Key Points
FXII-mediated activation of the contact pathway is increased in mice and patients with SCD at steady state.
FXII contributes to thrombin generation, inflammation, vascular stasis, venous thrombosis, and ischemic brain injury in SCD mice.
Abstract
A hypercoagulable state, chronic inflammation, and increased risk of venous thrombosis and stroke are prominent features in patients with sickle cell disease (SCD). Coagulation factor XII (FXII) triggers activation of the contact system that is known to be involved in both thrombosis and inflammation, but not in physiological hemostasis. Therefore, we investigated whether FXII contributes to the prothrombotic and inflammatory complications associated with SCD. We found that when compared with healthy controls, patients with SCD exhibit increased circulating biomarkers of FXII activation that are associated with increased activation of the contact pathway. We also found that FXII, but not tissue factor, contributes to enhanced thrombin generation and systemic inflammation observed in sickle cell mice challenged with tumor necrosis factor α. In addition, FXII inhibition significantly reduced experimental venous thrombosis, congestion, and microvascular stasis in a mouse model of SCD. Moreover, inhibition of FXII attenuated brain damage and reduced neutrophil adhesion to the brain vasculature of sickle cell mice after ischemia/reperfusion induced by transient middle cerebral artery occlusion. Finally, we found higher FXII, urokinase plasminogen activator receptor, and αMβ2 integrin expression in neutrophils of patients with SCD compared with healthy controls. Our data indicate that targeting FXII effectively reduces experimental thromboinflammation and vascular complications in a mouse model of SCD, suggesting that FXII inhibition may provide a safe approach for interference with inflammation, thrombotic complications, and vaso-occlusion in patients with SCD.
Introduction
Sickle cell disease (SCD), the most common inherited hemoglobinopathy, is caused by a single nucleotide mutation of the β-globin gene that results in the formation of misshapen rigid (“sickle”) red blood cells (RBCs) under hypoxic conditions.1-4 Although hemolytic anemia and vaso-occlusive crisis (VOC) are 2 primary pathologies caused by sickling of RBCs, SCD is also associated with chronic vascular inflammation and activation of coagulation.1,4 It is now well established that patients with SCD are at increased risk of venous and arterial thrombosis (including ischemic stroke and silent cerebral infarction) that are associated with increased morbidity and mortality.5-7
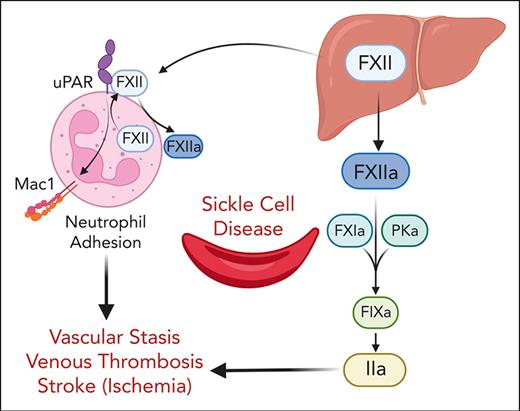
Tissue factor (TF)-initiated activation of coagulation contributes to systemic thrombin generation and inflammation,8,9 cardiovascular dysfunction,10 end-organ damage,11 and vascular stasis12 in mouse models of SCD. There is growing interest in targeting the coagulation factor (FXII) activated protease form (FXIIa)-initiated intrinsic pathway to prevent thrombosis, because unlike components of the extrinsic or common coagulation pathways, FXII is not required for hemostasis.13 FXII is primarily produced and secreted by hepatocytes; however, neutrophils have recently been identified as an additional source of FXII.14,15 In addition to its well-established role in initiation of the contact system that triggers the intrinsic coagulation and kallikrein-kinin-pathways,16,17 FXII plays an important role in leukocyte-mediated proinflammatory responses via interaction with its receptor, urokinase plasminogen activator receptor (uPAR) CD87.15
The role of FXII in the pathophysiology of SCD is largely unknown. Older reports demonstrated the reduction of plasma levels of FXII zymogen in patients with SCD compared with healthy controls,18-20 suggesting ongoing activation and consumption in vivo. Given the growing evidence that inhibiting zymogen FXII and its FXIIa spares hemostasis while simultaneously reducing thrombosis and inflammation, we set out to determine whether FXII/FXIIa contribute to the heightened thrombotic complications and VOC associated with SCD.
Materials and methods
Patient sample collection
Outpatients with SCD (n = 53) and healthy race-matched controls (n = 23) were recruited for studies on in vivo contact pathway activation (patient characteristics are shown in Table 1). Inclusion criteria were steady state disease (>1 month from last pain crisis), no transfusion within previous 3 months, and no current use of oral contraceptives, anticoagulants, or antiplatelet agents. Blood samples were obtained by clean venipuncture using a 21G butterfly needle. Platelet-poor plasma was prepared from blood drawn into 3.2% sodium citrate (ratio of blood to anticoagulant, 9:1) and stored at −80°C until analysis. The study was approved by the University of North Carolina's Institutional Review Board for human subjects (UNC IRB# 13-1906), and written informed consent was obtained from all study participants.
Demographic and descriptive data of study participants
| Parameter . | Controls (n = 23) . | SCD (n = 53) . |
|---|---|---|
| Age, y, median (range) | 28 (19-54) | 33.3 (19.1-59.4) |
| Gender | 16 female, 7 male | 27 female, 26 male |
| Hematocrit (%) | 39 (34-45) | 27.5 (19.5-34.4)∗∗∗∗ |
| Hb (g/dL) | 13 (10.8-14.8) | 9.4 (6.5-11.5)∗∗∗∗ |
| MCV (fL) | 89 (80.8-97.0) | 98.5 (69-122)∗ |
| Platelet (109/μL) | 253 (167-352) | 329 (191-695)∗∗ |
| White blood cells (103/μL) | 5.4 (3.2-8.5) | 8.75 (7.1-11.9)∗∗∗∗ |
| TAT complexes (ng/mL) | 1.44 (0.02-8.80) | 8.13 (0.16-32.00)∗∗∗ |
| D-dimer (ng/mL) | 226.2 (43.7-1347.0) | 1865 (213.5-7659.0)∗∗∗∗ |
| HU (%) | 0 | 79 (42/53) |
| Parameter . | Controls (n = 23) . | SCD (n = 53) . |
|---|---|---|
| Age, y, median (range) | 28 (19-54) | 33.3 (19.1-59.4) |
| Gender | 16 female, 7 male | 27 female, 26 male |
| Hematocrit (%) | 39 (34-45) | 27.5 (19.5-34.4)∗∗∗∗ |
| Hb (g/dL) | 13 (10.8-14.8) | 9.4 (6.5-11.5)∗∗∗∗ |
| MCV (fL) | 89 (80.8-97.0) | 98.5 (69-122)∗ |
| Platelet (109/μL) | 253 (167-352) | 329 (191-695)∗∗ |
| White blood cells (103/μL) | 5.4 (3.2-8.5) | 8.75 (7.1-11.9)∗∗∗∗ |
| TAT complexes (ng/mL) | 1.44 (0.02-8.80) | 8.13 (0.16-32.00)∗∗∗ |
| D-dimer (ng/mL) | 226.2 (43.7-1347.0) | 1865 (213.5-7659.0)∗∗∗∗ |
| HU (%) | 0 | 79 (42/53) |
HU, hydroxyurea; MCV, mean corpuscular volume.
∗P < .05, ∗∗P < .01, ∗∗∗P < .001, ∗∗∗∗P < .0001 vs control samples by unpaired Student t test. These cohorts were previously described.21
Neutrophils were isolated from whole blood obtained from healthy individuals and patients with SCD in accordance with a University Hospitals Cleveland Medical Center approved protocol. Inclusion criteria were >18 years of age, male or female, not on medications (immunosuppressive or nonsteroidal anti-inflammatory agents), and without a diagnosis of an acute illness in the past 4 weeks. Eligible patients with SCD were at steady state disease (>1 month from the last pain crisis), had not received transfusion within 30 days, and were not on anticoagulation, oral contraceptives, or antiplatelet therapy. Whole blood was drawn by venipuncture into 3.2% sodium citrate (9:1).
Mouse model of SCD
The Townes knockin model of SCD (hα/hα, hγ/hγ, hβA/hβS) was used for these studies.22,23 Mice were bred by intercrossing HbAS parents to generate mice that exclusively express human globins α, γ, and either βS (HbSS, sickle) or βA (HbAA, wild type). Studies used 4- to 5-month-old mice, with males and females in equal proportions. Mice were phenotyped by hemoglobin (Hb) electrophoresis (Helena Laboratories, Beaumont, TX). All studies were in accordance with the Animal Care and Use Committees of University of North Carolina, Louisiana State University, and Brunel University London and complied with Animal Research: Reporting In Vivo Experiments guidelines.
Experimental procedures
The dorsal skinfold chamber model of vascular stasis, femoral vein electrolytic injury venous thrombosis model, transient middle cerebral artery occlusion (tMCAO) stroke model, bone marrow (BM) transplantation, isolation of mouse and human neutrophils, immunofluorescence and flow cytometry studies, FXIIa and TF activity assays, collection of plasma and tissue samples, histological analysis of liver and kidney paraffin sections, and microscale thermophoresis are described in detail in the supplemental Materials, available on the Blood website.
Results
Markers of contact and intrinsic coagulation pathway activation are elevated in patients with SCD during steady state
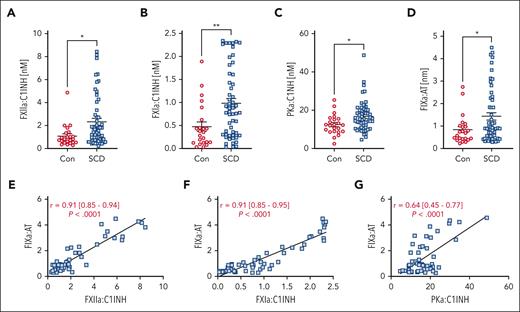
Previous studies reported decreased plasma levels of zymogen FXII, plasma prekallikrein (PK), and high–molecular weight kininogen in patients with SCD,18-20 suggesting that these proteins may be consumed due to chronic activation. We obtained plasma samples from matched controls and patients with SCD (Table 1).21 Patients with SCD had lower hematocrit and Hb levels, increased platelet and white blood cell counts, and significantly elevated thrombin-antithrombin (TAT) complexes and D-dimer levels compared with controls (Table 1). To determine whether components of the contact and intrinsic pathways are activated in patients with SCD, we used enzyme-linked immunosorbent assays to detect FXIIa, PKa, FXIa, or FIXa, bound to their endogenous plasma inhibitors C1 esterase inhibitor (C1INH) (for FXIIa, FXIa, and PKa) or antithrombin (AT) (for FIXa).24,25 We found that patients with SCD had significantly elevated plasma levels of FXIIa:C1INH, FXIa:C1INH, PKa:C1INH, and FIXa:AT complexes compared with healthy controls (Figure 1A-D), none of which correlated with RBC count, hematocrit, or Hb levels in patients with SCD (supplemental Figure 1). In the SCD group, we observed highly significant correlations between FXIIa:C1INH and FXIa:C1INH with FIXa:AT complexes (Figure 1E-F). Interestingly, a significant correlation was also observed between plasma levels of PKa:C1INH and FIXa:AT complexes (Figure 1G). These data suggest that in patients with SCD, FXIIa activates both FXI and PK. Furthermore, correlation analyses suggest that activation of FIX is not only mediated by FXIa but also, in part, by PKa. Consistent with increased plasma levels of FXIIa:C1INH complexes in patients with SCD, immunoblot analysis demonstrated an increase in FXIIa in HbSS mouse plasma compared with HbAA controls (supplemental Figure 2).
Markers of contact and intrinsic pathway activation are elevated in patients with SCD compared with that in healthy controls. Plasma from healthy controls (n = 23) and patients with SCD (n = 53) was assayed for (A) FXIIa-C1INH, (B) FXIa:C1INH, (C) PKa:C1INH, and (D) FIXa-AT complexes. Data are presented as mean ± SEM and analyzed by Student t test. ∗P < .05, ∗∗P < .01. Correlations between (E) FXIIa:C1INH, (F) FXIa:C1INH, and (G) PKa:C1INH with FIXa-AT were calculated and analyzed by linear regression. Mean r with range and P value is reported on graphs. AT, antithrombin.
Markers of contact and intrinsic pathway activation are elevated in patients with SCD compared with that in healthy controls. Plasma from healthy controls (n = 23) and patients with SCD (n = 53) was assayed for (A) FXIIa-C1INH, (B) FXIa:C1INH, (C) PKa:C1INH, and (D) FIXa-AT complexes. Data are presented as mean ± SEM and analyzed by Student t test. ∗P < .05, ∗∗P < .01. Correlations between (E) FXIIa:C1INH, (F) FXIa:C1INH, and (G) PKa:C1INH with FIXa-AT were calculated and analyzed by linear regression. Mean r with range and P value is reported on graphs. AT, antithrombin.
FXII contributes to thrombin generation and inflammation in HbSS mice during steady state
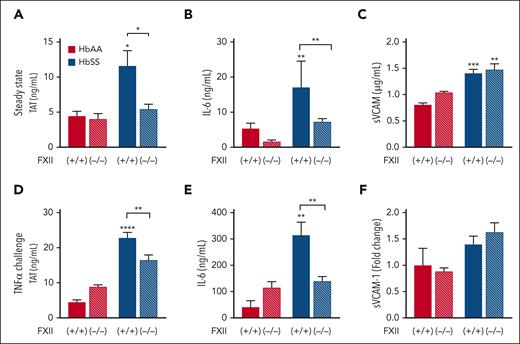
Given the above evidence suggesting chronic in vivo activation of FXII in patients with SCD and mice, we determined whether activation of FXII contributes to thromboinflammation at steady state. BM from HbAA and HbSS mice was transplanted into irradiated wild-type (FXII+/+) and FXII-deficient (FXII−/−) mice. This experimental approach generates HbAA and HbSS mice with either normal levels of FXII (HbAA/FXII+/+ and HbSS/FXII+/+) or with FXII deficiency only in nonhematopoietic cells (HbAA/FXII−/− and HbSS/ FXII−/−). Four months after BM transplantation, plasma levels of TAT, interleukin 6 (IL-6), and soluble vascular cell adhesion molecule-1 (sVCAM-1) were elevated in HbSS/FXII+/+ over HbAA/FXII+/+ mice (Figure 2A-C). Deficiency of FXII in nonhematopoietic cells completely attenuated TAT and IL-6 in HbSS mice to the same level observed in HbAA/FXII+/+ controls but had no effect on sVCAM-1 (Figure 2A-C). Using the same BM transplantation approach, we generated HbSS mice lacking FXI or PK in all nonhematopoietic cells. In contrast to what we observed in HbSS/FXII−/− mice, plasma TAT levels in HbSS/FXI−/− and HbSS/PK−/− mice were only modestly reduced (supplemental Figure 3), suggesting that eliminating only 1 of the FXIIa-dependent pathways is not sufficient to significantly attenuate systemic thrombin generation.
Nonhematopoietic FXII contributes to increased thrombin generation and inflammation in HbSS mice at steady state and after TNFα challenge. FXII−/− and FXII+/+ mice were irradiated and received transplantation with HbAA or HbSS BM. Four months later, plasma was collected for analysis of (A) TAT complexes, (B) IL-6, and (C) sVCAM-1. In a separate study, all mice were treated with TNFα (2 μg/kg, intraperitoneally). Five hours later, plasma was collected for analysis of (D) TAT complexes, (E) IL-6, and (F) sVCAM-1. Data are represented as mean ± SEM, n = 10 to 26 per group. ∗P < .05, ∗∗P < .01, and ∗∗∗∗P < .0001 by two-way analysis of variance (ANOVA) and Tukey post-hoc test. Asterisks above bars indicate significance within the same FXII genotype. Asterisks above lines indicate difference between Hb genotype within FXII genotype.
Nonhematopoietic FXII contributes to increased thrombin generation and inflammation in HbSS mice at steady state and after TNFα challenge. FXII−/− and FXII+/+ mice were irradiated and received transplantation with HbAA or HbSS BM. Four months later, plasma was collected for analysis of (A) TAT complexes, (B) IL-6, and (C) sVCAM-1. In a separate study, all mice were treated with TNFα (2 μg/kg, intraperitoneally). Five hours later, plasma was collected for analysis of (D) TAT complexes, (E) IL-6, and (F) sVCAM-1. Data are represented as mean ± SEM, n = 10 to 26 per group. ∗P < .05, ∗∗P < .01, and ∗∗∗∗P < .0001 by two-way analysis of variance (ANOVA) and Tukey post-hoc test. Asterisks above bars indicate significance within the same FXII genotype. Asterisks above lines indicate difference between Hb genotype within FXII genotype.
TF does not contribute to enhanced thromboinflammation observed in HbSS mice after tumor necrosis factor α (TNFα) challenge
Challenging HbSS mice with low-dose TNFα induces neutrophil activation, increases levels of circulating cell free (CF)-DNA, and promotes vaso-occlusion.26,27 Consistent with these data, we also observed that TNFα challenge reduced the number of circulating neutrophils and dramatically increased plasma levels of CF-DNA in HbSS mice (supplemental Figure 4A-B). Moreover, TNFα challenge further enhanced the plasma levels of TAT and IL-6 in HbSS mice (supplemental Figure 4C-D), without affecting levels of sVCAM-1 (supplemental Figure 4E).
We previously demonstrated that inhibition of TF, the initiator of the extrinsic coagulation pathway, attenuates thrombin generation and vascular inflammation in HbSS mice at steady state.9,10 To determine whether TF also contributes to the enhanced thromboinflammation after TNFα challenge, HbAA and HbSS mice received an inhibitory antimouse TF antibody, 1H19,28 (generously provided by Daniel Kirchhoffer, Genentech, South San Francisco, CA) or control immunoglobulin G (IgG) (25 mg/kg, intraperitoneally) 1 hour before TNFα administration. TF inhibition had no effect on TAT or IL-6 levels in TNFα-challenged HbSS mice and did not reverse the TNFα-mediated reduction in circulating neutrophils (supplemental Figure 5A-C). This lack of effect occurred despite sufficiently high plasma levels of 1H1 to completely attenuate TF-mediated procoagulant activity in an in vitro cell-based assay (supplemental Figure 5D). These data indicate that TF does not significantly contribute to thromboinflammation in TNFα-challenged HbSS mice.
FXII deficiency in nonhematopoietic cells attenuates thromboinflammation in HbSS mice after TNFα challenge
We sought to determine whether the intrinsic coagulation pathway is activated in HbSS mice. Although we were unable to develop mouse equivalents for all assays used on clinical samples, we successfully developed an enzyme-linked immunosorbent assay to measure murine FIXa:AT complexes in plasma. HbSS mice had a modest increase in FIXa:AT complexes compared with HbAA controls, and TNFα challenge significantly increased FIXa:AT plasma levels in HbSS mice (supplemental Figure 4F). We also observed a strong positive correlation between FIXa:AT and TAT complexes in all HbSS mice (r2 = 0.62, P < .001). We then evaluated whether FXII deficiency in nonhematopoietic cells affects these parameters in TNFα-challenged HbSS mice. FXII deficiency in nonhematopoietic cells resulted in partial but significant attenuation of plasma levels of TAT and IL-6 in TNFα-challenged HbSS/FXII−/− mice compared with HbSS/FXII+/+ mice (Figure 2D-E). sVCAM-1 levels were not elevated by TNFα in HbSS mice and were unaffected by FXII deficiency (Figure 2F). These data indicate that nonhematopoietic FXII contributes to the increased levels of TAT and IL-6 observed in HbSS mice, both at steady state and after TNFα challenge.
Degradation of CF-DNA with DNAse improves survival of HbSS mice after TNFα challenge.26 Because CF-DNA and neutrophil extracellular traps are thought to activate FXII, we set out to determine whether DNAse treatment attenuates systemic thrombin generation. Surprisingly, DNAse had no effect on plasma levels of TAT in HbSS mice after TNFα challenge (supplemental Figure 6), undermining the contribution of CF-DNA and neutrophil extracellular traps to systemic thrombin generation in this model.
FXII contributes to vascular stasis in HbSS mice
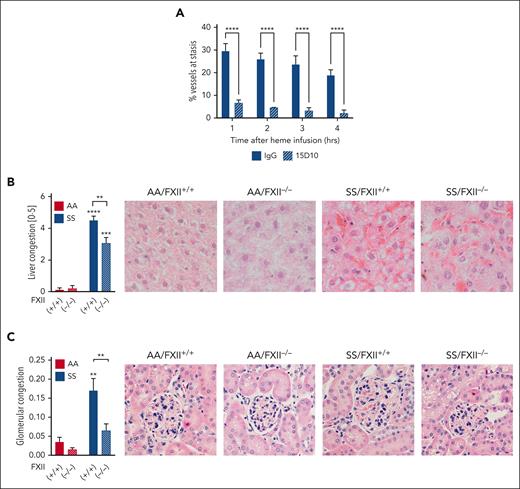
To determine whether FXII contributes to VOC, we used the well-established dorsal skinfold chamber model.29,30 HbSS mice were infused with control IgG or 15D10 antibodies (10 mg/kg, IV) 30 minutes before Hb infusion (1 μmol/kg, IV). 15D10 is a mouse anti-FXII antibody that reacts with FXII from multiple species, including mouse and human, and significantly attenuates FXIIa formation and FXIIa-driven coagulation by binding to the heavy chain of FXII.31 In our studies, intraperitoneal or IV dosing of 15D10 prolonged the activated partial thromboplastin time (aPTT) in C57 mice (supplemental Figure 7A-B). In IgG-treated HbSS mice, Hb induced stasis in 29.8% ± 3.0%, 26.2% ± 2.5%, 23.9% ± 3.6%, 19.1% ± 2.2% (mean ± standard error of the mean [SEM]) of preselected microvessels at 1, 2, 3, and 4 hours after infusion, respectively. This was significantly reduced in HbSS mice pretreated with 15D10 (Figure 3A) at all time points. In addition, pathologic scoring of liver and kidney sections revealed a significant increase in vascular congestion in HbSS/FXII+/+ mice compared with HbAA/FXII+/+ controls; this difference was attenuated by FXII deficiency in nonhematopoietic cells (Figure 3B-C). Further histological analysis of kidney sections revealed modest pathological changes in HbSS/FXII+/+ mice that were either FXII dependent (glomerular sclerosis, interstitial fibrosis, mesangial expansion, medullary congestion; supplemental Figure 8A-D) or independent (brush border loss; supplemental Figure 8E).
FXII contributes to vascular stasis and congestion in HbSS mice. (A) Townes HbSS mice were implanted with dorsal skinfold chambers. Using intravital microscopy, between 20 and 25 subcutaneous venules were selected and mapped. Mice were treated with IgG or 15D10 (10 mg/kg, IV) 30 minutes before challenge with stroma-free Hb (1 μmol/kg, IV). The preselected venules were marked as flowing or static at 1, 2, 3, and 4 hours after Hb infusion, and the percentage static venules was calculated. n = 4 mice per group, data represent mean ± SEM. ∗∗∗∗P < .0001 vs IgG/SS at each time point by two-way ANOVA. Paraffin sections of livers and kidneys from AA/FXII+/+, AA/FXII−/−, SS/FXII+/+, and SS/FXII−/− mice 4 months after BM transplantation were stained with hematoxylin and eosin. (B) Sinusoidal congestion and (C) glomerular congestion were scored by blinded pathologists. Representative images are shown; n = 4 to 8 mice per group, data represent mean ± SEM. ∗∗P < .01, ∗∗∗P < .001, ∗∗∗∗P < .0001 by two-way ANOVA and Tukey post-hoc test. Asterisks above bar represent difference from HbAA, asterisks above line indicate difference between FXII genotype. Red staining on representative images showed in panels B and C demonstrates presence of RBC within congested vessels. ANOVA, analysis of variance.
FXII contributes to vascular stasis and congestion in HbSS mice. (A) Townes HbSS mice were implanted with dorsal skinfold chambers. Using intravital microscopy, between 20 and 25 subcutaneous venules were selected and mapped. Mice were treated with IgG or 15D10 (10 mg/kg, IV) 30 minutes before challenge with stroma-free Hb (1 μmol/kg, IV). The preselected venules were marked as flowing or static at 1, 2, 3, and 4 hours after Hb infusion, and the percentage static venules was calculated. n = 4 mice per group, data represent mean ± SEM. ∗∗∗∗P < .0001 vs IgG/SS at each time point by two-way ANOVA. Paraffin sections of livers and kidneys from AA/FXII+/+, AA/FXII−/−, SS/FXII+/+, and SS/FXII−/− mice 4 months after BM transplantation were stained with hematoxylin and eosin. (B) Sinusoidal congestion and (C) glomerular congestion were scored by blinded pathologists. Representative images are shown; n = 4 to 8 mice per group, data represent mean ± SEM. ∗∗P < .01, ∗∗∗P < .001, ∗∗∗∗P < .0001 by two-way ANOVA and Tukey post-hoc test. Asterisks above bar represent difference from HbAA, asterisks above line indicate difference between FXII genotype. Red staining on representative images showed in panels B and C demonstrates presence of RBC within congested vessels. ANOVA, analysis of variance.
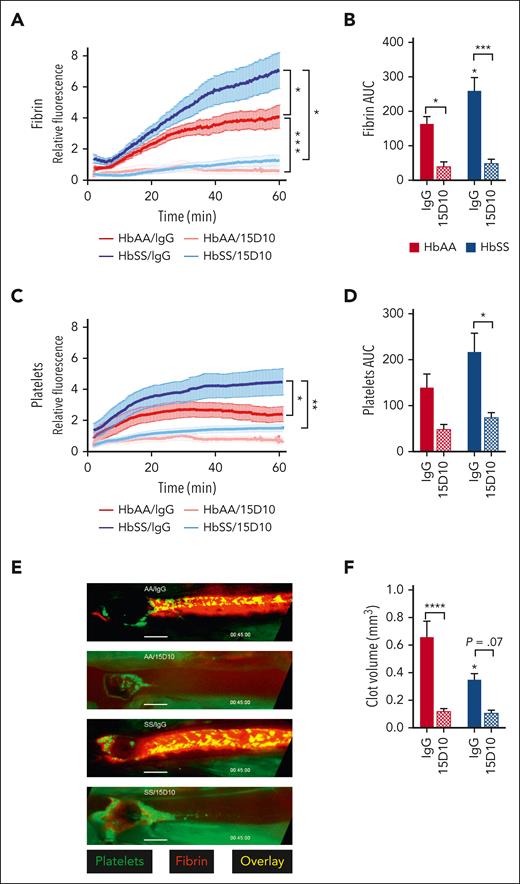
FXII inhibition reduces fibrin and platelet accumulation in venous clots of HbAA and HbSS mice
Using a model of venous thrombosis induced by electrolytic injury, we previously showed that HbSS mice have enhanced fibrin deposition in thrombi formed in the femoral vein.32 Because FXII contributes to thrombin generation both at steady state and after TNFα challenge, and that FXII contributes to experimental venous thrombosis in animal models,17,33-35 we investigated the role of FXII in venous thrombosis in HbSS mice. HbAA and HbSS mice received control IgGκ1 or 15D10 (5 mg/kg, IV); 30 minutes later, electrolytic injury was applied to the femoral vein. The intensity of fibrin and platelet deposition was measured by intravital microscopy over time within a defined length of the femoral vein (supplemental Figure 9). Consistent with previously published data,32 mean fluorescent fibrin intensity and total fibrin deposition (measured by area under the curve) were significantly increased in HbSS mice compared with HbAA mice treated with IgGκ1. Importantly, 15D10 treatment significantly reduced these end points in both HbAA and HbSS mice (Figure 4A-B). FXII inhibition with 15D10 also blocked mean fluorescent platelet intensity and total platelet deposition (Figure 4C-D). Representative images of thrombi from each treatment group are shown in Figure 5E. Analysis of time-lapse videos from both HbAA and HbSS mice that received control IgG demonstrated that initial platelet deposition occurred at the site of injury and was followed by fibrin accumulation, additional platelet recruitment, and clot formation in the direction of blood flow (supplemental Movies 1-2). Inhibition of FXII autoactivation by 15D10 retarded both initial platelet accumulation and fibrin formation. Thus, targeting FXII interferes with initial clot formation resulting in reduction in overall clot size in both HbAA and HbSS mice (supplemental Movies 3-4). Consistent with these results, 15D10 significantly prolonged aPTT in HbAA and HbSS mice (supplemental Figure 7C).
FXII(a) inhibition attenuates femoral vein thrombosis induced by electrolytic injury. Townes HbAA and HbSS mice were treated with IgG or 15D10 (5 mg/kg, IV) 30 minutes before electrolytic injury to the femoral vein. Quantification of (A) relative fibrin intensity over time and (B) the total fibrin fluorescence measured by AUC. Quantification of (C) relative platelet intensity over time and (D) the total platelet fluorescence measured by AUC. Data represent mean ± SEM; n = 6 to 10 mice per group. (E) Representative images of fibrin (red) and platelet (green) accumulation taken 45 minutes after electrolytic injury. (F) Histomorphometric quantification of clot volume. Data represent mean ± SEM of clot volume (mm3); n = 6 to 10 mice per group. ∗P < .05, ∗∗P < .01, ∗∗∗P < .001 by two-way ANOVA and Tukey post-hoc test. ANOVA, analysis of variance; AUC, area under the curve.
FXII(a) inhibition attenuates femoral vein thrombosis induced by electrolytic injury. Townes HbAA and HbSS mice were treated with IgG or 15D10 (5 mg/kg, IV) 30 minutes before electrolytic injury to the femoral vein. Quantification of (A) relative fibrin intensity over time and (B) the total fibrin fluorescence measured by AUC. Quantification of (C) relative platelet intensity over time and (D) the total platelet fluorescence measured by AUC. Data represent mean ± SEM; n = 6 to 10 mice per group. (E) Representative images of fibrin (red) and platelet (green) accumulation taken 45 minutes after electrolytic injury. (F) Histomorphometric quantification of clot volume. Data represent mean ± SEM of clot volume (mm3); n = 6 to 10 mice per group. ∗P < .05, ∗∗P < .01, ∗∗∗P < .001 by two-way ANOVA and Tukey post-hoc test. ANOVA, analysis of variance; AUC, area under the curve.
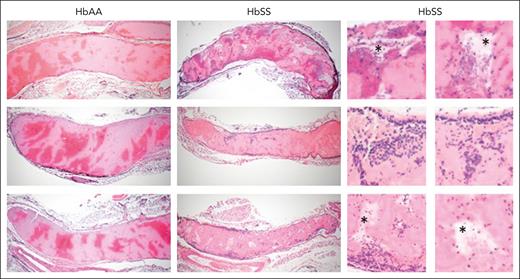
SCD affects morphology of venous clots. Representative, hematoxylin and eosin–stained images of femoral vein clots harvested from HbAA and HbSS mice 1 hour after electrolytic injury, (original magnification ×4). In the clots, areas rich in RBCs stain dark red whereas areas of fibrin and platelets stain light pink. In the enlarged images on the right, asterisks denote acellular empty spaces within the clots; dark blue staining denotes inflammatory cells within the clots.
SCD affects morphology of venous clots. Representative, hematoxylin and eosin–stained images of femoral vein clots harvested from HbAA and HbSS mice 1 hour after electrolytic injury, (original magnification ×4). In the clots, areas rich in RBCs stain dark red whereas areas of fibrin and platelets stain light pink. In the enlarged images on the right, asterisks denote acellular empty spaces within the clots; dark blue staining denotes inflammatory cells within the clots.
Inhibition of FXII generation reduces the volume of venous clots formed in HbAA and HbSS mice
The relatively small size of the thrombi that develop in the femoral vein prevents accurate clot weight quantification. Therefore, we harvested the femoral vein with the entire clot in situ for histomorphometric reconstruction of total clot volume (supplemental Figure 9). Unexpectedly, despite a higher mean fluorescent fibrin intensity, the clot volumes in HbSS mice were significantly smaller compared with clots in HbAA mice (Figure 4F). Importantly, clot volume was largely reduced in both HbAA and HbSS mice treated with 15D10 (Figure 4F). Histological analysis of HbAA and HbSS clots revealed interesting differences in clot structure and cellular composition. Thrombi that formed in the femoral veins of HbAA mice demonstrated a classical pattern with well-defined RBC– and fibrin/platelet-rich areas (Figure 5). In contrast, sickle clots were characterized by increased neutrophil incorporation at the interface of the clot and the endothelium, and within the core of the clot. Clot structure was also less organized and less compact in the red cell–rich areas, with multiple acellular spaces observed in HbSS mice compared with HbAA controls.
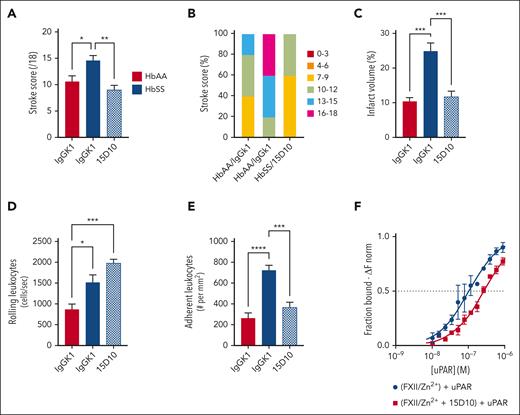
FXII contributes to enhanced neuronal damage and neurologic deficit observed in HbSS mice subjected to brain ischemia reperfusion injury
Ischemic stroke and silent cerebral infarction frequently occur in SCD, leading to neurological deficits and cognitive impairment.7 HbAA and HbSS mice were infused with control IgG or 15D10 antibodies (10 mg/kg, IV) 30 minutes before tMCAO (1-hour transient occlusion) and 6 hours into reperfusion. After 24 hours of reperfusion, brain injury and neurologic deficit were assessed in HbAA/IgG, HbSS/IgG, and HbSS/15D10 mice. The mean stroke severity score and proportion of mice at highest severity scores were increased in HbSS/IgG compared with that in HbAA/IgG mice; both parameters were significantly decreased in the HbSS/15D10 group (Figure 6A-B). Infarct volume was also increased in HbSS/IgG mice compared with that in HbAA/IgG counterparts, and it was significantly decreased by 15D10 treatment (Figure 6C). Intravital microscopy showed significantly more rolling leukocytes in the cerebral microcirculation in HbSS/IgG vs HbAA/IgG mice, likely due to neutrophilia in HbSS mice; this was not affected by 15D10 pretreatment (Figure 6D). In contrast, enhanced leukocyte adhesion to the endothelium observed within cerebral pial vessels of HbSS/IgG mice was significantly reduced by 15D10 administration (Figure 6E), suggesting that FXII contributes to leukocyte adhesion after tMCAO. We previously showed that in isolated neutrophils, FXII signals through uPAR and thereby upregulates the surface expression of αMβ2 (CD11b-CD18) integrin,15 a critical regulator of neutrophil firm adhesion to the vessel wall. Therefore, we investigated whether the binding kinetics of FXII and uPAR differed in the presence of 15D10. We used microscale thermophoresis, an assay that captures binding-induced changes in thermophoretic mobility.36,37 For these studies, murine His-tagged FXII was fluorescently labeled with RED-tris-nitriloacetic acid and subsequently incubated with serially diluted murine uPAR (final concentrations: 6.85 × 10−9 M to 2.6 × 10−6 M), in the absence or presence of 1 μM 15D10 (Figure 6F). The fluorescence intensity of labeled FXII is monitored before, during, and after infrared laser excitation, and the initial fluorescence intensity before heating is used to normalize fluorescence changes (ΔFnorm representing the bound fraction). Based on microscale thermophoresis measurements, a binding constant (Kd) of 0.89 × 10−7 M was determined for FXII and uPAR. In the presence of 15D10, Kd rose to ∼3 × 10−7 M, suggesting that 15D10 interferes with the FXII-uPAR interaction (Figure 6F; supplemental Figure 10).
FXII inhibition attenuates stroke severity in HbSS mice after tMCAO. Quantification of (A) stroke score, (B) proportion of stroke severity, (C) and brain infarct in HbAA and HbSS mice subjected to brain ischemia/reperfusion injury after treatment with IgG or 15D10 antibodies (10 mg/kg, IV). Intravital microscopy analysis was performed to assess the number of (D) rolling and (E) adherent leukocytes. Data represent mean ± SEM; n = 6 mice per group. ∗P < .05, ∗∗P < .01, ∗∗∗P < .001 by one-way ANOVA and Tukey post-hoc test. (F) Microscale thermophoresis was performed to measure direct binding between uPAR and FXII, in the absence (blue line) or presence (red line) of 15D10. Recombinant His-tagged murine FXII was fluorescently labeled with RED-tris-nitrilotriacetic acid and subsequently incubated with 15 μM Zn2+ and serially diluted murine uPAR. Where indicated, 1 μM of 15D10 was added to the reaction mixture. Initial fluorescence intensity of RED-FXII was used to normalize fluorescence changes (ΔFnorm representing the bound fraction). Binding constants (Kd) over time were determined for FXII-uPAR based on triplicate measurements of n = 3 individual experiments. ANOVA, analysis of variance.
FXII inhibition attenuates stroke severity in HbSS mice after tMCAO. Quantification of (A) stroke score, (B) proportion of stroke severity, (C) and brain infarct in HbAA and HbSS mice subjected to brain ischemia/reperfusion injury after treatment with IgG or 15D10 antibodies (10 mg/kg, IV). Intravital microscopy analysis was performed to assess the number of (D) rolling and (E) adherent leukocytes. Data represent mean ± SEM; n = 6 mice per group. ∗P < .05, ∗∗P < .01, ∗∗∗P < .001 by one-way ANOVA and Tukey post-hoc test. (F) Microscale thermophoresis was performed to measure direct binding between uPAR and FXII, in the absence (blue line) or presence (red line) of 15D10. Recombinant His-tagged murine FXII was fluorescently labeled with RED-tris-nitrilotriacetic acid and subsequently incubated with 15 μM Zn2+ and serially diluted murine uPAR. Where indicated, 1 μM of 15D10 was added to the reaction mixture. Initial fluorescence intensity of RED-FXII was used to normalize fluorescence changes (ΔFnorm representing the bound fraction). Binding constants (Kd) over time were determined for FXII-uPAR based on triplicate measurements of n = 3 individual experiments. ANOVA, analysis of variance.
Basal FXII activity is elevated in neutrophils from patients with SCD and HbSS mice compared with controls
Hepatocytes are the primary source of circulating FXII.38 We performed western blot analysis on plasma from FXII+/+ and FXII−/− mice that received transplantation with BM from FXII-expressing HbAA and HbSS mice. There was no detectable zymogen FXII in the plasma of either HbAA/FXII−/− or HbSS/FXII−/− mice (supplemental Figure 11), indicating that neutrophil-derived FXII does not significantly contribute to the pool of circulating FXII. However, we previously showed that FXII becomes activated on the surface of primed neutrophils.15,39 Here, we examined whether neutrophil-derived FXII can raise local FXIIa generation. Healthy human neutrophils (final count, 30 000 cells per well) were coincubated with normal human plasma or FXII-deficient plasma and 200 μM of FXIIa chromogenic substrate S-2302. Where indicated, reactions were supplemented with 15 μM Zn2+ or aPTT reagent (positive control) and the optical density at 405 nm was monitored. These studies demonstrated that adding neutrophils to normal human plasma in the presence of Zn2+, which supports FXII cell binding, resulted in robust FXIIa generation even in the absence of aPTT reagent (Figure 7A). Importantly, when healthy neutrophils were incubated with FXII-deficient plasma, there was detectable chromogenic substrate conversion to ∼40% of that observed when cells were mixed with normal human plasma (Figure 7A). These data support that neutrophils are a source of functional FXII and can partially sustain FXII procoagulant responses in settings of heightened neutrophil activation.
Neutrophil-derived FXII contributes to local FXIIa formation and enhanced neutrophil integrin activation in SCD. (A) Normal human plasma (NHP) or FXII-deficient plasma (F12−/− plasma) were incubated with aPTT-R or neutrophils supplemented with 15 μM Zn2+. The generation of FXIIa was determined by monitoring the cleavage of S2302 (200 μM) over time. NHP incubated with aPTT-R was used as positive control. n = 3 individual experiments, analyzed in triplicate. (B) Visualization of FXII (green) and 4′,6-diamidino-2-phenylindole (blue) in neutrophils isolated from healthy controls (AA) and patients with SCD. Images shown are representative of 3 individual experiments. Images shown at ×20 original magnification, scale: 10 μm. Mouse HbAA and HbSS neutrophils (n = 3-4, analyzed in duplicate) were assessed for FXIIa activity (S2302 cleavage) over 4 hours. Reaction rate of FXIIa activity was calculated in pM/s (C) per 250 000 neutrophils or (D) multiplied by the total number of circulating neutrophils in each individual mouse before isolation (pM/s × polymorphonuclear leukocyte [PMN]). Data represent mean ± SEM; ∗P < .05 vs HbAA by Student t test. Flow cytometry analysis of (E) uPAR, (F) total αMβ2 integrin, and (G) active αMβ2 integrin surface expression on untreated (Veh) and FXII/Zn2+–stimulated neutrophils isolated from healthy controls (Con) and patients with SCD. Data represent mean ± SEM; n = 4 to 9; ∗P < .05, ∗∗P < .01, ∗∗∗P < .001 by Kruskal-Wallis one-way analysis of variance. aPTT-R, aPTT reagent.
Neutrophil-derived FXII contributes to local FXIIa formation and enhanced neutrophil integrin activation in SCD. (A) Normal human plasma (NHP) or FXII-deficient plasma (F12−/− plasma) were incubated with aPTT-R or neutrophils supplemented with 15 μM Zn2+. The generation of FXIIa was determined by monitoring the cleavage of S2302 (200 μM) over time. NHP incubated with aPTT-R was used as positive control. n = 3 individual experiments, analyzed in triplicate. (B) Visualization of FXII (green) and 4′,6-diamidino-2-phenylindole (blue) in neutrophils isolated from healthy controls (AA) and patients with SCD. Images shown are representative of 3 individual experiments. Images shown at ×20 original magnification, scale: 10 μm. Mouse HbAA and HbSS neutrophils (n = 3-4, analyzed in duplicate) were assessed for FXIIa activity (S2302 cleavage) over 4 hours. Reaction rate of FXIIa activity was calculated in pM/s (C) per 250 000 neutrophils or (D) multiplied by the total number of circulating neutrophils in each individual mouse before isolation (pM/s × polymorphonuclear leukocyte [PMN]). Data represent mean ± SEM; ∗P < .05 vs HbAA by Student t test. Flow cytometry analysis of (E) uPAR, (F) total αMβ2 integrin, and (G) active αMβ2 integrin surface expression on untreated (Veh) and FXII/Zn2+–stimulated neutrophils isolated from healthy controls (Con) and patients with SCD. Data represent mean ± SEM; n = 4 to 9; ∗P < .05, ∗∗P < .01, ∗∗∗P < .001 by Kruskal-Wallis one-way analysis of variance. aPTT-R, aPTT reagent.
Given the prominent role of neutrophils in SCD pathology, we used immunofluorescence to detect FXII in peripheral blood neutrophils isolated from healthy individuals or patients with SCD at steady state. Neutrophils from individuals with SCD exhibited higher surface exposure of FXII than cells from healthy controls (Figure 7B). We also found that neutrophil-derived FXIIa activity was significantly higher in neutrophils isolated from HbSS mice compared with HbAA controls (Figure 7C). In addition, when the reaction rate was adjusted to the number of neutrophils in each sample, HbSS neutrophils exhibited a significant, near 7-fold increase in FXIIa activity compared with HbAA neutrophils (Figure 7D).
Next, we set out to determine whether the enhanced FXII expression in SCD neutrophils confers these cells with upregulated CD11b expression and, consequently, an increased adhesive potential. Neutrophils isolated from healthy individuals and patients with SCD were purified and either left untreated or stimulated with 200 nM FXII and 15 μM ZnCl2. As assessed by flow cytometry, constitutive surface expression of uPAR, total and active CD11b were significantly higher in neutrophils of patients with SCD compared with that in healthy controls (Figure 7E-G). Stimulation of control neutrophils with FXII modestly increased uPAR expression and significantly enhanced total and active CD11b surface expression (Figure 7E-G), whereas FXII stimulation of neutrophils from patients with SCD did not result in a further increase in surface expression of uPAR or CD11b integrin (Figure 7E-G). We posit that heightened basal neutrophil activation in SCD, in part mediated by FXII, leads to saturable expression of adhesive receptors.
Discussion
Using genetic and pharmacologic approaches coupled with clinical samples and murine models, we demonstrated a role for FXII in several vascular complications associated with SCD. Specifically, to the best of our knowledge, this is the first report to: (1) directly evaluate and demonstrate increased activity of the contact pathway in patients with SCD; (2) show that FXII, but not TF, contributes to enhanced thrombin generation and inflammation in HbSS mice after TNFα challenge; (3) reveal that inhibition of FXII attenuates venous thrombosis, microvascular stasis, and ischemic brain injury in a mouse model of SCD; and (4) show that SCD neutrophils have higher levels of FXII, uPAR, and activated αMβ2 integrin that confers these cells with enhanced adhesive properties.
Previous reports found a reduction in circulating levels of FXII, PK, and high–molecular weight kininogen in patients with SCD, suggesting the possibility of zymogen consumption because of chronic activation.18,19 To the best of our knowledge, this study is the first to demonstrate activation of these proteases through the measurement of complexes of FXIIa, FXIa, and PKa bound to their principal serpins24,25 in the plasma of patients with SCD. Our correlation analyses suggest that FXIIa contributes to activation of both the intrinsic coagulation (FXIa/FIXa) and contact (PKa) pathways in SCD. In addition to the canonical activation of FIX through FXIa, we, and others, have reported that PKa can directly activate FIX, independent of FXIa.40-42 Our data show that PKa:C1INH levels correlate with FIXa:AT complexes, suggesting that this pathway of FIX activation may be operative in SCD. These observations, together with the data showing a significant reduction of plasma TAT levels in HbSS/FXII−/− but only partial attenuation of this marker in HbSS/FXI−/− and HbSS/PK−/− mice, strongly suggest that both FXII-dependent activation of FXI and PK contribute to the systemic thrombin generation in SCD.
Challenging sickle cell mice with TNFα26,43,44 or Hb29,30,45 are the 2 commonly used models mimicking vascular pathologies induced by either acute inflammatory or hemolytic events, respectively. We revealed that Hb-induced microvascular stasis in HbSS mice can be attenuated by blocking TF9 as well as FXII (Figure 3A). In contrast, FXII deficiency but not TF inhibition attenuated the thromboinflammatory state in TNFα-challenged HbSS mice. These observations suggest that inhibition of FXII/FXIIa may be more effective than blocking TF to attenuate vascular pathologies in SCD. Future studies will focus on identifying the cellular and molecular triggers of FXII activation in SCD, such as the unique properties of sickle RBCs and/or their microparticles,41,46,47 CF-DNA,48 or mast cell–derived glycosaminoglycans.49,50 It will also be important to determine the selective contribution of FXII cell signaling activities and FXIIa procoagulant functions to the pathology of SCD.
SCD is associated with an increased risk of venous thrombosis that is associated with a high risk of recurrence and mortality.6,51 In SCD, there is a modestly higher incidence of pulmonary embolism than deep vein thrombosis.52 We previously demonstrated that ex vivo clots from HbSS mice are more heterogeneous in structure than clots from HbAA mice because of the presence of sickled RBCs.32 Moreover, despite the higher abundance of fibrin deposition within the clots formed in the femoral veins after electrolytic injury,32 clots from HbSS mice had a significantly smaller clot volume compared with HbAA controls. Because the number of RBCs affects the overall size and volume of venous clots,53-55 the reduced clot volume observed in HbSS mice may reflect the reduced hematocrit in these animals. However, reduced clot volume could also be a result of decreased clot stability and resultant embolization. Data from our group, and others, suggest that morphological properties of sickle RBCs may lead to the formation of less stable clots that are prone to embolization,32,56 and this hypothesis may be further supported by morphologic differences in clot structure between HbAA and HbSS clots, as presented in Figure 5. Moreover, the decreased clot volume, despite the greater number of neutrophils in HbSS clots, suggests that the cellular mechanisms of venous thrombosis vary depending on disease state.
The standard treatment for patients with SCD with venous thromboembolism is systemic anticoagulation for a minimum of 3 months.51 However, a recent retrospective study of >6400 individuals with SCD highlights the fact that these patients have an inherently elevated risk of bleeding (particularly from the upper gastrointestinal tract), amounting to a 21% cumulative incidence of bleeding by age 40, and an associated 2-fold increased risk of death.57 Furthermore, a recent history of ischemic stroke or venous thromboembolism was an independent risk factor for bleeding that was presumed to relate to antithrombotic agent usage.57 Thus, it is clear that safe alternative anticoagulant agents are needed to prevent recurrent thrombosis in patients with SCD. One candidate target molecule is FXII/FXIIa. FXII deficiency attenuates experimental thrombosis in animal models, including the electrolytic injury femoral vein model.17,33-35,58-61 In this study, we found that pretreatment with 15D10 antibody attenuated fibrin and platelet deposition, and reduced clot volume in HbSS mice. The intravital microscopy time-lapse videos suggest that FXII inhibition blocks the initiation of fibrin deposition and platelet accumulation at the injury site; however, it is possible that FXII inhibition will also affect clot stability. Indeed, FXII is known to directly interact with fibrinogen and fibrin to increase clot stability.62,63 Thus, more studies will be required to determine whether reduced clot size seen with FXII inhibition increases embolic risk.
Stroke is a common and severe complication of SCD.64,65 Others have demonstrated that FXII plays a role in mouse models of ischemic stroke.35,66 Therefore, we investigated whether FXII inhibition also attenuates neurologic damage in HbSS mice. Consistent with our previous studies,67,68 we found that after tMCAO, brain injury was more severe in HbSS mice than in HbAA controls. Importantly, pretreating HbSS mice with 15D10 significantly attenuated neuronal damage and improved behavioral deficits caused by brain ischemia/reperfusion injury. These protective effects may be attributed to the suppression of procoagulant responses occurring after brain ischemia/reperfusion injury.35,66,69-71 However, given that significantly lower numbers of adherent leukocytes were observed in brain pial vessels of HbS mice treated with 15D10, and because neutrophils have previously been shown to enhance brain injury after tMCAO,68,72 an additional hypothesis is that FXII regulates the brain inflammatory milieu after ischemic injury. In this respect, we previously showed that FXII-uPAR signaling in neutrophils upregulates αMβ2 integrin to promote neutrophil adhesion.15,39 Here, we found constitutively higher expression of FXII and uPAR in neutrophils from individuals with SCD, which correlated with higher basal αMβ2 integrin expression and activity. Finally, a microscale thermophoresis analysis revealed that the presence of 15D10 antibody interferes with the FXII-uPAR interaction. Although additional investigations are required, these data suggest that the signaling properties of FXII may also contribute to brain injury in SCD.
Although clinical trials of FXII/FXIIa inhibitors are presently focused on hereditary angioedema and thrombosis of extracorporeal medical devices (NCT 04278885, NCT04653766, NCT04934891), the unique features of SCD-related thrombosis32 and the evidence presented in this communication suggest that FXII(a) inhibition may be a rational and safe antithrombotic and antiadhesive strategy in SCD.
Acknowledgments
The authors thank Daniel Kirchhofer and Genentech, Inc for the anti-TF antibody 1H1.
This work was funded in part by the National Institutes of Health (NIH), National Heart, Lung, and Blood Institute (NHLBI) grants R01s HL142604 (R.P.), HL157441 (R.P., N.S.K.) and U01 HL117659 (R.P., N.S.K.). E.M.S. is supported by NIH, NHLBI grant R01 HL 155193. M.K. was supported by NIH, NHLBI grant R00HL144817 and University of Alabama at Birmingham AMC21 grant MULTIPI6724. G.M.V. and J.D.B. were supported by research funding from NIH, NHLBI grant 5R01 HL114567. T.R. acknowledges the Deutsche Forschungsgemeinschaft (DFG, German Research Foundation) grants P6 - KFO306, 80750187 - SFB841 and 470698011 -SFB877. D.G. was supported by NIH, NHLBI grant R35 HL140025. F.E.G. was supported by the Royal Society Wolfson Foundation (RSWF∖R3∖183001). A.G. and M.W. were supported by NIH, NHLBI grant R44 HL126235. This work was supported by NIH, NHLBI grant R01 HL137695 (E.X.S.), Merit Review Awards BX003851 (E.X.S.), and the Oscar D. Ratnoff Endowed Professorship (E.X.S.).
The contents are solely the responsibility of the authors and do not necessarily represent the official views of the National Institutes of Health, the US Department of Veterans Affairs, or the US Government.
Authorship
Contribution: E.M.S. designed the research, performed experiments, analyzed data, and wrote the manuscript; M.W.H., M.M., C.A., A.I., F.T., N.R., S.V., D.B., K.L.B., M.P., C.C., M.K., and B.C. performed experiments and analyzed data; M.W., T.R., and A.G. provided valuable reagents; D.G. performed experiments and provided valuable reagents; N.S.K. and G.M.V. provided critical guidance on experimental procedures and revised the manuscript; F.E.G., J.D.B., and E.X.S. performed experiments, analyzed data, and critically reviewed the manuscript; and R.P. designed the research, analyzed data, and wrote the manuscript.
Conflict-of-interest disclosure: R.P. and E.M.S. have received research funding from CSL. A.G. and M.W. are employees of Aronora, Inc. J.D.B. and G.M.V. have received research funding from Omeros, CSL Behring, Hillhurst Biopharmaceuticals, and Astellas/Mitobridge. J.D.B. is a consultant for Astellas/Mitobridge. G.M.V. is a consultant for Sanofi and Astellas/Mitobridge. The remaining authors declare no competing financial interests.
Correspondence: R. Pawlinski, Division of Hematology, Department of Medicine, Blood Research Center, University of North Carolina at Chapel Hill, 116 Manning Dr, 8008C Mary Ellen Jones Bldg, Chapel Hill, NC 27599; e-mail: rafal_pawlinski@med.unc.edu.
References
Author notes
Data are available on request from the corresponding author, R. Pawlinski (rafal_pawlinski@med.unc.edu).
The online version of this article contains a data supplement.
There is a Blood Commentary on this article in this issue.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement”in accordance with 18 USC section 1734.

![Neutrophil-derived FXII contributes to local FXIIa formation and enhanced neutrophil integrin activation in SCD. (A) Normal human plasma (NHP) or FXII-deficient plasma (F12−/− plasma) were incubated with aPTT-R or neutrophils supplemented with 15 μM Zn2+. The generation of FXIIa was determined by monitoring the cleavage of S2302 (200 μM) over time. NHP incubated with aPTT-R was used as positive control. n = 3 individual experiments, analyzed in triplicate. (B) Visualization of FXII (green) and 4′,6-diamidino-2-phenylindole (blue) in neutrophils isolated from healthy controls (AA) and patients with SCD. Images shown are representative of 3 individual experiments. Images shown at ×20 original magnification, scale: 10 μm. Mouse HbAA and HbSS neutrophils (n = 3-4, analyzed in duplicate) were assessed for FXIIa activity (S2302 cleavage) over 4 hours. Reaction rate of FXIIa activity was calculated in pM/s (C) per 250 000 neutrophils or (D) multiplied by the total number of circulating neutrophils in each individual mouse before isolation (pM/s × polymorphonuclear leukocyte [PMN]). Data represent mean ± SEM; ∗P < .05 vs HbAA by Student t test. Flow cytometry analysis of (E) uPAR, (F) total αMβ2 integrin, and (G) active αMβ2 integrin surface expression on untreated (Veh) and FXII/Zn2+–stimulated neutrophils isolated from healthy controls (Con) and patients with SCD. Data represent mean ± SEM; n = 4 to 9; ∗P < .05, ∗∗P < .01, ∗∗∗P < .001 by Kruskal-Wallis one-way analysis of variance. aPTT-R, aPTT reagent.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/141/15/10.1182_blood.2022017074/6/m_blood_bld-2022-017074-gr7.jpeg?Expires=1769883500&Signature=zFRyiKZBBxajRbQ2lxBf-hUB3itrIGAnypmszvAlDbziWghUJAPyJiGA1YMh7s9yLXL46R3HjKWBm6yBlgS64K8839Pdh5tBGBWuM--lmSgGnoq4aAso8bbmRSbHwPP4C4rfIvzDb7Pr8am8Ql9P5ENwimwEFwpXqcXvKdWXqw8nOoY1CeB7FRTX-2fBKiNIs8oPJzX5ZnUU3YYAuRgMbwk9NQ6qMmInjprc9FFw0WlqJPf-D5DICz65wThqfYuMDX3kp-GU2ZhLGEdeTzCOfFIZkjdyU6D4Zl17TMegWyqZS4s4rNcjEVxlaJVrOsnSTJp0GzCfoOZdqcFIDee7XQ__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)
This feature is available to Subscribers Only
Sign In or Create an Account Close Modal