events could be involved in the selective pressure after chemotherapy (supplemental Figure 7A). In addition, another DLBCL data set6 revealed an increased frequency of KLHL6 mutations in patients receiving R-CHOP therapy (supplemental Figure 7B). Overall survival analysis of patients with DLBCL exhibiting a partial response to R-CHOP showed a worse prognosis when KLHL6 was underexpressed than when it was overexpressed (Figure 7A). Furthermore, overall survival of patients harboring NOTCH2 double-degron mutations displayed a significantly worse prognosis as compared with those harboring NOTCH2 (WT) (Figure 7B), suggesting that ablation of 2 degrons could be more oncogenic.
Clinical relevance and combinatorial treatment for CHOP-resistant DLBCL. (A) Kaplan-Meier survival curves based on KLHL6 expression (top 20% vs bottom 20%) data for DLBCL tumors with partial response to R-CHOP (GSE31312) is shown. The P value from log-rank (Mantel-Cox) test is shown (n = 11 per group). (B) Kaplan-Meier survival analysis of DLBCL patients harboring NOTCH2 (WT) or mutations abolishing the FBXW7-degron alone (NOTCH2 [ΔdegFBXW7]) or the KLHL6-degron and FBXW7-degron (NOTCH2 [ΔdegKLHL6/ΔdegFBXW7]) (NOTCH2 [WT], n = 896; NOTCH2 [ΔdegFBXW7], n = 16; NOTCH2 [ΔdegKLHL6/ΔdegFBXW7]; n = 5; Mantel-Cox test).24 (C) 1 × 107 U2932 cells were xenografted in the flanks of NSG mice. After the average tumor volumes reached 100 mm3, mice were treated with cyclophosphamide (30 mg/kg), doxorubicin (2.48 mg/kg), and vincristine (0.38 mg/kg) IV on day 1 and prednisone (0.2 mg/kg) was given via oral gavage daily with ipataserbib (50 mg/kg) or nirogacestat (100 mg/kg), for 5 days and 7 days respectively, or combination. The experimental end point was reached when the tumor volume reached 1000 mm3. Each line represents one tumor in one mouse (n = 5 per group, two-way ANOVA). (D) Kaplan-Meier survival analysis of mice shown in (C), (n = 5 per group, the Mantel-Cox test). (E) Same as that in panel C, but U2932 cells stably expressing the indicated NOTCH2 cDNA were xenografted. (F) Kaplan-Meier survival analysis of mice shown in panel E (n = 5 per group; the Mantel-Cox test).
Clinical relevance and combinatorial treatment for CHOP-resistant DLBCL. (A) Kaplan-Meier survival curves based on KLHL6 expression (top 20% vs bottom 20%) data for DLBCL tumors with partial response to R-CHOP (GSE31312) is shown. The P value from log-rank (Mantel-Cox) test is shown (n = 11 per group). (B) Kaplan-Meier survival analysis of DLBCL patients harboring NOTCH2 (WT) or mutations abolishing the FBXW7-degron alone (NOTCH2 [ΔdegFBXW7]) or the KLHL6-degron and FBXW7-degron (NOTCH2 [ΔdegKLHL6/ΔdegFBXW7]) (NOTCH2 [WT], n = 896; NOTCH2 [ΔdegFBXW7], n = 16; NOTCH2 [ΔdegKLHL6/ΔdegFBXW7]; n = 5; Mantel-Cox test).24 (C) 1 × 107 U2932 cells were xenografted in the flanks of NSG mice. After the average tumor volumes reached 100 mm3, mice were treated with cyclophosphamide (30 mg/kg), doxorubicin (2.48 mg/kg), and vincristine (0.38 mg/kg) IV on day 1 and prednisone (0.2 mg/kg) was given via oral gavage daily with ipataserbib (50 mg/kg) or nirogacestat (100 mg/kg), for 5 days and 7 days respectively, or combination. The experimental end point was reached when the tumor volume reached 1000 mm3. Each line represents one tumor in one mouse (n = 5 per group, two-way ANOVA). (D) Kaplan-Meier survival analysis of mice shown in (C), (n = 5 per group, the Mantel-Cox test). (E) Same as that in panel C, but U2932 cells stably expressing the indicated NOTCH2 cDNA were xenografted. (F) Kaplan-Meier survival analysis of mice shown in panel E (n = 5 per group; the Mantel-Cox test).
To inhibit NOTCH signaling, we used nirogacestat,46 an oral, selective, reversible, noncompetitive GSI that showed efficacy and safety in a phase 3, randomized, placebo-controlled trial involving patients with progressing desmoid tumors.47 Notably, nirogacestat is also being tested in the treatment of multiple myeloma, in combination with belantamab mafodotin in a phase 1 study (NCT05556798). Firstly, we confirmed that nirogacestat inhibited NOTCH1 cleavage effectively (supplemental Figure 7C). Next, we performed a nirogacestat-sensitivity assay on U2932 cells isolated from CHOP-treated tumors shown in Figure 6A and found a general desensitization toward nirogacestat (supplemental Figure 7D; IC50 values were >3 μM).
Based on the data that stabilization of NOTCH2 can induce activation of the BCR/RAS pathway, we hypothesized that combinatorial inhibition of NOTCH with inhibitors of the RAS cascade could promote a stronger antitumor effect. We initially screened the combinatorial effect of vemurafenib (a RAF inhibitor),48 trametinib (an MEK1/2 inhibitor),49 ulixertinib (an extracellular signal-regulated kinase 1/2 inhibitor),50 alpelisib (a PI3K inhibitor),51 and ipatasertib (an AKT inhibitor)52 in combination with nirogacestat (supplemental Figure 7E). Viability analysis of U2932 cells isolated from CHOP-treated tumors revealed that the ipatasertib and nirogacestat combination had the strongest antiproliferation activity.
Ipatasertib is a potent, small-molecule kinase inhibitor highly specific to AKT53 which demonstrates efficacy in various cancer cells (eg, prostate cancer54 and breast cancer55). To assess the effect of ipatasertib and nirogacestat on DLBCL cells expressing NOTCH2 cancer–associated mutants, we treated U2932 cells isolated from mice that were treated with CHOP therapy with a low nanomolar dosage of ipatasertib and nirogacestat (supplemental Figure 7F). Single-agent treatments had weak effects on U2932 cell viability; however, the combination of the 2 drugs induced an overall decrease in cell viability in all biological conditions. Combination index calculation revealed that U2932 cells were sensitive to the drug combination, revealing a synergistic effect at both nanomolar dosages (supplemental Figure 7G).
Next, we transplanted U2932 cells into the flanks of NSG mice and treated these mice with CHOP, followed by either monotherapy or combination therapy (Figure 7C). Tumor volume assessment over time revealed that ipatasertib and nirogacestat drug combination significantly delayed tumor growth (Figure 7C) and resulted in an increased overall survival (Figure 7D). A similar experiment was performed in mice that received transplantation with degron mutants NOTCH2 (S2156A), NOTCH2 (R2400∗), and NOTCH2 (Q2140∗) (Figure 7E). The combinatorial treatment was effective in these tumors as well as in cells expressing the dual-degron mutant, NOTCH2 (Q2140∗) (Figure 7E), resulting in a significantly increased overall survival (Figure 7F). Importantly, the ipatasertib and nirogacestat drug combination was well tolerated by the mice, based on the body weight assessed at the experimental end point (supplemental Figure 7H).
Altogether, our data show that the combinatorial inhibition of NOTCH and AKT synergistically produces an antitumor effect in CHOP-resistant DLBCL.
Discussion
R-CHOP is a therapeutic regimen effective in ∼60% of patients with DLBCL; however, ∼40% of patients fail to respond, displaying a poor prognosis. Several trials are assessing response in R-CHOP rrDLBCLs by targeting Bruton tyrosine kinase, spleen tyrosine kinase, EZH2, PI3K, mammalian target of rapamycin, JAK, and BCL proteins.11
The genetic changes in rrDLBCLs are of critical importance for patient treatment. However, because of the lack of complete clinical data and follow-up in these reports,5,24,39,40,56 the clinical impact of the genetic mutations is still unknown. KLHL6 is a bric-a-brac, tramtrack, broad complex (BTB)-Kelch domain–containing protein expressed specifically in B cells, and cancer-associated mutations are detected in B-cell malignancies, including DLBCL.5,24,39-42 Most somatic mutations localize to the BTB domain, resulting in the loss of cullin-3 interaction and E3 ligase activity.25 Furthermore, the overall survival rates in patients with DLBCL exhibiting a partial response to R-CHOP suggests a worse prognosis when KLHL6 is underexpressed. In addition, analysis of mutational profiles in a small subset of paired diagnostic-relapse samples showed recurrent KLHL6 mutations in relapse.57
In this study, we demonstrated that inactivation of KLHL6 promotes chemoresistance to DLBCL. Mechanistically, we identified KLHL6 as a novel E3 ubiquitin ligase of NOTCH2. KLHL6 specifically binds, ubiquitylates, and triggers the degradation of plasma membrane NOTCH2. Importantly, KLHL6-KO DLBCL tumors display a CHOP-resistance phenotype with upregulation of RNA signatures, such as MYC- and KRAS-activated genes and p53-repressed genes in a NOTCH2-dependent manner. Notably, KLHL6 mutations cluster in DLBCLs of C4,6 suggesting that the inactivation of KLHL6 cooperates with BCR/PI3K, NF-κB, or RAS signaling.
In DLBCL, most mutations in either NOTCH1 or NOTCH2 lead to the deletion of the C-terminal PEST domain, inhibiting NICD degradation via ubiquitylation by the E3 ligase FBXW7.5,24 However, our biochemical analysis revealed that KLHL6 binds NOTCH2 in a motif that is upstream of the PEST domain. Patients with DLBCL display nonsense mutations leading to a truncation of the PEST domain alone (ie, R2400∗), or the KLHL6-binding motif and the PEST domain simultaneously (ie, Q2140∗).5,24,39-42 This double-degron NOTCH2 (Q2140∗) mutant displays significantly increased levels and half-life over NOTCH2 (WT), or single degron mutants in DLBCL. DLBCLs expressing double-degron NOTCH2 mutant relapse earliest to CHOP therapy and induce KRAS-activated genes and p53-repressed genes leading to activation of the AKT/ERK pathway in CHOP-resistant DLBCL. Consistently, chromatin immunoprecipitation followed by sequencing analysis revealed that DLBCLs expressing double-degron NOTCH2 mutant display an increased association of RBP-J in the proximity of the transcription start site and gene body of the BCR pathway, such as FYN, KRAS, and SOS1 as well as MYC, BCL6, and IRF4.
Activation of AKT by NOTCH1 has been described previously in GSI-sensitive T-cell acute lymphoblastic leukemia as a mechanism in which the expression of the phosphatase and tensin homolog is repressed by HES1.58,59 These findings are in agreement with that of our analysis that hyper-NOTCH activation in CHOP-resistant DLBCL leads to RAS/AKT activation. More evidence suggests that high levels of phosphorylated AKT have an adverse prognostic impact on patients with DLBCL treated with R-CHOP.60 As such, components of the PI3K-AKT signaling pathway are therapeutic targets in DLBCL. Importantly, an AKT inhibitor, ipatasertib, has been used in phase 3 clinical trial for prostate cancer.61
Although clinical studies with GSIs showed NOTCH-associated adverse side-effects,62,63 an oral, selective, reversible, noncompetitive GSI, nirogacestat, was well tolerated in patients with desmoid tumors.46,64 Furthermore, nirogacestat has been used to overcome resistance to tyrosine kinase inhibitors in epidermal growth factor receptor–driven lungadenocarcinoma.65
To our knowledge, our study for the first time, evaluated the effect of nirogacestat and ipatasertib in conjunction with the CHOP regimen. We showed that combinatorial inhibition of NOTCH and AKT synergistically produces an antitumor effect in combination with CHOP therapy, suggesting this combination as potential therapeutic regimen in rrDLBCL. Further clinical studies will be required to evaluate this hypothesis.
Altogether, this study reveals the existence of a novel posttranslational regulation of NOTCH2 via the ubiquitin pathway, opening a door to the use of an alternative therapeutic regimen to treat CHOP-resistant DLBCL.
Acknowledgments
The authors thank Ashley N. Hughes for critically reading the manuscript and Stephen C. Blacklow for kindly providing human NOTCH2 full-length complementary DNA.
This work was supported by Berman Family Fund (M.R.), National Cancer Institute, National Institutes of Health (NCI/NIH) grants R37 CA262362 (M.R.), R50 CA221838A (H.-Y.T.), 2R01CA207513 (L.B.), and 1DP2HG012443 and R00CA226399 (L.W.); National Institute of Allergy and Infectious Diseases, NIH grants F30 AI136325 (E.P.) and R01-AI091627 (I.M.), a Health Research Formula Fund from the Pennsylvania Department of Health (I.M.), American Cancer Society grant RSG-19-199-01 L.B.), and Leukemia & Lymphoma Society Career Development (L.B.). L.W. was supported by the Pew-Stewart Scholars Program for Cancer Research, the V Foundation, and the American Society of Hematology. Y.L. was supported by the Abramson Family Cancer Research Institute Postdoctoral Fellowship. The Wistar Proteomics and Metabolomics Facility was supported by NCI/NIH grant P30 CA010815.
Authorship
Contribution: L.B. conceived and directed the project and oversaw the results; N.Z. designed and performed most experiments; J.C. constructed Roquin2 knockdown cell lines and identified FLAG-KLHL6 interactome; G.G. helped with sgRNA for PCNA (gPCNA) construct and designing the CRISPR library; B.-J.K. helped with the administration of drugs into mice; J.S., D.R., and Z.C. helped with CRISPR library construction and screening; J.S. kindly provided LRG2.1 and LRCherry2.1 vectors; T.B. and H.-Y.T. performed the mass spectrometry analysis of TurboID-KLHL6 interactome; E.P. and I.M. kindly provided OP9-DLL1 cells as well as experimental advice R.B. helped with RNA-seq; M.R. and A.I. provided the primary DLBCL samples; Y.L., Q.L., and L.W. helped with chromatin immunoprecipitation followed by sequencing; and L.B. and N.Z. wrote the manuscript.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
The current affiliation for J.C. is Lymphoid Malignancies Branch, Center for Cancer Research, National Cancer Institute, National Institutes of Health, Bethesda, MD.
The current affiliation for B.-J.K. is Department of Surgery, Columbia University Irving Medical Center, New York, NY.
Correspondence: Luca Busino, Department of Cancer Biology, Perelman School of Medicine, University of Pennsylvania, 421 Curie Blvd, Philadelphia, PA; e-mail: businol@upenn.edu.
References
Author notes
Next generation dequencing data are deposited in the Gene Expression Omnibus database (accession number GSE231625).
Original data are available on request from the corresponding author, Luca Busino (businol@upenn.edu).
The online version of this article contains a data supplement.
There is a Blood Commentary on this article in this issue.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.

![KLHL6 promotes chemosensitivity of DLBCL cells. (A) Schematic representation of the CRISPR screen using the CRL sgRNA library in U2932-Cas9 cells. (B) Volcano plot representing the CRISPR score and P value. The red circles represent the genes whose sgRNAs were significantly enriched in doxorubicin-treated cells (two-tailed Student t test; P ≤ .01). (C) Genes selected in panel B were evaluated for the percentages of genetic alterations in DLBCL (Duke Cancer Institute, n = 1001; Dana-Farber Cancer Institute, n = 135; Broad Institute, n = 58; The Cancer Genome Atlas PanCancer Atlas, n = 48; The Cancer Genome Atlas Firehose Legacy, n = 48; BC Cancer Canada’s Michael Smith Genome Sciences Centre, n = 53). (D) 1 × 107 OCI-LY10 KLHL6+/+ and KLHL6–/– cells were xenografted in the flanks of NSG mice. After the average tumor volumes reached 100 mm3, mice were treated with cyclophosphamide (30 mg/kg), doxorubicin (2.48 mg/kg), and vincristine (0.38 mg/kg) IV on day 1, and prednisone (0.2 mg/kg) was given via oral gavage daily for 5 days. The experimental end point was reached when the tumor volume reached 1000 mm3. Each line represents 1 tumor in 1 mouse (n = 5 per group, two-way analysis of variance [ANOVA]). (E) Kaplan-Meier survival curves of OCI-LY10 KLHL6+/+ and KLHL6–/– xenografts as shown in panel D (n = 5 per group; the Mantel-Cox test). DMSO, dimethyl sulfoxide.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/142/11/10.1182_blood.2022018752/1/m_blood_bld-2022-018752-gr1.jpeg?Expires=1770213797&Signature=eZzaWfzsUOwQ1Gv8sfxtIsFvosPb02oteyJvZosKtXpoqF0HsDJRmjLRvhCK3f0tN2FWgYPAvk0p9mUJ8FrLdUdMI5uopTwzBKQuE6hPhsmHvXJFBuFb8Mgu1uH66godzVsb6G87VVr3VPf~GqPmr5rBLZlA6NSSLk9AiHoMrXECepvuQQVQo0JFcPHW7-4XnZNXJmEBpM3OBkYzmDw3E3MOu3OYRjXQzQGo7sH-OuQpS3Bv5B-tsGqDAxegueR4uTPGH3Dd7np75kAZ5~MLimf3JAcX7oKpKxy6u54ADnVcq-J4xX4vSEiFie48g9XfFZ-8z9XRm1Obi4krvNXaDw__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)
![Clinical relevance and combinatorial treatment for CHOP-resistant DLBCL. (A) Kaplan-Meier survival curves based on KLHL6 expression (top 20% vs bottom 20%) data for DLBCL tumors with partial response to R-CHOP (GSE31312) is shown. The P value from log-rank (Mantel-Cox) test is shown (n = 11 per group). (B) Kaplan-Meier survival analysis of DLBCL patients harboring NOTCH2 (WT) or mutations abolishing the FBXW7-degron alone (NOTCH2 [ΔdegFBXW7]) or the KLHL6-degron and FBXW7-degron (NOTCH2 [ΔdegKLHL6/ΔdegFBXW7]) (NOTCH2 [WT], n = 896; NOTCH2 [ΔdegFBXW7], n = 16; NOTCH2 [ΔdegKLHL6/ΔdegFBXW7]; n = 5; Mantel-Cox test).24 (C) 1 × 107 U2932 cells were xenografted in the flanks of NSG mice. After the average tumor volumes reached 100 mm3, mice were treated with cyclophosphamide (30 mg/kg), doxorubicin (2.48 mg/kg), and vincristine (0.38 mg/kg) IV on day 1 and prednisone (0.2 mg/kg) was given via oral gavage daily with ipataserbib (50 mg/kg) or nirogacestat (100 mg/kg), for 5 days and 7 days respectively, or combination. The experimental end point was reached when the tumor volume reached 1000 mm3. Each line represents one tumor in one mouse (n = 5 per group, two-way ANOVA). (D) Kaplan-Meier survival analysis of mice shown in (C), (n = 5 per group, the Mantel-Cox test). (E) Same as that in panel C, but U2932 cells stably expressing the indicated NOTCH2 cDNA were xenografted. (F) Kaplan-Meier survival analysis of mice shown in panel E (n = 5 per group; the Mantel-Cox test).](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/142/11/10.1182_blood.2022018752/1/m_blood_bld-2022-018752-gr7.jpeg?Expires=1770213797&Signature=fcBmCkXujhh02J0YDhc2TpEaj4Uw9RhPKeoo1qV2GJvutEWoZqad7E6-DenGPEG1htPgN2-Gooy3sCiLfDlJsev1rCts3CrLcsulR5SVmilxw7-8TK~-uoUxf93ZhNAeCU8SFk6myPmFj8CPJVUYmtWDIuCiaThiyZ1Vd62ATwsSCc0Xw415M6Imr8ktO6jjhpnQgPtebWBukdWlwMjszGWfLA1PzHVo2Wnu-81O6vfsFUd5vpOhRUCQPThbYVrw05AJf3hWbUTcUS65OsLmtaziPH8o5~tXYGJqLnY7bUiFODpZPCyKdie5SUettJeRks3gq2xxOFGuFYmyTybHWA__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)
This feature is available to Subscribers Only
Sign In or Create an Account Close Modal