In this issue of Blood, Zhou et al reveal the role of KLHL6 inactivation in chemoresistance in diffuse large B-cell lymphoma (DLBCL).1 KLH6 is a cullin-ring ubiquitin type 3 ligase and a central player in the ubiquitin proteasome system, a cellular system that targets proteins for subsequent degradation, thereby limiting their half-life and activity. Type 3 ligases, such as KLHL6, attach the ubiquitin chain to the target protein.
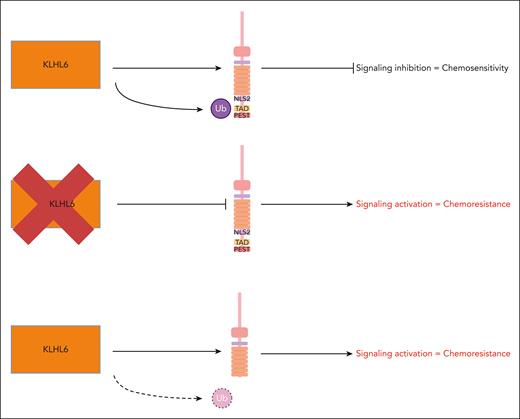
KLHL6 mutations have been described in many cancers, including DLBCL, in which mutations have been reported in 7% to 15% of patients. These genetic variants are usually loss of function, supporting a tumor-suppressor role for the enzyme.2 A link between loss of activity of KLHL6 and DLBCL generation was previously made by the same investigators, who showed that loss of enzyme activity resulted in NF-κB signaling through a molecular circuit involving roquin2, an RNA-binding protein that promotes RNA decay.3 Now, by using elegant genetic and biochemical approaches, the authors show that KLHL6 ubiquitinates NOTCH2, resulting in its degradation and terminating signaling. Thus, inactivating mutations in KLHL6 result in the lack of NOTCH2 ubiquitination and prolonged signaling. Likewise, NOTCH2 mutations evade KLHL6-mediated ubiquitination, with the same overall result of overactivation of NOTCH2-regulated RAS-dependent oncogenic pathways (see figure).
Schematic representation of the main findings reported by Zhou et al. The authors show that NOTCH2 is a target of the cullin-ring ubiquitin type 3 ligase KLHL6. Ubiquitination of the intracellular part of NOTCH2 causes protein degradation and pathway inhibition. Loss-of-function mutations in KLHL6 or NOTCH2 mutations in the C-terminal domains result in both failed ubiquitination and failed pathway inhibition, leading to activation of a RAS/extracellular signal-regulated kinase–dependent signaling and chemoresistance. NLS, nuclear localization signal; PEST, proline/glutamic acid/serine/threonine; TAD, transcriptional activation domain; Ub, ubiquitination.
Schematic representation of the main findings reported by Zhou et al. The authors show that NOTCH2 is a target of the cullin-ring ubiquitin type 3 ligase KLHL6. Ubiquitination of the intracellular part of NOTCH2 causes protein degradation and pathway inhibition. Loss-of-function mutations in KLHL6 or NOTCH2 mutations in the C-terminal domains result in both failed ubiquitination and failed pathway inhibition, leading to activation of a RAS/extracellular signal-regulated kinase–dependent signaling and chemoresistance. NLS, nuclear localization signal; PEST, proline/glutamic acid/serine/threonine; TAD, transcriptional activation domain; Ub, ubiquitination.
NOTCH2 codes for a ligand-activated receptor-activated transcription factor that is recurrently mutated in DLBCL. According to 2 seminal articles that recently reclassified DLBCL based on their molecular lesions, NOTCH2 mutations are present in more than 20% of all cases, defining specific disease subsets.4,5 From the clinical standpoint, a subsequent large study performed on 928 unselected patients determined that NOTCH2-mutated DLBCLs are a mixture of activated B-cell, germinal center B-cell, and unclassified lymphomas, with an intermediate prognosis.6 Conversely, KLHL6 mutations are typical of the subset of DLBCLs enriched in SGK1/TET2 mutations, which in some, but not all, studies are associated with a favorable prognosis. As they impact the same oncogenic pathways, NOTCH2 and KLHL6 mutations do not overlap. Although it is interesting that the molecular subgroups with NOTCH2 and KLHL6 mutations present with similarities to splenic marginal zone lymphomas (SMZL) in which NOTCH2 mutations are among the most common genetic event, their biology and behavior are highly different, and no DLBCL occurs in a preexisting SMZL.
A link between activation of NOTCH2-regulated pathways and chemoresistance was previously made in other experimental models, including breast cancer7 and neural stem cells,8 but never in DLBCL. The link to chemoresistance is important. Despite recent advances in our understanding of the molecular architecture of DLBCL, initial therapy for routine clinical care is not based on the molecular architecture. The mainstay of therapy remains a combination of chemotherapeutic agents, steroids, and anti-CD20 monoclonal antibodies (R-CHOP). A significant proportion of patients acquire resistance to this regimen. Currently, there are 146 clinical trials actively recruiting patients with DLBCL (https://clinicaltrials.gov/), most of them investigating therapy of relapsed/refractory disease.
By elegantly joining their molecular data to patient data set analyses, the authors determined a connection between the presence of KLHL6 mutations, resistance to R-CHOP, and poor overall survival. These observations provide the rationale for overcoming resistance using nirogacestat, which inhibits NOTCH2 and the RAS pathway, which is directly affected by NOTCH2 mutations. Nirogacestat is a novel and highly promising γ secretase inhibitor, which very recently showed significant benefits in adult patients with progressing desmoid tumors. In that study, in which the drug was used as a single agent, side effects of treatment, although frequent, were usually well tolerated.9 Clinical exploration in DLBCL is still far off as the drug needs to be studied in other models, such as patient-derived xenografts, before being considered for moving to the clinic.
Many questions remain open, such as defining the exact role of the NOTCH2 pathway in DLBLC. In the current report, the authors concentrate on early cellular consequence of NOTCH2 mutations in DLBCL, showing that R-CHOP–resistant NOTCH2-mutated cells have an overactivation of the AKT/ERK pathway. Thus, there are complex genetic effects that still need to be identified and studied. Identifying the specific molecular intermediates will be essential to tailor therapy to the causative molecular lesion in patients who are chemoresistant.
Conflict-of-interest disclosure: The author declares no competing financial interests.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal