Key Points
In Ph+ ALL, allogeneic transplant in first remission does not improve survival for patients achieving a deep molecular remission.
The use of transplant was associated with lower incidence of disease relapse but increased treatment-related mortality.
Abstract
Historically, Philadelphia chromosome-positive (Ph+) acute lymphoblastic leukemia (ALL) has been associated with poor outcomes, and allogeneic hematopoietic cell transplantation (allo-HCT) is recommended in first complete remission (CR1). However, in the tyrosine kinase inhibitor (TKI) era, rapid attainment of a complete molecular remission (CMR) is associated with excellent outcomes without allo-HCT, suggesting transplant may not be required for these patients. To test this hypothesis, we retrospectively identified adult patients with Ph+ ALL treated with induction therapy, including TKIs, and attained CMR within 90 days of diagnosis at 5 transplant centers in the United States. We compared outcomes of those who did and did not receive allo-HCT in first remission. We identified 230 patients (allo-HCT: 98; non-HCT: 132). The allo-HCT cohort was younger with better performance status. On multivariable analysis (MVA), allo-HCT was not associated with improved overall survival (adjusted hazard ratio [aHR]: 1.05; 95% CI, 0.63-1.73) or relapse-free survival (aHR: 0.86; 95% CI, 0.54-1.37) compared with non-HCT treatment. Allo-HCT was associated with a lower cumulative incidence of relapse (aHR: 0.32; 95% CI, 0.17-0.62) but higher non-relapse mortality (aHR: 2.59; 95% CI, 1.37-4.89). Propensity score matching analysis confirmed results of MVA. Comparison of reduced-intensity HCT to non-HCT showed no statistically significant difference in any of the above endpoints. In conclusion, adult patients with Ph+ ALL who achieved CMR within 90 days of starting treatment did not derive a survival benefit from allo-HCT in CR1 in this retrospective study.
Medscape Continuing Medical Education online

In support of improving patient care, this activity has been planned and implemented by Medscape, LLC and the American Society of Hematology. Medscape, LLC is jointly accredited with commendation by the Accreditation Council for Continuing Medical Education (ACCME), the Accreditation Council for Pharmacy Education (ACPE), and the American Nurses Credentialing Center (ANCC), to provide continuing education for the healthcare team.
Medscape, LLC designates this Journal-based CME activity for a maximum of 1.0 AMA PRA Category 1 Credit(s)™. Physicians should claim only the credit commensurate with the extent of their participation in the activity.
Successful completion of this CME activity, which includes participation in the evaluation component, enables the participant to earn up to 1.0 MOC points in the American Board of Internal Medicine's (ABIM) Maintenance of Certification (MOC) program. Participants will earn MOC points equivalent to the amount of CME credits claimed for the activity. It is the CME activity provider's responsibility to submit participant completion information to ACCME for the purpose of granting ABIM MOC credit.
All other clinicians completing this activity will be issued a certificate of participation. To participate in this journal CME activity: (1) review the learning objectives; (2) study the education content; (3) take the post-test with a 75% minimum passing score and complete the evaluation at https://www.medscape.org/journal/blood; and (4) view/print certificate. For CME questions, see page 2181.
Disclosures
Laurie Barclay, MD, freelance writer and reviewer, Medscape, LLC, has disclosed the following relevant financial relationships: stock, stock options, or bonds: AbbVie Inc. (former).
Learning objectives
Upon completion of this activity, participants will:
Describe outcomes of adult patients with Philadelphia chromosome-positive (Ph+) acute lymphoblastic leukemia (ALL) treated with induction therapy, including tyrosine kinase inhibitors (TKIs), who attained complete molecular remission (CMR) within 3 months of diagnosis, comparing patients who did and did not receive allogeneic hematopoietic cell transplantation (allo-HCT) in first complete remission (CR1), according to a multicenter retrospective analysis
Determine impact of reduced-intensity conditioning and salvage treatment on outcomes of adult patients with Ph+ ALL treated with induction therapy, including TKIs, who attained CMR within 3 months of diagnosis, comparing patients who did and did not receive allo-HCT in CR1, according to a multicenter retrospective analysis
Identify clinical implications of outcomes of adult patients with Ph+ ALL treated with induction therapy, including TKIs, who attained CMR within 3 months of diagnosis, comparing patients who did and did not receive allo-HCT in CR1, according to a multicenter retrospective analysis
Release date: November 17, 2022; Expiration date: November 17, 2023
Introduction
The Philadelphia chromosome (Ph+) is the most common cytogenetic abnormality in adult acute lymphoblastic leukemia (ALL), occurring in ∼25% of patients at diagnosis.1 Prior to the introduction of tyrosine kinase inhibitors (TKIs) targeting the BCR-ABL fusion protein, Ph+ ALL was associated with a poor prognosis, with an anticipatedlong-termsurvival of <20% with chemotherapy only and ∼40% with consolidation allogeneic hematopoietic cell transplantation (allo-HCT).2,3 With the integration of imatinib into the induction and consolidation phases of ALL therapy, outcomes have significantly improved.4-8 Smaller studies employing “next-generation” TKIs (dasatinib, ponatinib, or nilotinib) have reported even better outcomes, with overall survival (OS) ranging from 46% to 86%. Multiple studies in the pre- and post-TKI era have suggested improved survival with consolidation allo-HCT in first complete remission (CR1).3-5,9,10 However, given the rarity of adult ALL, randomized data are lacking, and the current recommendations for early allo-HCT are based on single-arm observational studies or biologic randomization studies.9,11
Attaining complete molecular response (CMR) after induction has long been recognized as a powerful prognostic factor in this population.12-15 CMR rates have also improved with integration of TKIs into induction, from 38% in the pre-TKI era to 60% to 80% in the post-TKI era.1-3,16 A case series by Short et al reported that Ph+ patients with ALL who achieve a CR with CMR, defined as BCR-ABL transcript level <0.01% by quantitative polymerase chain reaction (qPCR), had long-term survival comparable to prior cohorts undergoing allo-HCT (4-year OS rate: 66%).17 This study, combined with previous studies, demonstrated favorable long-term outcomes with deep molecular response following induction therapy for Ph+ ALL.12-14 However, prior analyses have been limited by small sample size and absence of comparator cohorts.
Consequently, we undertook a multicenter, retrospective study examining outcomes in adult patients with Ph+ ALL who achieve a CMR within 90 days of diagnosis and compared outcomes in those who received allo-HCT with those who did not receive allo-HCT, with the objective of clarifying the role of allo-HCT as consolidation therapy in these patients in the modern era.
Methods
Patients
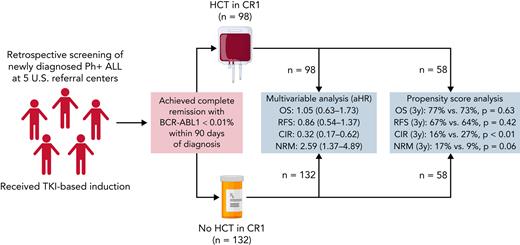
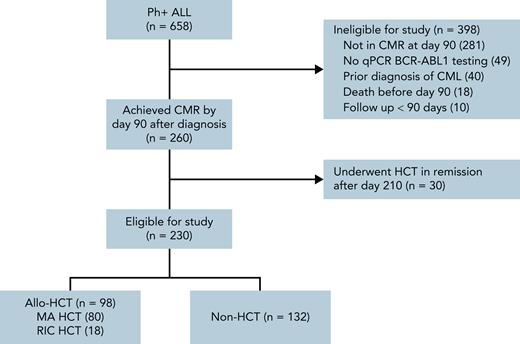
Adult (age ≥18) patients diagnosed with Ph+ ALL at participating institutions (Washington University School of Medicine, MD Anderson Cancer Center, City of Hope Cancer Center, H. Lee Moffitt Cancer Center, and Memorial Sloan Kettering Cancer Center) were retrospectively identified (Figure 1). Patients were eligible for inclusion if they were diagnosed with Ph+ ALL from May 2001 to December 2018 and achieved a complete remission with CMR within 90 days of diagnosis by qPCR and remained undetectable on subsequent evaluations up to 90 days after diagnosis. Patients undergoing allo-HCT before day 90 in CMR and patients with molecular relapse after day 90 but before allo-HCT were eligible. Day 90 was used for the landmark analysis based on prior work by Short et al.17 Patients were required to receive a TKI as part of initial therapy, but were otherwise included regardless of induction, consolidation, and maintenance regimen. Patients with a prior diagnosis of chronic myeloid leukemia (CML), early mortality (defined as death <90 days after diagnosis), or <90 days of follow-up were excluded. We designed our analysis to retrospectively simulate an “intent to transplant” after achieving CMR by day 90 and consequently only included patient transplanted within 210 days of diagnosis to allow a reasonable interval (4 months) for transplant evaluation and planning. Institutional review boards at all participating centers provided a waiver of consent for the study. Primary data analysis was performed by F.G. with assistance from A.G. and M.S. All authors had access to the full data set and reviewed the analysis.
Study screening flowchart for cohort selection. All adult (age ≥18) patients with confirmed Ph+ ALL and available clinical records at participating centers were screened for inclusion. Patients were identified via pathology reports and, where available, querying institutional leukemia- and transplant-specific databases. As described in the “Methods,” patients were excluded if they died or were lost to follow-up before day 90, had a prior diagnosis of CML, failed to achieve CR or complete molecular remission (CMR) prior to day 90 or, having achieved CR or CMR, experienced molecular or morphologic relapse prior to day 90. Patients without sufficient testing to establish CMR were also excluded. Furthermore, to improve cohort homogeneity, patients who achieved CR1/CMR prior to day 90 and underwent allo-HCT in remission after day 210 were excluded. Created with BioRender.com.
Study screening flowchart for cohort selection. All adult (age ≥18) patients with confirmed Ph+ ALL and available clinical records at participating centers were screened for inclusion. Patients were identified via pathology reports and, where available, querying institutional leukemia- and transplant-specific databases. As described in the “Methods,” patients were excluded if they died or were lost to follow-up before day 90, had a prior diagnosis of CML, failed to achieve CR or complete molecular remission (CMR) prior to day 90 or, having achieved CR or CMR, experienced molecular or morphologic relapse prior to day 90. Patients without sufficient testing to establish CMR were also excluded. Furthermore, to improve cohort homogeneity, patients who achieved CR1/CMR prior to day 90 and underwent allo-HCT in remission after day 210 were excluded. Created with BioRender.com.
Definitions
CMR was defined as BCR-ABL1 transcript <0.01% via qPCR assay in clinical use at the treating center. In contrast to the standard definition of CMR in CML, only 1 qPCR test was required to establish CMR.18 Morphologic relapse was used for primary analysis and defined as extramedullary disease and/or bone marrow blasts ≥5% unrelated to hematopoietic progenitor cell recovery. Molecular relapse was defined as a ≥1 log increase in BCR-ABL1 transcripts by qPCR. Induction regimens were classified as adolescent and young adult–inspired (intensive induction containing asparaginase), intensive (multiagent chemotherapy without asparaginase), or nonintensive (steroids and TKIs with or without blinatumomab or vincristine). Conditioning intensity was classified as previously described.19 Maintenance therapy was defined as TKI use without evidence of disease (morphologic, cytogenetic, or molecular). Acute graft-versus-host disease (aGVHD) was graded per established criteria.20 Chronic graft-versus-host disease (cGVHD) was graded according to the Seattle criteria.21 OS was defined as the time from diagnosis to death from any cause. Relapse-free survival (RFS) was defined as the time from diagnosis to relapse or death. Graft-versus-host disease (GVHD) –free relapse-free survival (GRFS) was defined as time from diagnosis to grade 3 to 4 aGVHD, cGVHD requiring systemic immunosuppressive treatment, relapse, or death from any cause.22 Although GRFS is a composite endpoint for which the non-HCT cohort was not at risk for the GVHD component endpoint, it was included as a surrogate marker of survival without evidence of disease and with good quality of life (QoL), based on the association between cGVHD and patient-reported QoL.23
Statistical analysis
The distributions of OS, RFS, and GRFS were described using Kaplan-Meier product limit methods and log-rank tests. Cumulative incidences of relapse (CIR), nonrelapse mortality (NRM), aGVHD, and cGVHD were estimated using Gray's subdistribution method for competing risks. Death without relapse was a competing risk for relapse. Relapse was a competing risk for NRM. Relapse and death without GVHD were competing risks for GVHD. Multivariate analyses (MVAs) were performed to assess the association between treatment types and outcomes using Cox proportional hazards model for OS/RFS/GRFS and using Gray's subdistribution regression for relapse/NRM, while using backward selection procedure to adjust for risk factors significant in the univariate analysis or significantly imbalanced between groups. The proportional hazards assumption was assessed graphically based on residuals out of the corresponding regression models. γ-frailty models were used to assess the impact of heterogeneity across different institutions.24 Propensity score (PS) matching was performed as a secondary analysis for graphical presentation of outcomes across 2 cohorts. Specifically, a logistic regression model was fitted to estimate the PS of receiving allo-HCT given the aforementioned imbalanced baseline characteristics. Similarity among individuals were measured by the difference in the logit of the PS, and the matched pairs with or without allo-HCT were identified using a caliper width of 0.25.25 All the analyses were performed using SAS 9.4 (SAS Institutes, Cary, NC). Statistical significance was defined as a 2-tailed P value of <.05 for all analyses.
Results
Patients
After review, 230 patients (allo-HCT: 98; non-HCT: 132) were identified and included in the study cohort (Figure 1). Clinical and demographic characteristics for the cohorts are summarized in Table 1. Median follow-up for survivors was 87.6 months (range, 17.5-206.2) and 57.8 months (range, 4.3-191.7) for allo-HCT and non-HCT cohorts, respectively. Most patients (92%) received intensive chemotherapy, principally with hyper-CVAD (hyperfractionated Cyclophosphomide, Vincristine, Doxorubicine, and Dexamethasone) -based protocols (81%). Cohorts were well matched in terms of sex, year of diagnosis, induction intensity, CNS involvement, white blood cell, and bone marrow blast count at diagnosis. The allo-HCT cohort was younger (median: 47 vs 56 years; P < .001) with better Karnofsky performance scores (KPS) at diagnosis (KPS < 90%: 15% vs 36%; P < .001). A smaller proportion of patients with allo-HCT had cytogenetic abnormalities in addition to the Ph+ (37% vs 66%; P < .001) and received ponatinib as first-line TKI (6% vs 29%; P < .001).
Patient and treatment characteristics by allogeneic hematopoietic cell transplant (allo-HCT) vs non-HCT treatment
| . | Non-HCT . | Allo-HCT . | P . |
|---|---|---|---|
| n | 132 | 98 | |
| Age, y, median (range) | 56 (19-84) | 47 (19-71) | <.001 |
| Male sex, n (%) | 66 (50) | 58 (59) | >.05 |
| Year diagnosed, n (%) | >.05 | ||
| 2001-2009 | 39 (30) | 24 (24) | |
| 2010-2018 | 93 (70) | 74 (76) | |
| ECOG performance status, n (%) | <.001 | ||
| 0 | 23 (17) | 52 (53) | |
| 1 | 88 (67) | 45 (46) | |
| ≥2 | 21 (16) | 1 (1) | |
| KPS < 90%, n (%) | 48 (36) | 15 (15) | <.001 |
| WBC at diagnosis, n (%) | >.05 | ||
| <30 000 | 72 (55) | 47 (48) | |
| 30 000-100 000 | 33 (25) | 30 (31) | |
| >100 000 | 27 (20) | 21 (21) | |
| BM blasts, median (range) | 85 (4-98) | 81 (10-90) | >.05 |
| CNS involvement, n (%) | 9 (7) | 7 (7) | >.05 |
| BCR-ABL p190 transcript, n (%) | 110 (83) | 71 (82) | >.05 |
| Other cytogenetic changes, n (%) | 87 (67) | 36 (37) | |
| Induction regimen, n (%) | >.05 | ||
| Nonintensive induction | 11 (8) | 8 (8) | |
| Steroid + TKI only | 9 (7) | 8 (8) | |
| Intensive induction | 121 (92) | 90 (92) | |
| Hyper-CVAD-based | 113 (86) | 72 (73) | |
| AYA-inspired | 4 (3) | 12 (12) | |
| Other | 4 (3) | 6 (6) | |
| First-line TKI, n (%) | <.001 | ||
| Imatinib | 32 (24) | 39 (40) | |
| Dasatinib | 62 (47) | 53 (54) | |
| Ponatinib | 38 (29) | 6 (6) | |
| Maintenance TKI, n (%) | <.001 | ||
| Imatinib | 31 (23) | 15 (15) | |
| Dasatinib | 58 (44) | 25 (26) | |
| Ponatinib | 34 (26) | 2 (2) |
| . | Non-HCT . | Allo-HCT . | P . |
|---|---|---|---|
| n | 132 | 98 | |
| Age, y, median (range) | 56 (19-84) | 47 (19-71) | <.001 |
| Male sex, n (%) | 66 (50) | 58 (59) | >.05 |
| Year diagnosed, n (%) | >.05 | ||
| 2001-2009 | 39 (30) | 24 (24) | |
| 2010-2018 | 93 (70) | 74 (76) | |
| ECOG performance status, n (%) | <.001 | ||
| 0 | 23 (17) | 52 (53) | |
| 1 | 88 (67) | 45 (46) | |
| ≥2 | 21 (16) | 1 (1) | |
| KPS < 90%, n (%) | 48 (36) | 15 (15) | <.001 |
| WBC at diagnosis, n (%) | >.05 | ||
| <30 000 | 72 (55) | 47 (48) | |
| 30 000-100 000 | 33 (25) | 30 (31) | |
| >100 000 | 27 (20) | 21 (21) | |
| BM blasts, median (range) | 85 (4-98) | 81 (10-90) | >.05 |
| CNS involvement, n (%) | 9 (7) | 7 (7) | >.05 |
| BCR-ABL p190 transcript, n (%) | 110 (83) | 71 (82) | >.05 |
| Other cytogenetic changes, n (%) | 87 (67) | 36 (37) | |
| Induction regimen, n (%) | >.05 | ||
| Nonintensive induction | 11 (8) | 8 (8) | |
| Steroid + TKI only | 9 (7) | 8 (8) | |
| Intensive induction | 121 (92) | 90 (92) | |
| Hyper-CVAD-based | 113 (86) | 72 (73) | |
| AYA-inspired | 4 (3) | 12 (12) | |
| Other | 4 (3) | 6 (6) | |
| First-line TKI, n (%) | <.001 | ||
| Imatinib | 32 (24) | 39 (40) | |
| Dasatinib | 62 (47) | 53 (54) | |
| Ponatinib | 38 (29) | 6 (6) | |
| Maintenance TKI, n (%) | <.001 | ||
| Imatinib | 31 (23) | 15 (15) | |
| Dasatinib | 58 (44) | 25 (26) | |
| Ponatinib | 34 (26) | 2 (2) |
AYA, adolescent and young adult. BM, bone marrow; CNS, central nervous system; CVAD, Cyclophosphomide, Vincristine, Doxorubicine, and Dexamethasone; ECOG, Eastern Cooperative Oncology Group; WBC, white blood cell count.
TKI therapy
In the non-HCT cohort, TKIs received during induction were dasatinib (48%), ponatinib (27%), and imatinib (24%). Following induction and consolidation, 93% received maintenance. First-line maintenance was dasatinib, ponatinib, and imatinib in 44%, 26%, and 23%, respectively. Twenty-nine patients (22%) received >1 TKI during maintenance. Duration of maintenance was <1, 1 to 3, and >3 years in 20%, 37%, and 43% of patients, respectively.
In the allo-HCT cohort, TKIs received during induction were dasatinib (55%), imatinib (39%), and ponatinib (6%). Forty-two patients (43%) received maintenance after transplant, starting at a median of 70 days after HCT (range: 23 to 433 days). First-line maintenance was dasatinib, imatinib, and ponatinib in 26%, 15%, and 2%, respectively. Thirteen patients (13%) received >1 TKI during maintenance. Duration of maintenance was <1, 1 to 3, and >3 years in 19%, 13%, and 10% of patients, respectively. Of 7 patients who relapsed after receiving maintenance, 5 patients (71%) had continued TKI until progression.
Hematopoietic cell transplantation
Demographics and treatment parameters in our allo-HCT cohort are comparable to previous reports from the transplant registries (supplemental Table 1, available on the Blood website).26,27 Ninety-four percent of patients received peripheral blood hematopoietic cell grafts, and donor types included matched sibling donor (56%), matched unrelated donor (30%), haploidentical (6%), mismatched unrelated (5%), and cord blood (2%). GVHD prophylaxis was tacrolimus based in 92% of patients. Eighty-two percent of patients received myeloablative conditioning, and 54% of patients received total body irradiation as part of their conditioning regimen. Median time from diagnosis to transplant was 142 days (range: 61-209 days). After maintaining CMR for 90 days, 80 patients (82%) had additional qPCR BCR-ABL1 testing at a median of 21 days (range: 1 to 73 days) before transplant. Six patients (8%) had detectable BCR-ABL1 at a median of 102 days (range: 91 to 156) after diagnosis and 35.5 days (range: 19 to 48) before allo-HCT. Notably, 3 patients (2%) in the non-HCT cohort also experienced molecular relapse before day 210. Forty-seven percent of patients received posttransplant TKI maintenance, most commonly dasatinib (29%) or imatinib (15%); only 1 patient received ponatinib.
Unadjusted outcome analysis
On unadjusted analysis, 5-year OS (67% vs 59%; P = .26) and RFS (62% vs 54%; P = .15) were not significantly different between the allo-HCT and non-HCT cohorts. On univariate analysis, age, KPS, and ECOG performance score were associated with OS and RFS, and use of ponatinib was associated with RFS (supplemental Tables 2 and 3). Five-year NRM was similar in both groups (22% vs 18%; P = .32). However, the CIR was worse in the non-HCT group (29% vs 16%; P = .008) (Figure 2A-D).
Outcomes by unadjusted analysis. (A) OS, (B) RFS, (C) CIR, (D) NRM, and (E) GRFS. Hazard ratios are reported without adjustment for covariates. P values are reported per the log-rank test. Red line represents cohort receiving consolidation allogeneic hematopoietic cell transplant (allo-HCT). Blue line represents non-HCT cohort.
Outcomes by unadjusted analysis. (A) OS, (B) RFS, (C) CIR, (D) NRM, and (E) GRFS. Hazard ratios are reported without adjustment for covariates. P values are reported per the log-rank test. Red line represents cohort receiving consolidation allogeneic hematopoietic cell transplant (allo-HCT). Blue line represents non-HCT cohort.
In the allo-HCT cohort, the cumulative incidence of grade II to IV and III to IV aGVHD at 90 days was 39.6% and 12.7%, respectively. The cumulative incidence of limited and extensive stage cGVHD at 5 years was 51.9% and 32.3%. GRFS at 5 years was 30% vs 54% (P < .001) in the allo-HCT and non-HCT cohorts, respectively (Figure 2E).
MVA and PS analysis
Given the significant differences in baseline characteristics, we performed a multivariable analysis (MVA) adjusting for KPS, ECOG, age, and use of ponatinib as initial TKI as described above. In this analysis, the use of allo-HCT was not associated with improved OS (adjusted hazard ratio [aHR]: 1.05; 95% CI, 0.63-1.73; P = .86) or RFS (aHR: 0.86; 95% CI, 0.54-1.37; P = .53). CIR was significantly improved by use of allo-HCT (aHR: 0.32; 95% CI, 0.17-0.62; P < .001), whereas NRM was significantly increased (aHR: 2.59; 95% CI, 1.37-4.89; P = .003). As expected, GRFS was significantly worse in the allo-HCT group (aHR: 2.27; 95% CI, 1.51-3.41; P < .001).
Given the heterogeneity of our cohort, we performed several sensitivity analyses to better characterize these results. First, we performed sensitivity analysis employing the γ-frailty model for institutional heterogeneity as described above, which did not significantly impact the analysis.24 Second, given the impact of ponatinib use on MVA and the unequal distribution of these patients between the cohorts, we recapitulated our models after excluding patients receiving ponatinib as their initial TKI, which produced similar results to those described above (supplemental Table 4). Third, although age was included in our initial models as a continuous variable, age is often treated as a categorical variable in clinical practice. Consequently, we performed our analysis excluding patients age >50 years. Interestingly, this analysis amplified the differences in NRM (aHR: 11.12; 95% CI, 1.81-69.00) and CIR (aHR: 0.17; 95% CI, 0.07-0.39) without impacting OS (aHR: 0.93; 95% CI, 0.41-2.10) (supplemental Table 4).
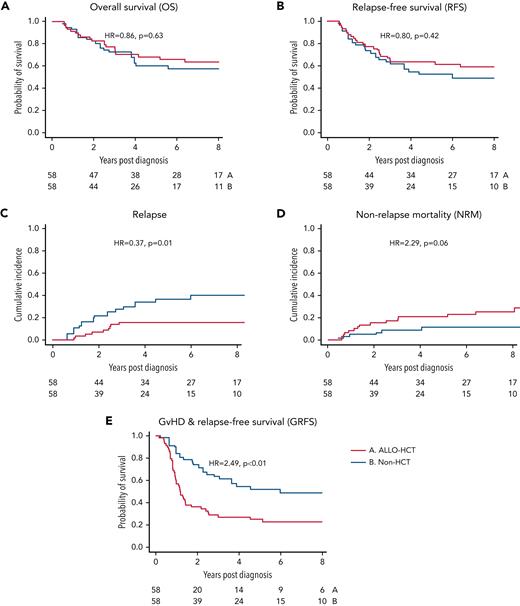
We also performed a confirmatory analysis utilizing the matched PS method as described above. Fifty-eight matched pairs were selected and were well balanced by demographics and model diagnostics (Table 2; supplemental Figures 1 and 2). PS matching analysis confirmed the results from MVA (Table 3). On this analysis, 5-year OS in the allo-HCT vs non-HCT group was 68% vs 61% (P = .63) and RFS was 63% vs 52% (P = .42) (Figure 3A-B). The CIR and NRM at 5 years were 16% vs 36% (P = .001) and 21% vs 11% (P = .06). Notably, relapse plateaued between the 3- and 5-year time points in the allo-HCT group, whereas a small number of non-HCT patients (9%) experienced late relapses. As expected, 5-year GRFS was significantly worse following allo-HCT (25% vs 52%; P < .001).
Patient characteristics in propensity matched cohorts
| . | Non-HCT . | Allo-HCT . | P . |
|---|---|---|---|
| n | 58 | 58 | |
| Age, y, median (range) | 51 (19-73) | 49.5 (19-71) | >.05 |
| Male sex, n (%) | 30 (52) | 31 (53) | >.05 |
| Year diagnosed, n (%) | >.05 | ||
| 2001-2009 | 18 (31) | 17 (29) | |
| 2010-2018 | 40 (69) | 41 (71) | |
| ECOG performance status, n (%) | >.05 | ||
| 0 | 15 (26) | 20 (34) | |
| 1 | 39 (67) | 37 (64) | |
| ≥2 | 4 (7) | 1 (2) | |
| KPS < 90%, n (%) | 13 (22) | 12 (21) | >.05 |
| WBC at diagnosis, n (%) | >.05 | ||
| <30 000 | 36 (62) | 26 (45) | |
| 30 000-100 000 | 13 (22) | 19 (33) | |
| >100 000 | 9 (16) | 13 (22) | |
| BM blasts, median (range) | 85 (4-98) | 82 (30-98) | >.05 |
| CNS involvement, n (%) | 4 (7) | 7 (12) | >.05 |
| BCR-ABL p190 transcript, n (%) | 48 (83) | 39 (81) | >.05 |
| Other cytogenetic changes, n (%) | 26 (45) | 27 (47) | |
| First-line TKI, n (%) | >.05 | ||
| Imatinib | 16 (28) | 25 (43) | |
| Dasatinib | 37 (64) | 27 (47) | |
| Ponatinib | 5 (9) | 6 (10) |
| . | Non-HCT . | Allo-HCT . | P . |
|---|---|---|---|
| n | 58 | 58 | |
| Age, y, median (range) | 51 (19-73) | 49.5 (19-71) | >.05 |
| Male sex, n (%) | 30 (52) | 31 (53) | >.05 |
| Year diagnosed, n (%) | >.05 | ||
| 2001-2009 | 18 (31) | 17 (29) | |
| 2010-2018 | 40 (69) | 41 (71) | |
| ECOG performance status, n (%) | >.05 | ||
| 0 | 15 (26) | 20 (34) | |
| 1 | 39 (67) | 37 (64) | |
| ≥2 | 4 (7) | 1 (2) | |
| KPS < 90%, n (%) | 13 (22) | 12 (21) | >.05 |
| WBC at diagnosis, n (%) | >.05 | ||
| <30 000 | 36 (62) | 26 (45) | |
| 30 000-100 000 | 13 (22) | 19 (33) | |
| >100 000 | 9 (16) | 13 (22) | |
| BM blasts, median (range) | 85 (4-98) | 82 (30-98) | >.05 |
| CNS involvement, n (%) | 4 (7) | 7 (12) | >.05 |
| BCR-ABL p190 transcript, n (%) | 48 (83) | 39 (81) | >.05 |
| Other cytogenetic changes, n (%) | 26 (45) | 27 (47) | |
| First-line TKI, n (%) | >.05 | ||
| Imatinib | 16 (28) | 25 (43) | |
| Dasatinib | 37 (64) | 27 (47) | |
| Ponatinib | 5 (9) | 6 (10) |
Clinical outcome by cohort
| . | Unadjusted analysis . | P . | |||||
|---|---|---|---|---|---|---|---|
| Allogeneic HCT, % (n = 98) . | Non-HCT, % (n = 132) . | ||||||
| 1 y . | 3 y . | 5 y . | 1 y . | 3 y . | 5 y . | ||
| OS | 90 | 76 | 67 | 92 | 72 | 59 | .26 |
| RFS | 88 | 70 | 62 | 85 | 65 | 54 | .15 |
| CIR | 2 | 13 | 16 | 8 | 23 | 29 | .01 |
| NRM | 10 | 17 | 22 | 7 | 13 | 18 | .32 |
| GRFS | 59 | 33 | 30 | 85 | 65 | 54 | <.0001 |
| . | Unadjusted analysis . | P . | |||||
|---|---|---|---|---|---|---|---|
| Allogeneic HCT, % (n = 98) . | Non-HCT, % (n = 132) . | ||||||
| 1 y . | 3 y . | 5 y . | 1 y . | 3 y . | 5 y . | ||
| OS | 90 | 76 | 67 | 92 | 72 | 59 | .26 |
| RFS | 88 | 70 | 62 | 85 | 65 | 54 | .15 |
| CIR | 2 | 13 | 16 | 8 | 23 | 29 | .01 |
| NRM | 10 | 17 | 22 | 7 | 13 | 18 | .32 |
| GRFS | 59 | 33 | 30 | 85 | 65 | 54 | <.0001 |
| . | PS matched analysis . | P . | |||||
|---|---|---|---|---|---|---|---|
| Allogeneic HCT, % (n = 58) . | Non-HCT, % (n = 58) . | ||||||
| 1 y . | 3 y . | 5 y . | 1 y . | 3 y . | 5 y . | ||
| OS | 91 | 77 | 68 | 93 | 73 | 61 | .63 |
| RFS | 88 | 67 | 63 | 84 | 64 | 52 | .42 |
| CIR | 3 | 16 | 16 | 11 | 27 | 36 | .001 |
| NRM | 9 | 17 | 21 | 5 | 9 | 11 | .06 |
| GRFS | 57 | 29 | 25 | 84 | 64 | 52 | .0001 |
| . | PS matched analysis . | P . | |||||
|---|---|---|---|---|---|---|---|
| Allogeneic HCT, % (n = 58) . | Non-HCT, % (n = 58) . | ||||||
| 1 y . | 3 y . | 5 y . | 1 y . | 3 y . | 5 y . | ||
| OS | 91 | 77 | 68 | 93 | 73 | 61 | .63 |
| RFS | 88 | 67 | 63 | 84 | 64 | 52 | .42 |
| CIR | 3 | 16 | 16 | 11 | 27 | 36 | .001 |
| NRM | 9 | 17 | 21 | 5 | 9 | 11 | .06 |
| GRFS | 57 | 29 | 25 | 84 | 64 | 52 | .0001 |
Includes 1-, 3-, and 5-y OS, RFS, CIR, NRM, and GRFS.
Outcomes by propensity matched analysis. Analysis of key endpoints following PS matching procedure in 58 matched pairs. (A) OS, (B) RFS, (C) CIR, (D) NRM, and (E) GRFS. P values are reported per the log-rank test. Red line represents cohort receiving consolidation allogeneic hematopoietic cell transplant (allo-HCT). Blue line represents non-HCT cohort.
Outcomes by propensity matched analysis. Analysis of key endpoints following PS matching procedure in 58 matched pairs. (A) OS, (B) RFS, (C) CIR, (D) NRM, and (E) GRFS. P values are reported per the log-rank test. Red line represents cohort receiving consolidation allogeneic hematopoietic cell transplant (allo-HCT). Blue line represents non-HCT cohort.
Impact of conditioning intensity
Given the excess NRM observed in the allo-HCT cohort, we hypothesized that reduced intensity conditioning (RIC) might provide a beneficial graft-versus-leukemia effect, lowering relapse while minimizing TRM. Consequently, we performed a subsequent analysis dividing the cohort into 3 groups: myeloablative HCT, RIC HCT, and non-HCT. Baseline characteristics are included in supplemental Table 5. We used the non-HCT cohort as the comparison group in our MVA, adjusting for the same factors (ECOG, KPS, age, ponatinib use) as above.
Contrary to our hypothesis, compared with the non-HCT cohort, RIC HCT was not associated with improved OS (aHR: 1.21; 95% CI, 0.55-2.65) or RFS (aHR: 1.22; 95% CI, 0.60-2.47). There was no difference in CIR (aHR: 0.70; 95% CI, 0.27-1.81) or NRM (aHR: 2.35; 95% CI, 0.81-6.79), although GRFS was significantly worse (aHR: 2.41; 95% CI, 1.25-4.62). Notably, analyzing the remaining 80 patients undergoing myeloablative allo-HCT separately yielded results similar to the previous analysis; myeloablative allo-HCT was associated with improved CIR (aHR: 0.25; P < .001), higher NRM (aHR: 2.67; P < .01), similar OS (aHR: 1.00; P = .99) and RFS (aHR: 0.77; P = .31), and inferior GRFS (aHR: 2.24; P < .001) (supplemental Figure 1A-E).
We also performed subset analysis of the 18 patients in the RIC allo-HCT cohort, using up to 3 PS-matched non-HCT patients as controls. Forty-three controls were identified using PS matching methods identical to those described above. No significant difference was observed between the 2 cohorts in regard to OS, PFS, TRM, or CIR (supplemental Figure 2).
Subsequent therapies
Of the 38 non-HCT patients who relapsed, 13 (34%) underwent subsequent allo-HCT. Other salvage therapies included additional cytotoxic chemotherapy (n = 24), inotuzumab ozogamicin (8), blinatumomab (7), and chimeric antigen receptor (CAR) T cells (1). In the 15 allo-HCT patients who relapsed, non-TKI therapy included CAR T cells (n = 5), blinatumomab (5), inotuzumab ozogamicin (3), and additional cytotoxic chemotherapy (2). None underwent a second allo-HCT. We did not observe any difference in OS following relapse between the 2 cohorts (supplemental Figure 3).
Discussion
Using a large, multicenter, retrospective cohort of adult patients with Ph+ ALL, our analysis demonstrates that, compared with non-HCT approaches with modern induction regimens and TKIs, allo-HCT in CR1 for patients achieving CMR within 90 days of diagnosis is not associated with an improvement in OS or RFS. This result persisted after adjustment for imbalances in baseline risk factors using traditional MVA and on PS matching. This difference was driven by an excess of NRM in the allo-HCT cohort, which was balanced by a decrease in the incidence of relapse. The use of allo-HCT was also associated with inferior GRFS, which could be interpreted as a surrogate marker for survival with a good QoL. A subset analysis of patients with RIC HCT showed similar OS, RFS, NRM, and CIR compared with non-HCT treatment.
Prior studies performed in unselected populations of adults with Ph+ ALL have consistently reported an OS benefit for allo-HCT in CR1. In the pre-TKI era LALA-94 study, patients achieving CR were “biologically randomized” based on availability of HLA-matched donors, with the no-donor group receiving chemotherapy with or without autologous HCT. Allo-HCT was performed in 93% of the “donor” group and was associated with a significantly improved 3-year OS (37% vs 12%; P = .02).2 The pre-TKI cohort of UKALLXII/ECOG E2993 employed a similar biologic randomization design and showed superior 5-year OS with transplant using matched sibling donor (44%) or matched unrelated donor (35%) vs chemotherapy alone (19%) (P = .001).3 A continuation of UKALLXII/ECOG E2993 into the TKI era showed the use of imatinib during induction or consolidation improved the proportion of allo-HCT in eligible patients from 31% to 46% while maintaining the benefit for allo-HCT vs chemotherapy (4-year OS: 50% vs 19%).4 A prospective study (GRAALL-2005) reported by Chalandon and colleagues showed similar results, with imatinib-based induction allowing 63% of eligible patients to receive allo-HCT. Receipt of allo-HCT was associated with an RFS (hazard ratio: 0.69; 95% CI, 0.49-0.98; P = .04) and OS benefit (hazard ratio: 0.65; 95% CI, 0.44-0.93; P = .02).28 Notably, the benefit of transplant was observed for patients in deep remission but not CMR.28 Other small prospective studies using imatinib have also suggested a benefit for allo-HCT in CR1 in unselected populations of Ph+ ALL.7,10,29 Notably, data with non-imatinib TKIs have been mixed, with 1 small study of dasatinib demonstrating improved OS with allo-HCT and 2 others using ponatinib and nilotinib finding no difference.14,16,30 Based on the above evidence, allo-HCT in CR1 for fit patients with an available donor remains the recommendation from the American Society of Transplant and Cellular Therapy.9,11
Notably, transplant eligibility in the above protocols only required achievement of CR, and none employed highly sensitive molecular techniques to guide therapy. Early data regarding the utility of deep molecular response following induction therapy for Ph+ ALL were mixed, employing variable definitions and techniques.31,32 With improved standardization, deeper molecular responses have increasingly been recognized as a useful prognostic factor.12-14,17 Multiple groups reported deep and maintained molecular response (defined as CMR or better) to TKI-based induction therapy is associated with long-term RFS after allo-HCT of >80%.12-14 However, benefit is not limited to patients undergoing allo-HCT. A recent analysis by Short et al in a cohort of 85 adult patients with Ph+ ALL who achieved a CR after chemotherapy followed by maintenance TKI without allo-HCT found that patients reaching CMR at 90 days was independently associated with improved OS compared with less deep responses.17 Four-year OS was 66% in this cohort, comparable with prior cohorts undergoing allo-HCT. They suggest that this may represent a subset of patients with different disease biology in whom allo-HCT in CR1 can be safely avoided, a conclusion supported by this analysis.
One major factor which may explain the difference between this and prior studies is the use of second- and third-generation TKIs. In this study, only the minority of patients received imatinib for induction in the allo-HCT (39%) and non-HCT (24%) cohorts. Imatinib in combination with intensive chemotherapy has historically been associated with a CMR rate of ∼50%, while significantly higher rates have been reported in small prospective studies of dasatinib (60%), ponatinib (83%), and nilotinib (87%).1,14,16,30,33 One randomized trial in children demonstrated improved OS with dasatinib vs imatinib in this setting, although generalizability to adults is unclear.34 Although we adjusted for TKI use, our study may suggest that next-generation TKIs may provide better up-front disease control, obviating consolidative allo-HCT. The depth of response needed to achieve this effect remains unclear. For example, 1 previous report found achieving MR5 (BCR-ABL1 < 0.001%) following nilotinib-based induction and maintenance without allo-HCT was associated with a disease-free survival of 64% at 2 years, compared with 0% for patients with <MR5.14
Another potential factor is the advances in the treatment of relapsed Ph+ ALL in the last 2 decades. Multiple TKIs are now available, and switching agents at relapse is effective in some patients.35,36 An analysis of adult Ph+ ALL in first relapse identified availability of later generation TKIs as associated with improved survival.37 RIC and alternate donor transplant have increased access to allo-HCT at relapse, although the role of allo-HCT in CR2 remains poorly defined.37 Notably, several novel agents for relapsed Ph+ ALL, including bispecific antibody (blinatumomab), antibody-drug conjugates (inotuzumab ozogamicin), and CAR T cells, were either approved near the end or after our study period.38-43 The availability of these salvage strategies may influence treatment decisions for patients who achieve deep molecular remissions in CR1. However, it should be noted that outcomes in the allo-HCT cohort reflect practice during our study period (2001-2018). Advances in graft manipulation, peritransplant supportive care, and GVHD prevention and treatment may reduce NRM and lead to a survival advantage for allo-HCT in CR1, regardless of remission depth. Consequently, frequent reevaluation of the role of allo-HCT in this rapidly evolving field is warranted.
Our study has several significant limitations. In the overall cohort, 92% of patients received intensive induction, followed by high-intensity consolidation in the non-HCT arm. Data from the GRAAPH 2014 study suggest long-term survival following reduced intensity chemotherapy without allo-HCT in this population is rare (<20%).44 Even less data are available for novel “chemotherapy-free” regimens combining blinatumomab, TKIs, and steroids.38,43 Consequently, generalizing our findings to patients undergoing low-intensity induction is unwarranted. Furthermore, the retrospective nature of this analysis has inherent limitations. Specifically, confounding by indication is a significant concern for retrospective analyses of transplant vs nontransplant approaches. In our study, the allo-HCT cohort was younger and had better performance status, which may bias outcomes in favor of transplant. Alternatively, it is possible that physicians preferentially recommend allo-HCT for high-risk disease. Although we did not observe imbalance in available baseline disease-specific factors, the possibility of unmeasured confounding factors remains. We took several precautions in our analysis to minimize this risk. First, by only including patients who achieved CMR by day 90, we produced a relatively homogenous cohort. Second, by only including patients who underwent allo-HCT within 210 days following diagnosis, we attempted to recapitulate a “best-case scenario” for planned transplant in CR1 and remove patients who went to transplant in CR1 owing to development of concerning clinical features. Third, we performed multiple types of analyses (including MVA and matched PS analysis) to adjust for measured confounders.
In summary, our analysis suggests that allo-HCT in CR1 may not be required in adult patients with Ph+ ALL who achieve CMR (defined as BCR-ABL1 transcript level <0.01%) within 90 days of diagnosis. Notably, our analysis does not demonstrate a survival benefit with transplant regardless of conditioning intensity. Lower relapse rate in myeloablative allo-HCT failed to improve RFS owing to higher NRM. In addition, RIC allo-HCT did not decrease relapse compared with non-HCT approaches. For some patients, the reduction in relapse (and potentially shorter duration of TKI therapy) may make transplant an attractive option. However, this benefit must be balanced against the increased risk of NRM and late effects that may impact QoL as represented by decreased GRFS.22 Early referral to a high-volume transplant center to evaluate transplant eligibility, identify potential allogeneic donors, and discuss the risks and benefits of allogeneic transplant as a therapeutic option remains an essential component of management in Ph+ ALL. If allo-HCT is deferred, patients should be monitored closely by qPCR for molecular relapse to facilitate early intervention and, potentially, salvage allo-HCT. However, this study provides reasonable evidence that allo-HCT may be deferred in patients with Ph+ ALL who achieve specifically a complete molecular response within 90 days of diagnosis. Whether patients meeting these criteria can be selected for transplant vs nontransplant approaches based on modern risk stratifications tools, such as copy number alteration profiling, mutation analysis with next-generation sequencing, and/or persistent abnormalities on next-generation flow, is an area of clinical interest and future research. Prospective, randomized trials are required to fully assess the role of allo-HCT in this population and may clarify a number of questions raised by this analysis.
Acknowledgments
This work was supported by the National Center for Advancing Translational Sciences of the National Institutes of Health, under Award Number TL1TR002344 (M.S.) and Award Number R25 CA190190 (M.S.).
No individual not listed as an author was involved in the writing, editing, or analysis of this manuscript.
Authorship
Contribution: A.G., P.K., and F.R. designed the study; M.S., A.G., and F.G. reviewed the data; F.G. performed the statistical analysis; A.G. and M.S. wrote the manuscript. A.G., M.S., H.K., J.A., I.A., K.A.M., E.J., R.F., B.S., F.L., W.F., J.H.P., N.J.S., F.G., G.L.U., P.W., J.F.D., R.E.C., M.M.A., F.R., and P.K. contributed data and critically reviewed and revised the design and manuscript and approved the final version. All authors agree to be accountable for the accuracy and integrity of this work.
N.S. reports receiving consulting fees from AstraZeneca, NGMBio, and Jazz Pharmaceuticals; research funding from Takeda Oncology, Astellas Pharma Inc, and Stemline Therapeutics, and honoraria from Amgen and Novartis. E.J. reports research grants and consultancy fees from Abbvie, Amgen, Takeda, Pfizer, BMS, Novartis, Genentech, and Adaptive Biotechnologies. R.F. reports scientific advising to Allogene, Amgen, Bluebird Bio, BMS/Celgene, Calibr, Cellular Biomedicine Group, GammaDelta Therapeutics, Iovance, Kite Pharma, Janssen, Legend Biotech, Novartis, Sana, Takeda, Wugen, and Umoja; research funding from Kite Pharma (Institutional), Allogene (Institutional), Novartis (Institutional), BlueBird Bio (Institutional), BMS (Institutional), National Cancer Institute, and the Leukemia and Lymphoma Society; consultancy fees from Cowen, EcoR1, Emerging Therapy Solutions, and the Gerson Lehrman Group (GLG); and educational/editorial activity for Aptitude Health, ASH, BioPharma Communications CARE Education, Clinical Care Options Oncology, Imedex, and the Society for Immunotherapy of Cancer. B.S. reports consultancy fees from Amgen, Pfizer, Novartis, BMS/Celgene/Juno, Kite/Gilead, Precision Biosciences, Jazz, Acrotech, Beigene, Pharmacyclics, Adaptive, Century Therapeutics, Autolus; research funding from Kite/Gilead, Jazz, Servier; and serves on the steering committee for PeproMene Bio. G.U. reports consultancy fees from Novartis, Abbvie, Agios, GSK, Jazz, and Genentech. F.R. reports research funding from Novartis and Kite/Gilead. P.W. reports serving on an advisory board for Pfizer. H.K. reports research funding from AbbVie, Amgen, Ascentage, BMS, Daiichi-Sankyo, Immunogen, Jazz, and Novartis and has consulted, served on an advisory board, or received honoraria from AbbVie, Amgen, Ascentage, Astellas, Astrazeneca, Biologix, Curis, Ipsen Biopharmaceuticals, KAHR Medical, Novartis, Pfizer, Precision Biosciences, Shenzhen Target Rx, and Taiho Pharma Canada. The remaining authors declare no competing financial interests.
Correspondence: Armin Ghobadi, Center for Gene and Cellular Immunotherapy (CGCI), Section of Stem Cell Transplant and Leukemia, Division of Medical Oncology, Washington University School of Medicine, 660 S. Euclid Ave, Campus Box 8007-29, St. Louis, MO 63110; e-mail: arminghobadi@wustl.edu.
References
Author notes
∗F.R. and P.K. are joint senior authors.
Requests for deidentified patient data will be evaluated by the corresponding author on a case-by-case basis.
The online version of this article contains a data supplement.
There is a Blood Commentary on this article in this issue.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.


This feature is available to Subscribers Only
Sign In or Create an Account Close Modal