Key Points
In advanced-stage T-cell lymphoma, the addition of etoposide to CHOP improved OS in ALK+ ALCL but not in ALK− ALCL, AITL, or PTCL NOS.
Consolidation with ASCT in the first-line setting significantly increased OS in ALK− ALCL, AITL, and PTCL NOS.
Abstract
Patients aged <65 years with peripheral T-cell lymphoma (PTCL) are treated with cyclophosphamide, doxorubicin, vincristine, and prednisone (CHOP). Although the addition of etoposide (CHOEP) and consolidation with autologous stem cell transplantation (ASCT) are preferred in some countries, randomized trials are lacking. This nationwide population-based study assessed the impact of etoposide and ASCT on overall survival (OS) among patients aged 18 to 64 years with stage II to IV anaplastic large-cell lymphoma (ALCL), angioimmunoblastic T-cell lymphoma (AITL), or PTCL not otherwise specified (NOS) diagnosed between 1989 and 2018 using the Netherlands Cancer Registry. Patients were categorized into 2 calendar periods, representing pre- and post-eras of etoposide and ASCT, respectively. A total of 1427 patients were identified (ALCL, 35%; AITL, 21%; and PTCL NOS, 44%). OS increased from 39% in the period from 1989 to 2009 to 49% in the period of 2009 to 2018 (P < .01). Five-year OS was superior for patients treated with CHOEP vs CHOP (64% and 44%, respectively; P < .01). When adjusted for subtype, International Prognostic Index score, and ASCT, the risk of mortality was similar between the 2 groups, except for patients with ALK+ ALCL, for whom the risk of mortality was 6.3 times higher when treated with CHOP vs CHOEP. Patients undergoing consolidation with ASCT had superior 5-year OS of 81% compared with 39% for patients not undergoing ASCT (P < .01), regardless of whether complete remission was achieved. In patients aged <65 years with advanced-stage ALK− ALCL, AITL, or PTCL, the use of ASCT consolidation, but not the addition of etoposide, was associated with improved OS.
Introduction
Peripheral T-cell lymphomas (PTCLs) comprise a heterogeneous group of mature lymphoproliferative diseases that account for ∼10% of newly diagnosed lymphomas worldwide.1 In adult patients, >20 distinct subtypes are currently recognized, with anaplastic large-cell lymphoma (ALCL), angioimmunoblastic T-cell lymphoma (AITL), and PTCL not otherwise specified (NOS) most prevalent in Europe and North America.2-4 The prognosis of patients with PTCL is generally poor, with median 5-year overall survival (OS) of 28% to 32%.2,3,5 Rare PTCL subtypes tend to have an even worse prognosis; the positive exception, however, is ALK+ ALCL, with median 5-year OS of 70% to 86%.2,3,6,7
The cyclophosphamide, doxorubicin, vincristine, and prednisone (CHOP) regimen has been the standard of care for a majority of T-cell non-Hodgkin lymphoma (NHL) subtypes for decades. Complete remission (CR) is achieved in 55% to 70% of patients.8 For patients in CR, 2-year OS is 75% to 80%, but patients who experience progression or relapse have dismal outcomes, with 3-year OS of >10%, because effective second-line therapies are lacking.9,10
Etoposide in addition to CHOP (CHOEP) is preferred in some countries, including the Netherlands, but evidence supporting this strategy is controversial.2,11-13 In White patients aged <65 years, an increase in progression-free survival (PFS) has been observed, but the effect on OS is less clear. A meta-analysis of Asian studies showed no benefit in number of CRs or partial remissions or in overall response rate in general, although more adverse events were seen.14
Young and fit patients often undergo consolidation in first remission with myeloablative autologous stem cell transplantation (ASCT).15,16 However, because of the different conditioning regimens used, the offering of ASCT only to patients with chemotherapy-sensitive disease, and the lack of randomized controlled trials, the exact impact of ASCT on OS in patients with PTCL is unclear.
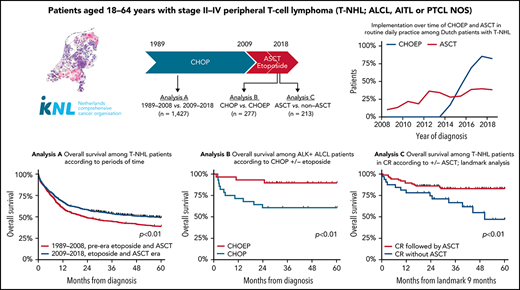
In the Netherlands, the opportunity to add etoposide to CHOP as well as to consolidate with ASCT in first-line treatment was adopted from 2009 onward.12,15 This nationwide population-based study evaluated the impact of etoposide and ASCT on OS among patients aged <65 years with 1 of the 3 major PTCL subtypes (ALCL, AITL, and PTCL NOS) at stage II to IV.
Patients and methods
Population-based registries
We obtained data from the nationwide population-based Netherlands Cancer Registry (NCR). Its validity has been reported previously.17,18 Additional details are provided in the data supplement (available on the Blood Web site). In brief, a minimal data set with basic information on demographic and clinical characteristics was routinely collected by trained registrars of the NCR through retrospective medical record review.
Study population
We identified adult patients (age 18-65 years) diagnosed with Ann Arbor stage II to IV ALCL, AITL, or PTCL NOS in the Netherlands between 1 January 1989 and 31 December 2018 from the NCR. Patients with other T-cell NHL subtypes and patients diagnosed through autopsy were excluded from all analyses. The age of 65 years was chosen as a cutoff because in the past, it was regarded as the maximum age at which to safely undergo ASCT.
The NCR records first-line treatment initiated within 12 months postdiagnosis. Primary therapy was initially grouped into 5 broad categories: (1) chemotherapy followed by consolidation with stem cell transplantation (SCT), (2) chemotherapy without SCT, (3) combination of chemotherapy and radiotherapy, (4) other, and (5) no antineoplastic therapy. For the study population in the period from 1989 to 2018, patients were categorized into 2 calendar periods (ie, 1989-2008 and 2009-2018), which represent the pre- and post-eras of etoposide and ASCT implementation, respectively, for T-cell NHL primary therapy strategies in the Netherlands. ALK+ and ALK− ALCLs were registered as distinct entities in the NCR as of 2008, according to WHO classification of 2008 (4th edition). Information on the exact therapeutic regimen was registered in the NCR for patients diagnosed as of 1 January 2014.
In analysis A, the pre- and post-eras of etoposide and ASCT were compared, as were the 5 subtypes (ALK+ ALCL, ALK− ALCL, ALCL [recoded as single entity in the NCR before 2008], AITL, and PTCL NOS), among 1427 patients diagnosed between 1989 and 2018 (supplemental Figure 1).
The impact of etoposide and ASCT, separately, was evaluated using the data of 352 patients diagnosed as of 2014 in analyses B and C, respectively (supplemental Figure 1). Analysis B compared CHOP and CHOEP, thereby excluding patients receiving cycles from both CHOP and CHOEP (n = 20), patients who received chemotherapy other than CHOP or CHOEP (n = 24), and patients who did not receive first-line treatment (n = 31), leaving 277 patients for this analysis. Patients with ALK+ ALCL (n = 58) were evaluated separately from patients with ALK− ALCL, AITL, or PTCL NOS. Analysis C explicitly compared ASCT with no ASCT. In routine practice, patients diagnosed with ALK+ ALCL are considered not to have an indication for ASCT. Therefore, this subgroup of patients was excluded from analysis C (n = 67). Furthermore, to facilitate a proper unbiased comparison, the duration of treatment had to be equal in both treatment groups. Therefore, analysis using a landmark approach was undertaken, in which a landmark cutoff time of 9 months postdiagnosis was set as the new start of follow-up. The cutoff time of 9 months was chosen as a clinically meaningful period of time, because a majority of patients with AITL, PTCL NOS, or ALCL have completed first-line treatment at 9 months postdiagnosis. Patients who died <9 months postdiagnosis were excluded (n = 72) to account for immortal time bias, finally leaving 213 patients for analysis C.
According to the Central Committee on Research Involving Human Subjects in the Netherlands, this type of observational study does not require approval from an ethics committee. The Privacy Review Board of the NCR approved the use of anonymous data for this study.
End points
The primary end point of the study was OS, defined as the time from diagnosis until death resulting from any cause or last date of follow-up, whichever occurred first. Patients alive were censored on 1 February 2021. The Kaplan-Meier method was used to estimate OS, and the log-rank test was used to examine differences in survival distributions. All survival analyses were restricted to 5 years of follow-up postdiagnosis. The secondary end point was best response (ie, complete response, partial response, or stable/progressive disease) to first-line treatment, as routinely collected by trained registrars of the NCR through retrospective medical record review. Best response was determined by physician assessment using the Lugano classification as of 2014 onward.
Statistical analyses
Descriptive statistics were used to present patient and treatment characteristics across the 2 calendar periods. The Pearson χ2 test was used to compare categorical covariates, and the Kruskal-Wallis test was used to compare nonnormally distributed continuous covariates across the 2 calendar periods. Trends in primary therapy over calendar periods were analyzed using nonparametric tests of trend across the described subgroups.
Risk of mortality was estimated in analysis B using age, sex, subtype of PTCL, International Prognostic Index (IPI) score, and ASCT as a time-varying covariate in Cox proportional hazards regression analyses. ASCT after chemotherapy has become an integral part of first-line treatment for patients with PTCL. However, only patients without refractory or progressive disease after chemotherapy are eligible for ASCT. In survival analyses, these patients seem immortal, because they can only end up in the ASCT group by surviving chemotherapy (ie, alive and event free until ASCT is completed). Therefore, ASCT was included as a time-varying covariate. Results from Cox regression analyses produce hazard ratios (HRs) with associated 95% confidence intervals (CIs). Proportional hazards assumption was tested based on Schoenfeld residuals. Covariates were introduced into regression models with a forward selection method, after adjusting for influence of those already selected. The final model was accomplished when the P value for entering an additional covariable was >.05.
A P value <.05 was considered statistically significant. All analyses were performed using STATA/SE 17.1 software (StataCorp LP, College Station, TX).
Results
Pre- vs post-era of etoposide and ASCT (analysis A)
Patient characteristics
A total of 1427 patients aged <65 years with advanced disease were included in analysis A. Baseline characteristics according to calendar period of diagnosis are presented in Table 1. A majority of patients were male (62%). Median age was 52 years (range, 18-64 years). Overall, 504 patients (35%) were diagnosed with ALCL, 294 (21%) with AITL, and 629 (44%) with PTCL NOS. Of note, of patients with ALCL diagnosed from 2008 onward, 139 (60%) had ALK+ disease, 89 (39%) had ALK− disease, and 3 (1%) had unknown ALK status. In total, the number of diagnoses per year went up from 39 cases per year in the first calendar period to 64 cases per year in the second calendar period (crude rates of 5.0 in 1 million and 7.7 in 1 million, respectively), a phenomenon observed globally.1,4 From 2009 to 2018, the proportion of AITL was higher compared with that from 1989 to 2008 (27% vs 15%), and the proportion with PTCL NOS lower (38% vs 48%; P < .01).
Patient characteristics: analysis A
| . | 1989-2008 (n = 785) . | 2009-2018 (n = 642) . | ||
|---|---|---|---|---|
| n . | % . | n . | % . | |
| Male sex | 494 | 63 | 398 | 62 |
| Age at diagnosis, y | ||||
| Median | 50 | 54 | ||
| Range | 18-64 | 18-64 | ||
| Subtype of T-cell NHL | ||||
| ALCL | 287 | 37 | 219 | 34 |
| PTCL NOS | 381 | 48 | 246 | 38 |
| AITL* | 117 | 15 | 177 | 27 |
| First-line therapy | ||||
| Chemotherapy and ASCT | 39 | 5 | 201 | 31 |
| Chemotherapy only | 591 | 75 | 367 | 57 |
| Radiotherapy | 9 | 1 | 1 | 0 |
| Chemotherapy and radiotherapy | 61 | 8 | 15 | 3 |
| Other or no therapy | 85 | 11 | 58 | 9 |
| Follow-up, mo | ||||
| Median | 22.4 | 32.0 | ||
| Range | 0.03-382.6 | 0.03-143.4 | ||
| . | 1989-2008 (n = 785) . | 2009-2018 (n = 642) . | ||
|---|---|---|---|---|
| n . | % . | n . | % . | |
| Male sex | 494 | 63 | 398 | 62 |
| Age at diagnosis, y | ||||
| Median | 50 | 54 | ||
| Range | 18-64 | 18-64 | ||
| Subtype of T-cell NHL | ||||
| ALCL | 287 | 37 | 219 | 34 |
| PTCL NOS | 381 | 48 | 246 | 38 |
| AITL* | 117 | 15 | 177 | 27 |
| First-line therapy | ||||
| Chemotherapy and ASCT | 39 | 5 | 201 | 31 |
| Chemotherapy only | 591 | 75 | 367 | 57 |
| Radiotherapy | 9 | 1 | 1 | 0 |
| Chemotherapy and radiotherapy | 61 | 8 | 15 | 3 |
| Other or no therapy | 85 | 11 | 58 | 9 |
| Follow-up, mo | ||||
| Median | 22.4 | 32.0 | ||
| Range | 0.03-382.6 | 0.03-143.4 | ||
Patients aged <65 y with stage II to IV disease at diagnosis with ALTL, AITL, and PTCL NOS in The Netherlands.
Including T-cell NHL, follicular helper T cells.
The proportion of patients receiving antineoplastic therapy was similar for both calendar periods (90% and 92%, respectively). Among these, the proportion of patients treated with chemotherapy only (without ASCT) decreased from 2009 to 2018 compared with from 1989 to 2008 (58% vs 75%, respectively). Accordingly, ASCT after chemotherapy was increasingly used in first-line treatment (ie, from 5% of patients in the first calendar period to 31% of patients from 2009 onward; P < .01). The pace of ASCT implementation as of 2008 is depicted in supplemental Figure 2A.
Outcomes
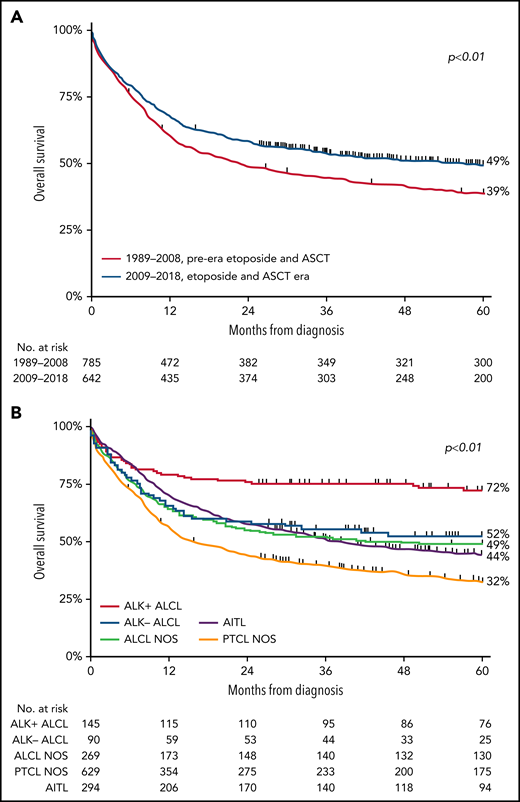
For the entire cohort, 5-year OS was 43% (range, 41% to 46%), with a median follow-up of 28.9 months (range, 0.03-383 months). Five-year OS significantly increased from 39% (95% CI, 35% to 42%) in the pre-era of etoposide and ASCT to 49% (95% CI, 45% to 53%; P < .001) in the era of etoposide and ASCT (Figure 1A). For the subtypes of PTCL, 5-year OS was superior in patients with ALK+ ALCL. In detail, 5-year OS in patients with ALK+ ALCL, ALK− ALCL, ALCL NOS, AITL, and PTCL NOS were 72%, 52%, 49%, 44%, and 32% (Figure 1B), respectively.
OS in patients aged <65 years with stage II to IV PTCL. (A-B) OS according to 2 calendar periods (1989-2008 and 2009-2018) (A) and according to histologic subtypes ALK− ALCL, ALK+ ALCL, ALCL NOS, AITL, and PTCL NOS (B).
OS in patients aged <65 years with stage II to IV PTCL. (A-B) OS according to 2 calendar periods (1989-2008 and 2009-2018) (A) and according to histologic subtypes ALK− ALCL, ALK+ ALCL, ALCL NOS, AITL, and PTCL NOS (B).
Multivariable assessment of OS showed that patients diagnosed between 2009 and 2018 had decreased risk of mortality (HR, 0.80; 95% CI, 0.69-0.94; P < .01) compared with patients diagnosed before 2009 (supplemental Table 1). Furthermore, older age was independently associated with higher mortality risk (HR, 1.03; 95% CI, 1.03-1.04; P < .01) as well as with ALK− ALCL (HR, 1.62; 95% CI, 1.06-2.47; P = .03), ALCL NOS (HR, 1.59; 95% CI, 1.10-2.30; P = .01), PTCL NOS (HR, 2.24; 95% CI, 1.61-3.14; P < .01), and AITL subtypes (HR, 1.58; 95% CI, 1.11-2.24; P = .01).
Impact of etoposide (analysis B)
Patient characteristics
To analyze the impact of etoposide on outcome, we compared CHOEP in analysis B with CHOP using data of patients diagnosed in the period from 2014 to 2018. The use of etoposide increased from 17% in 2014 to 82% in 2018 (supplemental Figure 1B). Baseline characteristics for patients with ALK+ ALCL and for patients with ALK− ALCL, AITL, or PTCL NOS are listed in Table 2. There were no significant differences in sex, median age, or other clinical variables between those with ALK+ ALCL treated with CHOP or CHOEP (Table 2) or between those ALK− ALCL, AITL, or PTCL NOS patients treated with CHOP or CHOEP (Table 2).
Patient characteristics: analysis B
| . | CHOP . | CHOEP . | ||
|---|---|---|---|---|
| n . | % . | n . | % . | |
| ALK+ ALCL | (n = 28) | (n = 30) | ||
| Male sex | 18 | 64 | 16 | 53 |
| Age at diagnosis, y | ||||
| Median | 43 | 49 | ||
| Range | 23-63 | 19-62 | ||
| Ann Arbor stage | ||||
| 2 | 8 | 29 | 10 | 33 |
| 3-4 | 20 | 71 | 20 | 67 |
| Elevated LDH | 7 | 25 | 10 | 33 |
| WHO performance score | ||||
| 0-2 | 14 | 50 | 21 | 70 |
| 3-4 | 1 | 4 | 1 | 3 |
| Unknown | 13 | 46 | 8 | 27 |
| >1 extranodal localization | 4 | 14 | 6 | 20 |
| IPI score | ||||
| 0-2 | 26 | 93 | 27 | 90 |
| 3-5 | 2 | 7 | 3 | 10 |
| ALK− ALCL, AITL, or PTCL NOS | (n = 85) | (n = 134) | ||
| Male sex | 54 | 64 | 86 | 64 |
| Age at diagnosis, y | ||||
| Median | 55 | 54 | ||
| Range | 26-62 | 19-60 | ||
| Subtype of T-cell NHL | ||||
| ALK− ALCL | 14 | 16 | 31 | 23 |
| ALCL NOS | 0 | — | 1 | 1 |
| PTCL NOS | 25 | 29 | 54 | 40 |
| AITL* | 46 | 54 | 48 | 36 |
| Ann Arbor stage | ||||
| 2 | 6 | 7 | 14 | 10 |
| 3-4 | 79 | 93 | 120 | 90 |
| Elevated LDH | 48 | 56 | 76 | 57 |
| WHO performance score | ||||
| 0-2 | 38 | 45 | 78 | 58 |
| 3-4 | 0 | 0 | 4 | 3 |
| Unknown | 47 | 55 | 52 | 39 |
| >1 extranodal localization | 25 | 29 | 37 | 28 |
| IPI score | ||||
| 0-2 | 52 | 61 | 92 | 69 |
| 3-5 | 33 | 39 | 42 | 31 |
| ASCT | 27 | 32 | 82 | 61 |
| . | CHOP . | CHOEP . | ||
|---|---|---|---|---|
| n . | % . | n . | % . | |
| ALK+ ALCL | (n = 28) | (n = 30) | ||
| Male sex | 18 | 64 | 16 | 53 |
| Age at diagnosis, y | ||||
| Median | 43 | 49 | ||
| Range | 23-63 | 19-62 | ||
| Ann Arbor stage | ||||
| 2 | 8 | 29 | 10 | 33 |
| 3-4 | 20 | 71 | 20 | 67 |
| Elevated LDH | 7 | 25 | 10 | 33 |
| WHO performance score | ||||
| 0-2 | 14 | 50 | 21 | 70 |
| 3-4 | 1 | 4 | 1 | 3 |
| Unknown | 13 | 46 | 8 | 27 |
| >1 extranodal localization | 4 | 14 | 6 | 20 |
| IPI score | ||||
| 0-2 | 26 | 93 | 27 | 90 |
| 3-5 | 2 | 7 | 3 | 10 |
| ALK− ALCL, AITL, or PTCL NOS | (n = 85) | (n = 134) | ||
| Male sex | 54 | 64 | 86 | 64 |
| Age at diagnosis, y | ||||
| Median | 55 | 54 | ||
| Range | 26-62 | 19-60 | ||
| Subtype of T-cell NHL | ||||
| ALK− ALCL | 14 | 16 | 31 | 23 |
| ALCL NOS | 0 | — | 1 | 1 |
| PTCL NOS | 25 | 29 | 54 | 40 |
| AITL* | 46 | 54 | 48 | 36 |
| Ann Arbor stage | ||||
| 2 | 6 | 7 | 14 | 10 |
| 3-4 | 79 | 93 | 120 | 90 |
| Elevated LDH | 48 | 56 | 76 | 57 |
| WHO performance score | ||||
| 0-2 | 38 | 45 | 78 | 58 |
| 3-4 | 0 | 0 | 4 | 3 |
| Unknown | 47 | 55 | 52 | 39 |
| >1 extranodal localization | 25 | 29 | 37 | 28 |
| IPI score | ||||
| 0-2 | 52 | 61 | 92 | 69 |
| 3-5 | 33 | 39 | 42 | 31 |
| ASCT | 27 | 32 | 82 | 61 |
Patients receiving CHOP vs CHOEP during first-line treatment.
Including T-cell NHL, follicular helper T cells.
Outcomes
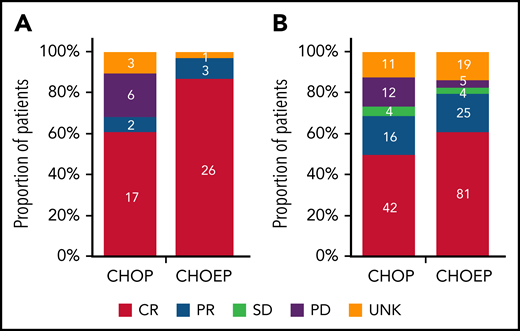
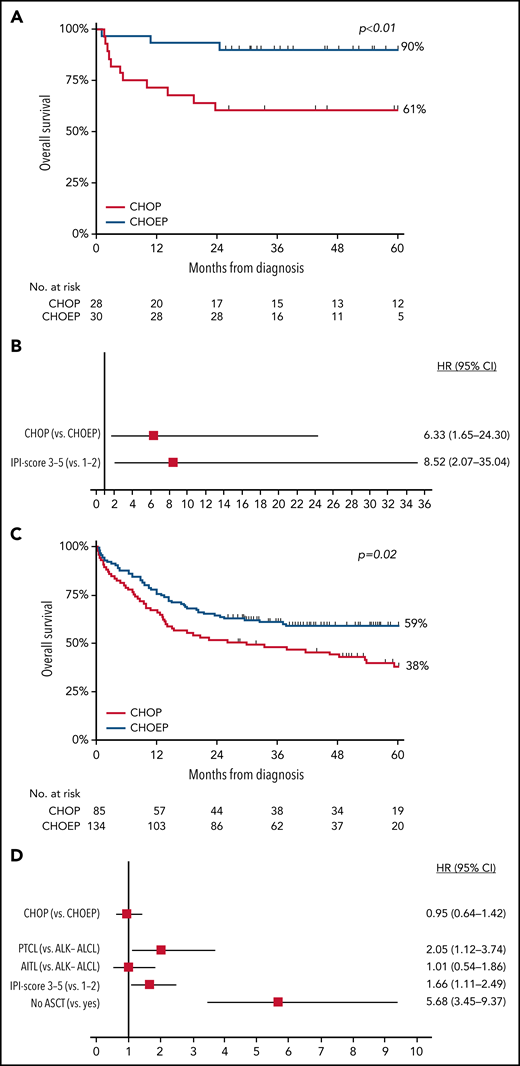
In patients with ALK+ ALCL who received CHOEP, CR rate was significantly higher than in patients who received CHOP (86% vs 61%; P = .03; Figure 2A). Overall, 5-year OS for patients with ALK+ ALCL who received CHOEP was superior to that in patients who received CHOP (90%; 95% CI, 72% to 97% vs 61%; 95% CI, 40% to 76%; P < .01; Figure 3A). When simultaneously adjusted for age at diagnosis and IPI score, risk of mortality was higher for patients treated with CHOP than for patients receiving CHOEP (HR, 6.33; 95% CI, 1.65-24.30; P < .01; Figure 3B) IPI score ≥3 adversely affected risk of mortality (univariable HRs and corresponding 95% CIs are presented in supplemental Table 3A).
Best tumor response in patients with ALK+ ALCL aged <65 years with stage II to IV disease. (A-B) Best response in patients who received CHOP with or without etoposide (A) and in those with ALK− ALCL, AITL, or PTCL NOS regardless of subsequent ASCT (B). Denominators include patients with ALK+ ALCL diagnosed in the period from 2014 to 2018 and treated with either CHOP of CHOEP (A) and patients with ALK− ALCL, AITL, or PTCL NOS diagnosed in the period from 2014 to 2018 and treated with either CHOP or CHOEP (B). PD, progressive disease; PR, partial remission; SD, stable disease; UNK, unknown.
Best tumor response in patients with ALK+ ALCL aged <65 years with stage II to IV disease. (A-B) Best response in patients who received CHOP with or without etoposide (A) and in those with ALK− ALCL, AITL, or PTCL NOS regardless of subsequent ASCT (B). Denominators include patients with ALK+ ALCL diagnosed in the period from 2014 to 2018 and treated with either CHOP of CHOEP (A) and patients with ALK− ALCL, AITL, or PTCL NOS diagnosed in the period from 2014 to 2018 and treated with either CHOP or CHOEP (B). PD, progressive disease; PR, partial remission; SD, stable disease; UNK, unknown.
OS in patients aged <65 years with stage II to IV disease. (A-D) OS in patients with ALK+ ALCL (A) and in those with ALK− ALCL, AITL, or PTCL NOS treated with CHOP or CHOEP in the first-line setting (C), with corresponding forest plots of HRs and 95% CIs (B,D), using multivariable Cox regression analysis.
OS in patients aged <65 years with stage II to IV disease. (A-D) OS in patients with ALK+ ALCL (A) and in those with ALK− ALCL, AITL, or PTCL NOS treated with CHOP or CHOEP in the first-line setting (C), with corresponding forest plots of HRs and 95% CIs (B,D), using multivariable Cox regression analysis.
In patients with ALK− ALCL, AITL, or PTCL NOS who received CHOEP, CR rate was significantly higher, although borderline, compared with that in patients who received CHOP (60% vs 49%; P = .06; Figure 2B). For patients who did not undergo consolidation with ASCT, 83% treated with CHOP and 57% treated with CHOEP achieved CR (supplemental Figure 4). Overall, 5-year OS for patients treated with CHOEP was superior to that for patients receiving CHOP (59%; 95% CI, 50% to 67% vs 38%; 95% CI, 27% to 49%; P < .01; Figure 3C). However, when simultaneously adjusted for age at diagnosis, PTCL subtype, IPI score, and ASCT as time-varying covariate, risk of mortality was similar for patients treated with CHOP (HR, 0.95; 95% CI, 0.64-1.42; P = .81) and patients treated with CHOEP (Figure 3D). Older age, PTCL NOS subtype, IPI score 3 to 5, and no SCT adversely affected risk of mortality (univariable HRs and corresponding 95% CIs are presented in supplemental Table 3B).
Impact of ASCT (analysis C)
Patient characteristics
In analysis C, we evaluated the impact of ASCT among patients diagnosed in the period from 2014 to 2018, regardless of whether these patients received CHOP with or without etoposide or another type of chemotherapy. Overall, 117 (52%) of 213 patients underwent ASCT. Baseline characteristics are presented in Table 3. Patients who underwent ASCT compared with patients who did not were less often male, more often had a lower IPI score, and were more often diagnosed with ALK− ALCL.
Patient characteristics: analysis C
| . | ASCT (n = 117) . | No ASCT (n = 96) . | ||
|---|---|---|---|---|
| n . | % . | n . | % . | |
| Male sex | 71 | 61 | 67 | 70 |
| Age at diagnosis, y | ||||
| Median | 54 | 54.5 | ||
| Range | 20-64 | 19-64 | ||
| Subtype of T-cell NHL | ||||
| ALK− ALCL | 28 | 24 | 16 | 17 |
| PTCL NOS | 41 | 35 | 34 | 35 |
| AITL* | 48 | 41 | 46 | 48 |
| Ann Arbor stage | ||||
| 2 | 13 | 11 | 13 | 13 |
| 3-4 | 104 | 89 | 83 | 87 |
| Elevated LDH | 56 | 48 | 50 | 52 |
| WHO performance score | ||||
| 0-2 | 73 | 63 | 42 | 45 |
| 3-4 | 2 | 1 | 4 | 4 |
| Unknown | 42 | 36 | 50 | 52 |
| >1 extranodal localization | 29 | 25 | 23 | 24 |
| IPI score | ||||
| 0-2 | 90 | 77 | 64 | 67 |
| 3-5 | 27 | 23 | 32 | 33 |
| . | ASCT (n = 117) . | No ASCT (n = 96) . | ||
|---|---|---|---|---|
| n . | % . | n . | % . | |
| Male sex | 71 | 61 | 67 | 70 |
| Age at diagnosis, y | ||||
| Median | 54 | 54.5 | ||
| Range | 20-64 | 19-64 | ||
| Subtype of T-cell NHL | ||||
| ALK− ALCL | 28 | 24 | 16 | 17 |
| PTCL NOS | 41 | 35 | 34 | 35 |
| AITL* | 48 | 41 | 46 | 48 |
| Ann Arbor stage | ||||
| 2 | 13 | 11 | 13 | 13 |
| 3-4 | 104 | 89 | 83 | 87 |
| Elevated LDH | 56 | 48 | 50 | 52 |
| WHO performance score | ||||
| 0-2 | 73 | 63 | 42 | 45 |
| 3-4 | 2 | 1 | 4 | 4 |
| Unknown | 42 | 36 | 50 | 52 |
| >1 extranodal localization | 29 | 25 | 23 | 24 |
| IPI score | ||||
| 0-2 | 90 | 77 | 64 | 67 |
| 3-5 | 27 | 23 | 32 | 33 |
Patients undergoing ASCT vs not undergoing ASCT during first-line treatment.
Including T-cell NHL, follicular helper T cells.
Outcomes
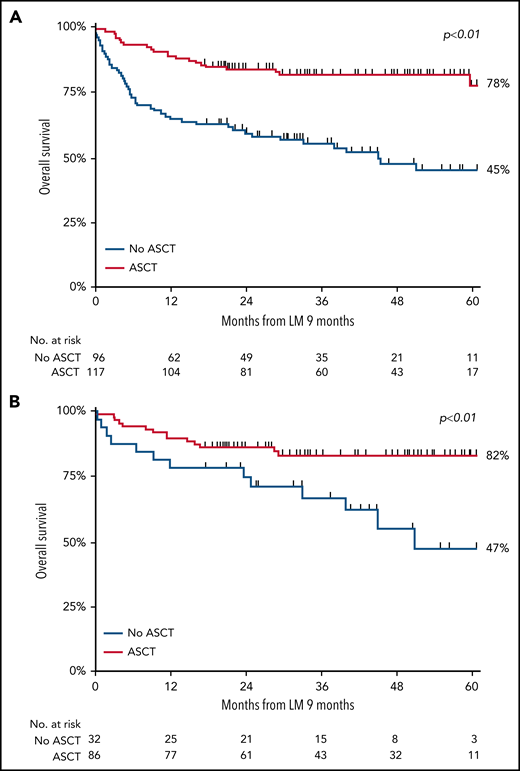
Using the landmark approach, 5-year OS of patients undergoing consolidation with ASCT was superior to that of patients receiving induction chemotherapy only (78% vs 45%, respectively; P < .01; Figure 4A). In a sensitivity analysis, we evaluated OS estimates among patients with or without ASCT who achieved CR. Baseline characteristics for these patients were balanced regarding age at diagnosis, LDH level, Ann Arbor stage, and number of extranodal localizations, and 5-year OS was superior in patients who underwent consolidation with ASCT to that in patients who did not undergo ASCT consolidation (82% and 47%, respectively; P < .01; Figure 4B). Superior 5-year OS with ASCT consolidation was also observed in each subtype separately (supplemental Table 4), although patient numbers were small.
OS in patients aged <65 years with stage II to IV disease. (A-B) OS in patients with ALCL, AITL, or PTCL NOS treated with or without consolidation with ASCT after chemotherapy in the first-line setting, measured in months after the 9-month landmark (LM) (A), and in patients who achieved CR in the first-line setting after induction chemotherapy with or without consolidation with ASCT (B).
OS in patients aged <65 years with stage II to IV disease. (A-B) OS in patients with ALCL, AITL, or PTCL NOS treated with or without consolidation with ASCT after chemotherapy in the first-line setting, measured in months after the 9-month landmark (LM) (A), and in patients who achieved CR in the first-line setting after induction chemotherapy with or without consolidation with ASCT (B).
Discussion
In this nationwide population-based study, we aimed to complement and expand on previously reported phase 2 studies of primary treatment and OS among patients with PTCL seen in routine clinical practice in the Netherlands, thereby identifying all patients with AITL, ALCL, or PTCL NOS diagnosed between 1989 and 2018, including less fit patients who are typically excluded in other studies. A statistically significant increase in 5-year OS from 39% in the first calendar period to 49% in the second calendar period was observed. In addition to the growing possibilities in first-line treatment strategies, access to more sensitive diagnostic modalities in routine daily practice, improved supportive care, and increased application of novel agents in subsequent treatment lines, such as allogeneic hematopoietic SCT and brentuximab vedotin, may have led to this observed improvement in outcomes.
Five-year OS in our study was higher when compared with that in other study populations of patients with PTCL, most likely because we restricted our analysis to patients aged <65 years and excluded rare PTCL subtypes, which generally have a worse prognosis.2,5,6,12,19 The increase in OS between the calendar periods applied to the group as a whole as well as to the different PTCL subtypes. Although the addition of etoposide to CHOP had no impact on OS, there was an OS benefit for patients with PTCL when first-line therapy included consolidation with ASCT.
After nonrandomized and population-based studies, etoposide was added to CHOP in first-line treatment for PTCL in the Netherlands from 2014 onward.2,12 We observed an increase in the use of etoposide from 2014 onward in our study population. Although studies showed an increase in PFS in patients aged <60 years when etoposide was added to CHOP, there was no significant OS benefit. Among patients treated between 2014 and 2018, CR rate was significantly higher for patients treated with CHOEP. For patients with ALK+ ALCL, we observed a significant OS difference in favor of etoposide. In patients with ALK− ALCL, AITL, or PTCL NOS, use of etoposide did not translate into an OS benefit when corrected for IPI score, PTCL subtype, and use of ASCT. Therefore, if there is any benefit of etoposide, it is short lived and does not improve OS.
Data supporting the use of ASCT in frontline therapy for PTCL go back to 2004, and although there have been prospective studies, randomized controlled trials are lacking.15,20 In our study, we observed a significant difference in 5-year OS between patients who underwent ASCT versus those who did not. There was a statistical benefit in OS among patients with PTCL in CR after first-line consolidation with ASCT. Our findings are partially in line with previous studies. Although ASCT consolidation in the first-line setting was associated with superior OS in a Swedish population-based study, this was not observed in a Scandinavian population-based study or in a French multicenter study.2,9,21
Until recently, alternative treatment strategies in PTCL had not been successful. First-line studies involving non–anthracycline-based regimens, such as gemcitabine, cisplatin, and methylprednisolone, have not reported improved outcomes in patients with PTCL.22 Although the addition of alemtuzumab to CHOP led to higher response rates, this did not translate into an OS benefit because of increased toxicity.23 Romidepsin added to CHOP did not improve PFS or OS in first-line treatment for patients with PTCL; however, patients with AITL did seem to benefit from this combination.24 Allogeneic SCT (alloSCT) remains a treatment option in the relapsed/refractory setting for patients with PTCL.21 Although retrospective studies have favored alloSCT in the first-line setting, a recent randomized phase 3 trial showed no significant differences between treatment arms.25-28 The strong graft-versus-lymphoma effect in the alloSCT group was nullified by transplantation-related mortality. Therefore, alloSCT should not be offered to patients as consolidation after first-line therapy.
The first prospective trial that showed an OS benefit in first-line treatment over CHOP in PTCL involved patients with CD30+ T-cell NHL (≥10% of cells by local review) who were treated with the combination of brentuximab vedotin, doxorubicin, cyclophosphamide, and prednisolone (A+CHP).29 CD30 expression varies across histologic subtypes (PTCL, 58%-64%; AITL, 63%-75%; and ALCL, 100%).30 As a result, 70% of patients in this study had ALCL. ASCT was offered to 22% of patients in the A+CHP group and 17% of patients in the CHOP group, based on physician choice. An exploratory study of PFS favored ASCT, but the effect on OS remains unconfirmed.31
The main strength of our study is the use of a nationwide population-based cancer registry with comprehensive data available on first-line treatment. Therefore, changing treatment practices over time could be assessed and directly linked to improvements in outcome. Limitations of our study mainly pertain to the lack of detailed information on first-line treatment for the period of 1989 to 2013; the lack of information on comorbidities, relapse, and subsequent therapy lines; and the potential misclassification of 1 subtype of PTCL as another. Furthermore, the reasons for not proceeding to ASCT are unknown and by themselves might have affected outcomes. Despite these limitations, cancer registries remain the standard for cancer surveillance activities and for population-based analysis of treatment outcomes because of the probable differences between real-world cohorts and phase 2 and 3 study populations.
In conclusion, in this large population-based study, we observed a significant increase in OS rate among patients with PTCL with advanced-stage disease over the last 2 decades, but their prognosis remains poor. The use of CHOEP compared with CHOP as first-line treatment did not increase OS, except in patients with ALK+ ALCL. Our data support the use of consolidation with ASCT in first-line treatment for patients with ALK− ALCL, AITL, or PTCL NOS.
Acknowledgments
The authors thank the registrars of the Netherlands Cancer Registry (NCR) for their dedicated data collection. The nationwide population-based NCR is maintained and hosted by the Netherlands Comprehensive Cancer Organisation.
Authorship
Contribution: M.N. and M.B. designed the study; M.B. collected the data; M.B., M.N., and F.O.M. analyzed the data and wrote the paper; and all authors revised the manuscript and accepted its final version.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Marcel Nijland, Department of Hematology, University Medical Center Groningen, University of Groningen, Hanzeplein 1, DA21, 9713 GZ Groningen, The Netherlands; e-mail: m.nijland@umcg.nl.
The online version of this article contains a data supplement.
There is a Blood Commentary on this article in this issue.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
REFERENCES
Author notes
M.B. and F.O.M. share first authorship.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal