TO THE EDITOR:
FMS-like tyrosine kinase-3 internal tandem duplication (FLT3-ITD) is identified in ∼25% of patients with acute myeloid leukemia (AML), making it one of the most common variants identified in this disease.1 Although frontline incorporation of the FLT3 inhibitor midostaurin has been shown to improve survival of patients with FLT3 mutant AML, it is broadly accepted that allogeneic hematopoietic cell transplant (allo-HCT), performed in approximately 25% to 30% of patients in first remission, is a key contributor to enhanced outcomes.2 Two studies have shown that detection of FLT3-ITD before transplant in morphologic remission using conventional fluorescence-based polymerase chain reaction (PCR) techniques with 1% to 5% sensitivity was associated with higher post-HCT relapse risk (39%-59%), compared with patients “negative” for FLT3-ITD (relapse risk 23%-41%).3,4 Assessment of FLT3-ITD by capillary electrophoresis (CE)-based approaches, however, has low sensitivity (∼1%), in contrast to high-coverage PCR and next-generation sequencing (NGS), which enables detection of FLT3-ITD with 100- to 1000-fold greater sensitivity.5-7 By using NGS to assess FLT3-ITD with a limit of detection of 10−4 to 10−5, the proportion of patients whose measurable residual disease (MRD) was negative after 2 cycles of intensive chemotherapy combined with midostaurin was 67%.8 Currently, it is not known whether detection of FLT3-ITD clones below the sensitivity of CE-based methods has clinical relevance in forecasting post-HCT relapse risk, especially if myeloablative conditioning (MAC) is administered. One study incorporating peripheral blood (PB) detection of FLT3-ITD before MAC or reduced-intensity conditioning HCT found that 9 of 10 patients positive for FLT3-ITD MRD (variant allele frequency [VAF], 0.03%-3.97%) relapsed, compared with 1 of 7 patients relapsing if FLT3-ITD MRD was negative pre-HCT.9
We sought to evaluate the prognostic impact of MRD assessment by PCR-NGS to detect FLT3-ITD with high sensitivity prior to allo-HCT and to determine the added value of this approach compared with CE. The study was approved by Alfred Health, Peter MacCallum Cancer Centre, Royal Melbourne Hospital (181/21), and All-Wales (08/MRE09/29) ethics committees. All subjects provided informed consent, and the study was conducted according to principles established by the Declaration of Helsinki. Patients with FLT3-ITD AML at diagnosis undergoing their first allo-HCT in morphologic remission (bone marrow [BM] blasts <5%) and with pre-HCT DNA available (92 BM and 12 PB) were included in this retrospective cohort (supplemental Figure 1, available on the Blood website). Strong concordance between BM and PB sampling was noted (supplemental Methods). None of the patients was exposed to FLT3 inhibitor maintenance post-HCT. Amplicon-based PCR-NGS of exons 14 to 15 detected FLT3-ITDs of at least 6 base pairs to a minimum depth of 500K reads, resulting in a limit of detection of 0.001% (supplemental Methods).7NPM1 MRD by quantitative reverse transcription PCR and NGS were determined as previously described.10,11 Kaplan-Meier survival estimates were calculated from the HCT date to the date of death or last follow-up (overall survival [OS]), and/or morphologic relapse (relapse-free survival [RFS]). Cumulative incidence of relapse (CIR) was estimated considering transplant-related mortality as a competing risk. Cox proportional hazards model was used for univariate and multivariate analyses.
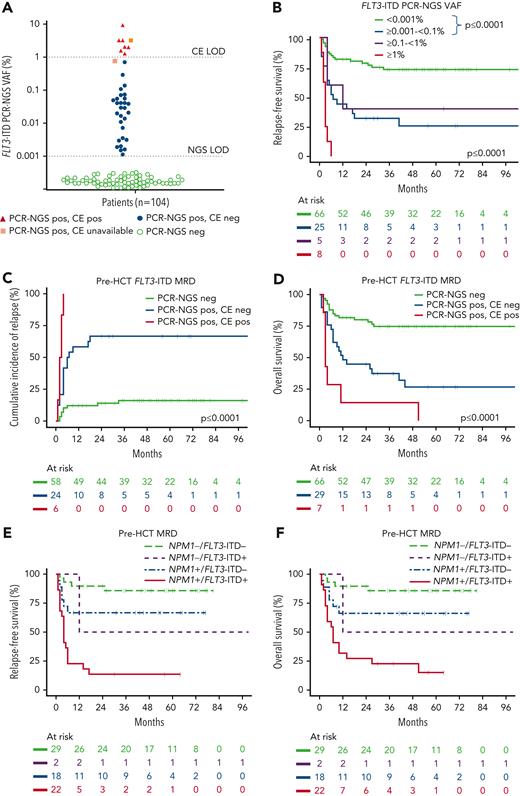
The cohort included 104 patients with a median age of 49 years (17-68). One-quarter had prior exposure to FLT3 inhibitors, 43% had a baseline FLT3-ITD allelic ratio ≥0.5, 75% had coexisting NPM1 mutation, 86% underwent transplantation in first remission (CR1), and 49% received MAC (Table 1). The higher than expected proportion of patients with NPM1 comutation is explained by the NCRI AML17 cohort being enriched for patients undergoing NPM1 MRD monitoring. Twelve patients received MRD-directed therapy before HCT to treat molecular NPM1mut relapse (supplemental Figure 2). Among the 104 patients in morphologic remission pre-HCT, 37% had FLT3-ITD MRD detected by PCR-NGS (median VAF 0.041% [range, 0.0011-9.352]). Of the cases who were FLT3-ITD positive by PCR-NGS, only 7 of 36 (19%) were positive by CE (Figure 1A). The median time from the date pre-HCT FLT3-ITD MRD was assessed to the date of HCT was 27.5 days (range, 1-87). The 2-year RFS was 78%, 32%, 40%, and 0% for FLT3-ITD MRD levels <0.001 (negative), ≥0.001 to <0.1%, ≥0.1 to <1%, and ≥1%, respectively (Figure 1B), with the corresponding CIR and OS according to the level of FLT3-ITD MRD shown in supplemental Figure 3. Therefore, post-HCT relapse was substantial even for patients with FLT3-ITD MRD levels <1%, below the detection threshold of CE. For patients positive for FLT3-ITD by both PCR-NGS and CE, CIR was 100% within the first 6 months post-HCT (Figure 1C for CIR and supplemental Figure 4 for RFS). For patients FLT3-ITD MRD positive by PCR-NGS but not by CE, the CIR post-HCT was 67% (Figure 1C). In contrast, patients with FLT3-ITD MRD levels <0.001% (negative) by PCR-NGS had a post-HCT relapse risk of 16%. OS post-HCT was also negatively impacted by the presence of FLT3-ITD MRD pre-HCT, with ≤26% alive at 4 years, compared with 74% if FLT3-ITD MRD was negative (Figure 1D). Similar to previously published results,9 MAC was not able to attenuate the high relapse risk associated with pre-HCT FLT3-ITD MRD, with RFS and OS comparable to that observed with reduced-intensity conditioned (supplemental Figure 5). Multivariate analysis considering factors listed in supplemental Table 1 demonstrated that pre-HCT FLT3-ITD MRD (hazard ratio [HR], 4.94, P < .0001), transplant in CR2 vs CR1 (HR, 2.39, P = .05), and T-cell depletion (HR, 2.80, P = .006) were the most important determinants of post-HCT relapse risk and survival.
Baseline characteristics
| Variables at AML diagnosis, n (%) unless specified . | All patients (n = 104) . | Pre-HCT: FLT3-ITD positive∗ . | Pre-HCT: FLT3-ITD negative . | |
|---|---|---|---|---|
| PCR-NGS positive and CE positive (n = 7) . | PCR-NGS positive, CE negative (n = 29) . | PCR-NGS negative (n = 66) . | ||
| Median age (range), y | 49 (17-68) | 44 (25-68) | 51 (26-68) | 47 (17-68) |
| Men | 47 (45) | 1 (14) | 18 (62) | 27 (42) |
| AML subtype | ||||
| De novo | 97/102 (95) | 7 (100) | 26 (90) | 62/64 (97) |
| Secondary | 5/102 (5) | — | 3 (10) | 1/64 (3) |
| Prior therapy | ||||
| No FLT3 inhibitor | 76 (73) | 6 (86) | 18 (62) | 50 (76) |
| FLT3 inhibitor | 28 (27) | 1 (14) | 11 (38) | 16 (24) |
| Karyotype risk† | ||||
| Favorable | — | — | — | — |
| Intermediate | 95/96 (99) | 7/7 (100) | 28/28 (100) | 59/59 (100) |
| Adverse | 1/96 (1) | — | — | — |
| FLT3-ITD allelic ratio (1 NA) | ||||
| <0.5 | 59/103 (57) | 3 (43) | 15/28 (54) | 40 (61) |
| ≥0.5 | 44/103 (43) | 4 (57) | 13/28 (46) | 26 (39) |
| NPM1 mutant | 78 (75) | 4 (57) | 21 (72) | 52 (79) |
| NPM1 wild-type | 26 (25) | 3 (43) | 8 (28) | 14 (21) |
| FLT3-TKD mutant | 4/96 (4) | 0 | 2/28 (7) | 2/59 (3) |
| FLT3-TKD wild-type | 92/96 (96) | 7/7 (100) | 26/28 (93) | 57/59 (97) |
| DNMT3A mutant‡ | 33/59 (56) | 0 | 9/16 (56) | 23/40 (57) |
| DNMT3A wild-type | 26/59 (44) | 2/2 (100) | 7/16 (44) | 17/40 (43) |
| Prior lines of therapy pre-HCT | ||||
| 1 | 75 (72) | 2 (29) | 19 (66) | 52 (79) |
| 2 | 29 (28) | 5 (71) | 10 (34) | 14 (21) |
| Conditioning§ | ||||
| Myeloablative | 40/82 (49) | 3/6 (50) | 12/22 (55) | 24/53 (44) |
| Reduced intensity | 42/82 (51) | 3/6 (50) | 10/22 (45) | 29/52 (56) |
| Transplant status | ||||
| CR1 | 89 (86) | 3 (43) | 25 (86) | 59 (89) |
| CR2 | 15 (14) | 4 (57) | 4 (14) | 7 (11) |
| T-cell depletion‖ | 41/81 (51) | 4/5 (80) | 12/22 (54) | 25/53 (47) |
| Unrelated donor¶ | 52/100 (52) | 3/6 (50) | 9/28 (32) | 40/65 (62) |
| Median time from last treatment to day 0 of HCT (range), d | 42.5 (12-147) | |||
| Variables at AML diagnosis, n (%) unless specified . | All patients (n = 104) . | Pre-HCT: FLT3-ITD positive∗ . | Pre-HCT: FLT3-ITD negative . | |
|---|---|---|---|---|
| PCR-NGS positive and CE positive (n = 7) . | PCR-NGS positive, CE negative (n = 29) . | PCR-NGS negative (n = 66) . | ||
| Median age (range), y | 49 (17-68) | 44 (25-68) | 51 (26-68) | 47 (17-68) |
| Men | 47 (45) | 1 (14) | 18 (62) | 27 (42) |
| AML subtype | ||||
| De novo | 97/102 (95) | 7 (100) | 26 (90) | 62/64 (97) |
| Secondary | 5/102 (5) | — | 3 (10) | 1/64 (3) |
| Prior therapy | ||||
| No FLT3 inhibitor | 76 (73) | 6 (86) | 18 (62) | 50 (76) |
| FLT3 inhibitor | 28 (27) | 1 (14) | 11 (38) | 16 (24) |
| Karyotype risk† | ||||
| Favorable | — | — | — | — |
| Intermediate | 95/96 (99) | 7/7 (100) | 28/28 (100) | 59/59 (100) |
| Adverse | 1/96 (1) | — | — | — |
| FLT3-ITD allelic ratio (1 NA) | ||||
| <0.5 | 59/103 (57) | 3 (43) | 15/28 (54) | 40 (61) |
| ≥0.5 | 44/103 (43) | 4 (57) | 13/28 (46) | 26 (39) |
| NPM1 mutant | 78 (75) | 4 (57) | 21 (72) | 52 (79) |
| NPM1 wild-type | 26 (25) | 3 (43) | 8 (28) | 14 (21) |
| FLT3-TKD mutant | 4/96 (4) | 0 | 2/28 (7) | 2/59 (3) |
| FLT3-TKD wild-type | 92/96 (96) | 7/7 (100) | 26/28 (93) | 57/59 (97) |
| DNMT3A mutant‡ | 33/59 (56) | 0 | 9/16 (56) | 23/40 (57) |
| DNMT3A wild-type | 26/59 (44) | 2/2 (100) | 7/16 (44) | 17/40 (43) |
| Prior lines of therapy pre-HCT | ||||
| 1 | 75 (72) | 2 (29) | 19 (66) | 52 (79) |
| 2 | 29 (28) | 5 (71) | 10 (34) | 14 (21) |
| Conditioning§ | ||||
| Myeloablative | 40/82 (49) | 3/6 (50) | 12/22 (55) | 24/53 (44) |
| Reduced intensity | 42/82 (51) | 3/6 (50) | 10/22 (45) | 29/52 (56) |
| Transplant status | ||||
| CR1 | 89 (86) | 3 (43) | 25 (86) | 59 (89) |
| CR2 | 15 (14) | 4 (57) | 4 (14) | 7 (11) |
| T-cell depletion‖ | 41/81 (51) | 4/5 (80) | 12/22 (54) | 25/53 (47) |
| Unrelated donor¶ | 52/100 (52) | 3/6 (50) | 9/28 (32) | 40/65 (62) |
| Median time from last treatment to day 0 of HCT (range), d | 42.5 (12-147) | |||
NA, not available.
Two without available sample for CE correlation.
Determined according to UK MRC criteria. Eight patients with unknown karyotype.
DNMT3A mutation testing in 59 patients only.
Conditioning intensity information available in 82 patients. Myeloablative conditioning regimens were busulfan and cyclophosphamide, cyclophosphamide and total body irradiation, and fludarabine and 4 days of busulfan.
T-cell depletion information available in 81 patients.
Donor source information available in 100 patients.
Pre-HCTFLT3-ITD MRD and outcomes. (A) The majority of FLT3-ITD MRD detected by PCR-NGS pre-HCT is below the threshold of conventional CE. The limit of detection for FLT3-ITD as assessed by CE and PCR-NGS is indicated. Orange boxes indicate 2 patients positive for FLT3-ITD by PCR-NGS but without available CE data. (B) Relapse risk post-HCT is associated with FLT3-ITD PCR-NGS VAF ≥0.001%. Kaplan–Meier estimates of relapse-free survival according to pre-HCT FLT3-ITD MRD levels showing highest relapse risk for FLT3-ITD MRD ≥1%, lowest risk for FLT3-ITD MRD <0.001%, and intermediate risk for levels between 0.001 and <1%. (C-D) Pre-HCT FLT3-ITD MRD is associated with inferior clinical outcomes. Kaplan-Meier estimates of (C) cumulative incidence of relapse (with transplant-related mortality as a competing risk in 14 patients not included in this curve) and (D) overall survival according to pre-HCT FLT3-ITD PCR-NGS and CE status. Two patients with positive FLT3-ITD by PCR-NGS lacking CE data were excluded. (E-F) Pre-HCT FLT3-ITD and NPM1 MRD are prognostic for clinical outcome post-HCT. Kaplan-Meier estimates of (E) relapse-free and (F) overall survival according to pre-HCT FLT3-ITD PCR-NGS MRD and NPM1 MRD in 71 of 78 co-mutated for both FLT3-ITD and NPM1. Seven patients with insufficient material for NPM1 MRD assessment were excluded.
Pre-HCTFLT3-ITD MRD and outcomes. (A) The majority of FLT3-ITD MRD detected by PCR-NGS pre-HCT is below the threshold of conventional CE. The limit of detection for FLT3-ITD as assessed by CE and PCR-NGS is indicated. Orange boxes indicate 2 patients positive for FLT3-ITD by PCR-NGS but without available CE data. (B) Relapse risk post-HCT is associated with FLT3-ITD PCR-NGS VAF ≥0.001%. Kaplan–Meier estimates of relapse-free survival according to pre-HCT FLT3-ITD MRD levels showing highest relapse risk for FLT3-ITD MRD ≥1%, lowest risk for FLT3-ITD MRD <0.001%, and intermediate risk for levels between 0.001 and <1%. (C-D) Pre-HCT FLT3-ITD MRD is associated with inferior clinical outcomes. Kaplan-Meier estimates of (C) cumulative incidence of relapse (with transplant-related mortality as a competing risk in 14 patients not included in this curve) and (D) overall survival according to pre-HCT FLT3-ITD PCR-NGS and CE status. Two patients with positive FLT3-ITD by PCR-NGS lacking CE data were excluded. (E-F) Pre-HCT FLT3-ITD and NPM1 MRD are prognostic for clinical outcome post-HCT. Kaplan-Meier estimates of (E) relapse-free and (F) overall survival according to pre-HCT FLT3-ITD PCR-NGS MRD and NPM1 MRD in 71 of 78 co-mutated for both FLT3-ITD and NPM1. Seven patients with insufficient material for NPM1 MRD assessment were excluded.
We next sought to determine whether FLT3-ITD MRD by PCR-NGS provided additional prognostic value among patients also known to be NPM1 mutant. Of 71 patients with both FLT3-ITD and NPM1 mutation at AML diagnosis with available pre-HCT MRD for both markers, RFS and OS were most favorable for those negative for both NPM1 and FLT3-ITD MRD pre-HCT (Figure 1E-F). In contrast, outcomes were dismal for patients double positive for both NPM1 and FLT3-ITD MRD. Interestingly, intermediate RFS and OS were observed for patients positive for either NPM1 or FLT3-ITD MRD before HCT, suggesting MRD assessment for NPM1 and FLT3-ITD had complementary clinical value (Figure 1E-F). In this FLT3-ITD cohort, low-level NPM1 MRD (<2%) was associated with inferior prognosis, despite allo-HCT (supplemental Figure 6).12
Our results highlight the dismal prognosis associated with pre-HCT detection of FLT3-ITD MRD using high-sensitivity PCR-NGS–based approaches. The poor prognosis associated with FLT3-ITD MRD was relevant even at very low MRD levels and was not ameliorated by MAC HCT. Therefore, FLT3-ITD MRD detection pre-HCT may be an indication for future MRD-directed therapeutic strategies in the pre- or post-HCT setting. Clinical studies in FLT3mut relapsed/refractory AML indicate that gilteritinib may reduce FLT3-ITD VAF to ≤10−4 in 29.2% of patients achieving complete remission or complete remission with partial hematologic recovery.13 Gilteritinib combined with the BCL-2 inhibitor venetoclax reduced FLT3-ITD levels to ≤10−2 in 56.7% of patients achieving morphologic remission.14 Although literature supports the clinical efficacy of posttransplant FLT3 inhibitor maintenance in reducing relapse risk and death,15 the efficacy of FLT3 inhibitors in suppressing FLT3-ITD MRD levels in the post-HCT setting has not been formally demonstrated. This is likely to be addressed by the BMT-CTN1506 study, which randomized patients post-HCT to either gilteritinib or placebo maintenance with PCR-NGS MRD assessments planned at pre- and post-HCT timepoints. An intriguing future question is whether FLT3-ITD MRD assessment by high-coverage PCR-NGS could identify patients most likely to benefit from FLT3-directed therapy before or after transplant. FLT3-ITD assessment by PCR-NGS has important clinical value and warrants further investigation as a guide for precision-based therapeutic strategies aimed at improving the natural history of patients identified to be MRD positive pre-HCT.
Acknowledgments
This work was supported by fellowships and grants from the Australian National Health and Medical Research Council 1162809 (A.H.W.), Metcalf Family Fellowship (A.H.W.), Medical Research Future Fund 1141460 (A.H.W.), and Australian Cancer Research Foundation and Cancer Research UK (AML M17).
Authorship
Contribution: A.H.W. and S.L. designed the research; A.I. designed and validated the PCR-NGS assay; A.I., N.S.A., N.P., J.J., M.R., M.M., and A.G. sequenced and analyzed the samples or provided other molecular analysis tools and interpretation; J.O. and S.L. performed clinical data collection; S.L. performed the statistical analysis; S.L., R.D., J.O., I.S.T., C.C.C., A.B., D.R., Z.H.Y., K.G., I.T., S.J., N.H.R., and A.H.W. contributed patients or analyzed and interpreted data; S.L. and A.H.W. wrote the manuscript; and all authors read and approved the manuscript.
Conflict-of-interest disclosure: A.H.W. and N.S.A. are employees of the Walter and Eliza Hall Institute, which receives milestone and royalty payments related to venetoclax. A.H.W. has received research funding from Servier and AbbVie and is a medical advisor to Astellas, AbbVie, and Servier. The remaining authors declare no competing financial interests.
Correspondence: Andrew H. Wei, Department of Haematology, Peter MacCallum Cancer Centre and Royal Melbourne Hospital, 305 Grattan St, Parkville, VIC 3000, Australia; e-mail: andrew.wei@petermac.org.
References
Author notes
∗S.L. and R.D. contributed equally to this work.
The data that support the findings of this study are available from the corresponding author, A.H.W., upon reasonable request.
The online version of this article contains a data supplement.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal