TO THE EDITOR:
The severity of COVID-19 disease, caused by severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2), is highly variable ranging from asymptomatic to a self-limited flulike illness to severe respiratory failure, often accompanied by cardiovascular events, coagulopathy, thrombosis, and high mortality.1-3 Although several risk factors for severe disease have been identified, including age, sex, ethnicity, genetic variation, and a range of comorbidities, these only partially predict disease severity and additional determinants remain to be identified.2-6
Clonal hematopoiesis (CH) describes the disproportionate expansion of a hematopoietic stem cell and its progeny, in association with leukemia-associated somatic mutations, most commonly affecting the genes for epigenetic regulators DNMT3A, TET2, and ASXL1.7,8 The prevalence and size of such clones rise with age, in association with changes in the driver gene landscape.9 CH is associated with an increased risk of hematologic malignancies, but also of cardiovascular disease (CVD), independently of other known CVD risk factors.10,11 The basis for this increased CVD risk has been linked to hyperinflammatory positive feedback loops driven by increased cytokine release from clonal myeloid cells, particularly interleukin-6 and interleukin-1β.12-16
The close association of CH with advancing age and chronic inflammation led us to hypothesize that it may be another factor associated with increased risk of severe COVID-19 disease, through hyperactivation of abnormal, clonally derived, myeloid cells, including monocytes and macrophages, following SARS-CoV-2 infection.
To investigate a possible association between CH and COVID-19 disease severity, we studied 568 patients aged 50 to 90 years old (median age, 64 years), including 120 nonhospitalized individuals with asymptomatic or mild disease, 241 hospitalized patients not requiring intensive care unit (ICU) support, and 207 critically ill patients who required ICU admission, or mechanical ventilation or went on to die (Table 1). All patients had laboratory-confirmed SARS-CoV-2 infection during the first 6 months of 2020. All participants provided written informed consent as part of ethics committee-approved studies (supplemental Note on the Blood Web site).
COVID-19 patients characteristics
| . | Nonhospitalized . | Hospitalized . | ICU . | Total . |
|---|---|---|---|---|
| Total N | 120 | 241 | 207 | 568 |
| Age, y | ||||
| 50-59 | 6 (5%) | 90 (37%) | 61 (29%) | 157 (28%) |
| 60-69 | 104 (86%) | 50 (21%) | 77 (37%) | 231 (41%) |
| 70-79 | 8 (7%) | 67 (28%) | 53 (26%) | 128 (23%) |
| 80+ | 2 (2%) | 34 (14%) | 16 (8%) | 52 (9%) |
| Sex | ||||
| Female | 71 (59%) | 107 (45%) | 48 (23%) | 226 (40%) |
| Male | 49 (41%) | 134 (55%) | 159 (77%) | 342 (60%) |
| Ethnicity | ||||
| White | 114 (95%) | 196 (81%) | 143 (69%) | 453 (80%) |
| Non-White | 6 (5%) | 27 (11%) | 35 (17%) | 68 (12%) |
| Other | 0 (0%) | 8 (3%) | 21 (10%) | 29 (5%) |
| NA | 0 (0%) | 10 (4%) | 8 (4%) | 18 (3%) |
| Smoking | ||||
| Never | 40 (33%) | 134 (56%) | 107 (52%) | 281 (49%) |
| Former | 26 (22%) | 69 (29%) | 62 (30%) | 157 (28%) |
| Current | 0 (0%) | 8 (3%) | 7 (3%) | 15 (3%) |
| Missing | 54 (45%) | 30 (12%) | 31 (15%) | 115 (20%) |
| Hypertension | ||||
| No | 38 (32%) | 24 (10%) | 21 (10%) | 83 (15%) |
| Yes | 31 (26%) | 24 (10%) | 33 (16%) | 88 (15%) |
| Missing | 51 (41%) | 193 (80%) | 153 (74%) | 397 (70%) |
| CVD | ||||
| No | 61 (51%) | 172 (71%) | 154 (74%) | 387 (68%) |
| Yes | 8 (6%) | 64 (27%) | 47 (23%) | 119 (21%) |
| Missing | 51 (43%) | 5 (2%) | 6 (3%) | 62 (11%) |
| COPD/asthma | ||||
| No | 56 (47%) | 194 (80%) | 174 (84%) | 424 (75%) |
| Yes | 14 (12%) | 41 (17%) | 27 (13%) | 82 (14%) |
| Missing | 50 (42%) | 6 (3%) | 6 (3%) | 62 (11%) |
| Diabetes | ||||
| No | 56 (47%) | 176 (73%) | 138 (67%) | 370 (65%) |
| Yes | 9 (7%) | 59 (24%) | 62 (30%) | 130 (23%) |
| Missing | 55 (46%) | 6 (3%) | 7 (3%) | 68 (12%) |
| Cancer (neoplasm and hematological) | ||||
| No | 66 (55%) | 208 (86%) | 190 (92%) | 464 (82%) |
| Yes | 4 (3%) | 22 (9%) | 11 (6%) | 38 (7%) |
| Missing | 50 (42%) | 11 (5%) | 5 (2%) | 66 (12%) |
| Immunodeficiency | ||||
| No | 71 (59%) | 204 (85%) | 181 (88%) | 456 (80%) |
| Yes | 0 | 30 (12%) | 17 (8%) | 47 (8%) |
| Missing | 49 (41%) | 7 (3%) | 9 (4%) | 65 (11%) |
| . | Nonhospitalized . | Hospitalized . | ICU . | Total . |
|---|---|---|---|---|
| Total N | 120 | 241 | 207 | 568 |
| Age, y | ||||
| 50-59 | 6 (5%) | 90 (37%) | 61 (29%) | 157 (28%) |
| 60-69 | 104 (86%) | 50 (21%) | 77 (37%) | 231 (41%) |
| 70-79 | 8 (7%) | 67 (28%) | 53 (26%) | 128 (23%) |
| 80+ | 2 (2%) | 34 (14%) | 16 (8%) | 52 (9%) |
| Sex | ||||
| Female | 71 (59%) | 107 (45%) | 48 (23%) | 226 (40%) |
| Male | 49 (41%) | 134 (55%) | 159 (77%) | 342 (60%) |
| Ethnicity | ||||
| White | 114 (95%) | 196 (81%) | 143 (69%) | 453 (80%) |
| Non-White | 6 (5%) | 27 (11%) | 35 (17%) | 68 (12%) |
| Other | 0 (0%) | 8 (3%) | 21 (10%) | 29 (5%) |
| NA | 0 (0%) | 10 (4%) | 8 (4%) | 18 (3%) |
| Smoking | ||||
| Never | 40 (33%) | 134 (56%) | 107 (52%) | 281 (49%) |
| Former | 26 (22%) | 69 (29%) | 62 (30%) | 157 (28%) |
| Current | 0 (0%) | 8 (3%) | 7 (3%) | 15 (3%) |
| Missing | 54 (45%) | 30 (12%) | 31 (15%) | 115 (20%) |
| Hypertension | ||||
| No | 38 (32%) | 24 (10%) | 21 (10%) | 83 (15%) |
| Yes | 31 (26%) | 24 (10%) | 33 (16%) | 88 (15%) |
| Missing | 51 (41%) | 193 (80%) | 153 (74%) | 397 (70%) |
| CVD | ||||
| No | 61 (51%) | 172 (71%) | 154 (74%) | 387 (68%) |
| Yes | 8 (6%) | 64 (27%) | 47 (23%) | 119 (21%) |
| Missing | 51 (43%) | 5 (2%) | 6 (3%) | 62 (11%) |
| COPD/asthma | ||||
| No | 56 (47%) | 194 (80%) | 174 (84%) | 424 (75%) |
| Yes | 14 (12%) | 41 (17%) | 27 (13%) | 82 (14%) |
| Missing | 50 (42%) | 6 (3%) | 6 (3%) | 62 (11%) |
| Diabetes | ||||
| No | 56 (47%) | 176 (73%) | 138 (67%) | 370 (65%) |
| Yes | 9 (7%) | 59 (24%) | 62 (30%) | 130 (23%) |
| Missing | 55 (46%) | 6 (3%) | 7 (3%) | 68 (12%) |
| Cancer (neoplasm and hematological) | ||||
| No | 66 (55%) | 208 (86%) | 190 (92%) | 464 (82%) |
| Yes | 4 (3%) | 22 (9%) | 11 (6%) | 38 (7%) |
| Missing | 50 (42%) | 11 (5%) | 5 (2%) | 66 (12%) |
| Immunodeficiency | ||||
| No | 71 (59%) | 204 (85%) | 181 (88%) | 456 (80%) |
| Yes | 0 | 30 (12%) | 17 (8%) | 47 (8%) |
| Missing | 49 (41%) | 7 (3%) | 9 (4%) | 65 (11%) |
COPD, chronic obstructive pulmonary disease; NA, not available.
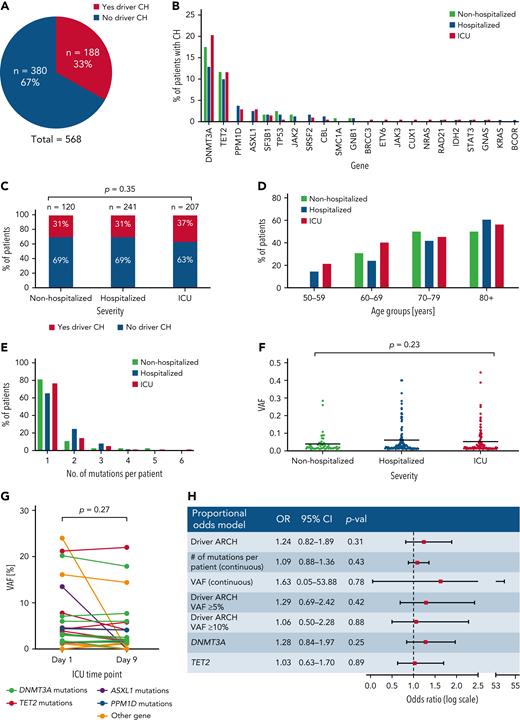
To identify individuals with CH, we performed error-corrected targeted sequencing of blood DNA for 56 genes implicated in CH using a custom set of RNA baits (Twist Bioscience design ID TE-99420296; supplemental Table 1). Sequences were mapped to human reference genome GRCh38 and CH somatic driver mutations with a variant allele fraction (VAF) of 1% to 40% were identified using Shearwater (SNV)17 and Mutect2 (Indels).18 With median sequencing coverage of 2000×, we detected 266 CH driver mutations within 22 genes (supplemental Tables 2 and 3), with 188/568 (33%) patients having at least 1 mutation (Figure 1A). DNMT3A and TET2 mutations were most common in all 3 groups, with no significant enrichment for particular genes in any group (Figure 1B).
Investigation of the impact of clonal hematopoiesis on COVID-19 disease severity. (A) Proportion of patients carrying at least 1 driver CH mutation in all 3 COVID-19 patient cohorts combined. (B) Distribution of CH driver mutations by gene in nonhospitalized, hospitalized, and ICU patients. (C) Proportion of patients carrying at least 1 driver mutation in nonhospitalized, hospitalized, and ICU COVID-19 patients. P value was calculated using χ2 test. (D) Proportion of patients at least 1 CH driver mutation in nonhospitalized, hospitalized, and ICU COVID-19 patients across different age groups. (E) Number of CH driver mutations per patient in nonhospitalized, hospitalized, and ICU COVID-19 patients. (F) VAF distribution of CH driver mutations in nonhospitalized, hospitalized, and ICU COVID-19 patients. P value is calculated using 1-way analysis of variance. (F) Driver CH mutation gene distribution in nonhospitalized, hospitalized, and ICU COVID-19 patients. (G) VAF of driver CH mutations on day 1 and day 9 of ICU admission in individual patients. P value is calculated using t test. (H) Multivariate proportional odds model shows that the presence of CH, number of mutations per patient, VAF, CH mutation of ≥ 5% VAF, CH mutation of ≥ 10% VAF, DNMT3A mutation, and TET2 mutation are not associated with an increased risk of COVID-19 hospitalization and ICU admission. Age, sex, ethnicity, diabetes, chronic obstructive pulmonary disease/asthma, CVD, cancer/neoplasm, immunodeficiency, and smoking status were adjusted for.
Investigation of the impact of clonal hematopoiesis on COVID-19 disease severity. (A) Proportion of patients carrying at least 1 driver CH mutation in all 3 COVID-19 patient cohorts combined. (B) Distribution of CH driver mutations by gene in nonhospitalized, hospitalized, and ICU patients. (C) Proportion of patients carrying at least 1 driver mutation in nonhospitalized, hospitalized, and ICU COVID-19 patients. P value was calculated using χ2 test. (D) Proportion of patients at least 1 CH driver mutation in nonhospitalized, hospitalized, and ICU COVID-19 patients across different age groups. (E) Number of CH driver mutations per patient in nonhospitalized, hospitalized, and ICU COVID-19 patients. (F) VAF distribution of CH driver mutations in nonhospitalized, hospitalized, and ICU COVID-19 patients. P value is calculated using 1-way analysis of variance. (F) Driver CH mutation gene distribution in nonhospitalized, hospitalized, and ICU COVID-19 patients. (G) VAF of driver CH mutations on day 1 and day 9 of ICU admission in individual patients. P value is calculated using t test. (H) Multivariate proportional odds model shows that the presence of CH, number of mutations per patient, VAF, CH mutation of ≥ 5% VAF, CH mutation of ≥ 10% VAF, DNMT3A mutation, and TET2 mutation are not associated with an increased risk of COVID-19 hospitalization and ICU admission. Age, sex, ethnicity, diabetes, chronic obstructive pulmonary disease/asthma, CVD, cancer/neoplasm, immunodeficiency, and smoking status were adjusted for.
CH mutations in at least 1 gene were identified in 37 (31%) nonhospitalized, 74 (31%) hospitalized, and 77 (37%) critically ill patients (Figure 1C). There was no significant difference in the prevalence of CH between groups (P = .35, χ2 test). We next examined CH prevalence by age and found that, although this increased with advancing age in all 3 groups, the groups did not differ significantly when comparing individuals in the same age ranges (Figure 1D). In addition, with most CH carriers harboring 1 or 2 mutations, there were no differences between the 3 groups with regard to the mean number of mutations per patient (P = .80, 1-way analysis of variance test; Figure 1E) or the average clone size as measured by VAF (P = .23, 1-way analysis of variance test; Figure 1F). To investigate whether mutation-bearing myeloid cells were preferentially mobilized or expanded during the acute clinical course of COVID-19, we studied available paired samples, taken 8 days apart, from 54 critically ill patients. CH was identified in 16 (32%). Comparison of VAFs between day 1 and day 9 samples did not differ significantly (P = .27, pairwise t test) (Figure 1G), indicating that there was no preferential expansion of myeloid progeny arising from the CH clone.
To take account for covariates previously implicated in COVID-19 disease severity including age, sex, ethnicity, diabetes, chronic obstructive pulmonary disease/asthma, CVD, cancer/neoplasm, immunodeficiencies, and smoking status, we applied a multivariable proportional odds model to retest for a possible association between COVID-19 disease severity and CH (supplemental Table 4). We found that male sex (adjusted odds ratio [OR] = 2.84; 95% confidence interval [CI], 1.91-4.24; P < .01), diabetes (adjusted OR = 1.56; 95% CI, 1.05-2.44; P = .044), cardiovascular disease (adjusted OR = 1.64; 95% CI, 1.01-2.44; P = .046), and immunodeficiency (adjusted OR = 2.10; 95% CI, 1.11-4.07; P = .024) were all significantly associated with COVID-19 hospitalization and ICU admission, consistent with previous findings.5 However, even after adjusting for these factors, the presence of CH was not associated with an increased risk of severe COVID-19 (OR = 1.24; 95% CI, 0.82-1.89; P = .31; Figure 1H). Similarly, neither were the number of mutations per patient (adjusted OR = 1.09; 95% CI, 0.88-1.36; P = .43) nor the CH clone size (adjusted OR = 1.63; 95% CI, 0.05-53.88; P = .78) (Figure 1H).
Given the reported links between TET2 and DNMT3A mutations and hyperinflammation or response to infection,15,16,19 these 2 gene mutations were interrogated individually for a possible association with COVID-19 severity using a proportional odds model. Mutations in TET2 and DNMT3A were also not associated with COVID-19 disease severity (Figure 1H). Finally, given that large clone size is more strongly associated with CVD,10 we analyzed the risk associated with large CH clones (VAF ≥ 5%), and again found no association with COVID-19 disease severity (OR = 1.29; 95% CI, 0.69-2.42; P = .42; Figure 1H). Similarly, we found no association between CH with VAF ≥ 10% and COVID-19 disease severity (OR = 1.06; 95% CI, 0.50-2.28; P = .88).
In summary, our study found no evidence that CH is associated with COVID-19 disease severity, even after adjusting for covariates known to affect the risk of severe disease. Previous studies examining the association between COVID-19 disease severity and clonal hematopoiesis CH have produced conflicting results. Three smaller studies concluded that CH is not associated with COVID-19 disease severity.20-22 However, conclusions were less definitive because of comparisons only to historical non-COVID-19 controls,20 small sample size/power to detect potentially relevant associations,21 different sequencing platforms used for cases and controls,20 and/or limited availability of additional comorbidity/risk factor data.21 A larger study (n = 413) examined the relationship among patients with solid cancers at various stages during treatment (MSK-IMPACT cohort), and reported that nonputative driver clonal hematopoiesis mutations were associated with COVID-19 disease severity.23 This finding may have reflected the impact of prior cancer treatment on patients with reduced hematopoietic stem cell numbers/reserve. The same study used an independent noncancer cohort (n = 112) for validation, and found no significant associations within this smaller cohort, although fixed-effects meta-analysis of the combined 2 cohorts remained positive, likely driven by the larger MSC-IMPACT cohort.23 Also, a recent study reported an association between mosaic chromosomal alterations, a distinct form of CH, and risk of COVID-19 hospitalization.24 Our current study attempted to mitigate these prior limitations by studying the largest number of patients to date, directly comparing relevant patient groups (asymptomatic/mild, hospitalized, critically ill), incorporating covariates from well-characterized additional risk factors, and performing sequencing and mutation calling using the same platforms. Overall, we found no evidence of an association between CH and COVID-19 severity, resolving much of the uncertainty surrounding this question. Although it is never possible to rule out an association with absolute certainty, our study indicates that the clinical impact of any theoretical association is unlikely to be substantial (Figure 1H).
Acknowledgments
The authors are grateful to all physicians, nurses, and health care workers treating COVID-19 patients under very challenging circumstances and still being able to help with providing data for this paper. The authors are also very grateful to the 2648 frontline National Health Service clinical and research staff and volunteer medical students who collected these data in challenging circumstances. The authors thank the generosity of the participants and their families for their individual contributions in these difficult times. The authors acknowledge Lucy Lorris for extracting clinical information for ISARIC4C patients; Tovah Klein for providing samples and clinical information for NorthShore Hospital patients; Mary Magliocco, Michael Stack, Elana Shaw, Jason Barnett, Smilee Samuel, and Sandhya Xirasagar for maintaining LabKey database for NIAID Immune Response to COVID patients; Charla Andrews, Britta Flach, Emily Coates, Obrimpong Amoa-Awua, Maria Burgos Florez, Lasonji Holman, and Renunda Hicks for providing samples and clinical information for VRC 200 patients; and Elizabeth Laidlaw and Silvia Lage for providing samples and clinical information for CALYPSO patients. The authors thank Xiaolin Wu, Arati Raziuddin at the National Cancer Institute (NCI) Frederick Sequencing Facility, Yuesheng Li, Yan Luo, and Patrick Burr at the National Heart, Lung and Blood Institute (NHLBI) DNA Sequencing and Genomics Core for preparing sequencing library and sequencing. The authors are grateful for the assistance provided by Helen Matthews, Sarah Weber, James Chappell, and Wilna Oosthuyzen and thank Shu Huang at Cavendish Laboratory, University of Cambridge, for discussion on data processing. The authors also acknowledge the support of Jeremy J. Farrar and Nohoko Shindo.
This work used the computational resources of the National Institutes of Health (NIH) High Performance Computing Biowulf cluster and Wellcome-MRC Cambridge Stem Cell Institute High Performance Computing cluster. The NorthShore University Health System COVID-19 Convalescent Plasma collection program was initially supported by grants to the NorthShore Foundation, including a donation from the Rice Foundation, and support from NorthShore University HealthSystem Research Institute. Subsequently, NorthShore received funding from the Department of Defense (W911QY2090012-D.S.). The ISARIC4C is supported by grants from the NIHR (award CO-CIN-01), MRC (grant MC_PC_19059), NIHR Imperial Biomedical Research Centre (grant P45058), HPRU in Respiratory Infections at Imperial College London, and NIHR HPRU in Emerging and Zoonotic Infections at the University of Liverpool, in partnership with Public Health England (NIHR award 200907), Wellcome Trust, Department for International Development (215091/Z/18/Z), Bill & Melinda Gates Foundation (OPP1209135), Liverpool Experimental Cancer Medicine Centre (grant C18616/A25153), NIHR Biomedical Research Centre at Imperial College London (IS-BRC-1215–20013), and EU Platform for European Preparedness Against (Re-) Emerging Epidemics (PREPARE, FP7 project 602525).
This research was funded in whole, or in part, by the Wellcome Trust 203151/Z/16/Z] and the UKRI Medical Research Council [MC_PC_17230]. For the purpose of open access, the author has applied a CC BY public copyright license to any Author Accepted Manuscript version arising from this submission. This work was supported by a European Hematology Association COVID-19 in Hematology Research Grant 2020 and a National Institutes of Health Intramural Targeted Anti-COVID-19 (ITAC) Award. G.S.V. is funded by a Cancer Research UK Senior Cancer Research Fellowship (C22324/A23015). The work was also supported in part by the Intramural Research Program of the National Heart, Lung, and Blood Institure and National Institute of Allergy and Infectious Diseases.
Authorship
Contribution: G.S.V., C.E.D., and Z.M. conceived the study; G.S.V., C.E.D., J.K.B., Z.M., and Y.Z. designed and supervised the study; Y.Z., R.S., C.O.W., M.G., M.A.F., and P.M.Q. performed and advised on the bioinformatic and statistical analysis; S.N.R., S.Y., T.-H.S., A.D., W.D., S.A., W.L., and M.C. prepared library and sequenced samples; A.L.G., J.P., J.H., E.J., A.C., L.A., F.R.-L., B.T., A.F., I.J.G., L.N., L.S., M.R.G., A.L., I.S., T.J.G., A.B., P.B., L.I., C.L.D., Y.Z., K.D., H.C.S., L.D.N., P.J.M.O., and M.G.S. provided samples and clinical information; P.J.M.O., M.G.S., and J.K.B. set up the ISARIC4C cohort; and G.S.V., C.E.D., and Y.Z. wrote the manuscript with input from all coauthors.
Conflict-of-interest disclosure: G.S.V. is a consultant with STRM.BIO and receives a research grant from AstraZeneca. During 2021, T.J.G. was a paid consultant for Frenwal/Fresenius Kabi and became a full-time employee of the company in March 2022. The remaining authors declare no competing financial interests.
A complete list of the ISARIC4C Investigators appears in supplemental Appendix A.
Correspondence: Cynthia E. Dunbar, Translational Stem Cell Biology Branch, National Heart, Lung and Blood Institute, National Institutes of Health, Room 5E-3332, Bldg 10-CRC, 9000 Rockville Pike, Bethesda, MD 20892; e-mail: dunbarc@nhlbi.nih.gov; and George S. Vassiliou, Haematological Medicine, Wellcome-MRC Cambridge Stem Cell Institute, University of Cambridge, Puddicombe Way, Cambridge, CB2 0AW, UK; e-mail: gsv20@cam.ac.uk.
References
Author notes
∗Z.M., K.B., C.E.D., and G.S.V. contributed equally to this work.
For access to the original sequencing data, please contact: cmdl_ngs@medschl.cam.ac.uk.
The online version of this article contains a data supplement.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal