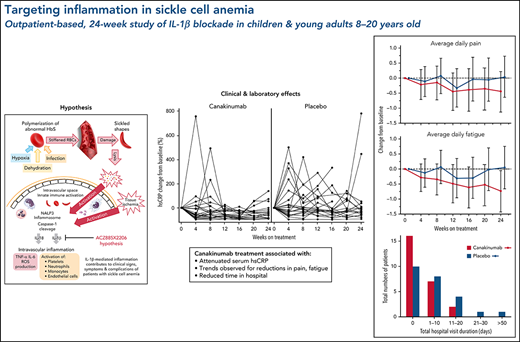
Key Points
Canakinumab treatment of sickle cell anemia did not meet its primary endpoint of a clinically relevant reduction in average daily pain.
Canakinumab in combination with hydroxyurea was well tolerated and reduced systemic inflammation with potential for clinical benefits.
Abstract
Excessive intravascular release of lysed cellular contents from damaged red blood cells (RBCs) in patients with sickle cell anemia (SCA) can activate the inflammasome, a multiprotein oligomer promoting maturation and secretion of proinflammatory cytokines, including interleukin-1β (IL-1β). We hypothesized that IL-1β blockade by canakinumab in patients with SCA would reduce markers of inflammation and clinical disease activity. In this randomized, double-blind, multicenter phase 2a study, patients aged 8 to 20 years with SCA (HbSS or HbSβ0-thalassemia), history of acute pain episodes, and elevated high-sensitivity C-reactive protein >1.0 mg/L at screening were randomized 1:1 to received 6 monthly treatments with 300 mg subcutaneous canakinumab or placebo. Measured outcomes at baseline and weeks 4, 8, 12, 16, 20, and 24 included electronic patient-reported outcomes, hospitalization rate, and adverse events (AEs) and serious AEs (SAEs). All but 1 of the 49 enrolled patients were receiving stable background hydroxyurea therapy. Although the primary objective (prespecified reduction of pain) was not met, compared with patients in the placebo arm, patients treated with canakinumab had reductions in markers of inflammation, occurrence of SCA-related AEs and SAEs, and number and duration of hospitalizations as well as trends for improvement in pain intensity, fatigue, and absences from school or work. Post hoc analysis revealed treatment effects on weight, restricted to pediatric patients. Canakinumab was well tolerated with no treatment-related SAEs and no new safety signal. These findings demonstrate that the inflammation associated with SCA can be reduced by selective IL-1β blockade by canakinumab with potential for therapeutic benefits. This trial was registered at www.clinicaltrials.gov as #NCT02961218.
Introduction
Sickle cell anemia (SCA) is the most common, severe inherited disorder, with >300 000 affected babies born each year.1 It is caused by a point mutation in the β globin gene (HBB; c.20A>T, p.Glu7Val) changing a glutamic acid to valine, resulting in sickle hemoglobin (HbS) with tendency to polymerize when deoxygenated.2 This leads to abnormally shaped sickle cell erythrocytes having reduced membrane elasticity with increased propensity for lysis and adherence. Heterozygous individuals carrying 1 copy of the HbS variant (sickle cell trait) usually have no overt disease. However, individuals who are homozygous for the mutation (HbSS) experience a range of disease severity and complications, depending on the amount of fetal hemoglobin (HbF) produced, other coexisting condition such as G6PD deficiency or α thalassemia, and exposure to factors conducive to sickling, such as infection, dehydration, and acidosis.
Lifelong disease manifestations include anemia, chronic pain, and fatigue, punctuated by episodic disease flares, termed acute vaso-occlusive events. Accumulation over time of severe, end-organ damage contributes to early mortality, with life expectancy reduced by at least 20 years in high-income countries3 and up to 80% of patients dying during childhood in many low-income countries.4 Hydroxyurea (HU), a chemotherapeutic agent that increases HbF levels, offers some clinical benefits,5 and more recent therapies targeting adhesion molecules6 or increasing hemoglobin affinity for oxygen7 have demonstrated further important yet still limited benefits for patients. Thus, there remains for patients with SCA an urgent need to develop additional therapeutic strategies acting by different mechanisms.
Well-established evidence exists in patients with SCA for presence of ongoing inflammatory responses, with correlations to disease severity8-12 and association with risk for early death.13,14 Proinflammatory markers such as leukocyte count and high-sensitivity C-reactive protein (hsCRP) are elevated in pediatric and adult patients under steady-state conditions, with further increases observed in patients hospitalized for acute vaso-occlusive events8-10 and with complications such as acute chest syndrome (ACS) and silent cerebral infarction.15,16 Moreover, evidence suggests that patients with HbSS or HbSβ0-thalassemia have higher hsCRP levels compared with patients with other sickle cell disease (SCD) variants such as HbSC, characterized by less anemia and correspondingly milder disease symptoms.9,13,15 It remains unclear, however, to what extent intravascular inflammation contributes to the numerous SCD clinical manifestations.
The inflammasome is a multiprotein oligomer expressed in myeloid cells as an innate immunity component responding to a range of infectious and sterile stimuli (supplemental Data, available on the Blood Web site).17 Inflammasome activation results in an inflammatory cascade that includes cleavage by caspase-1 of pro-interleukin (IL)-1β and pro-IL-18 into their active forms, triggering other inflammatory processes. Therapeutic IL-1β blockade has been effectively used to treat several autoinflammatory syndromes, chronic diseases having in common either genetic mutations or other conditions leading to increased inflammasome activation.18 Recurrent flares associated with these autoinflammatory conditions are typically accompanied by increased levels of innate immune cytokines and neutrophilic inflammation, marked by elevated hsCRP.
Since first posited by Wanderer,19 a growing body of evidence supports the hypothesis that inflammasome activity is constitutively upregulated in patients with SCA, leading to exaggerated, proinflammatory responses triggered by ischemic reperfusion injury, intravascular hemolysis, and other stimuli.14,20,21 However, therapeutic targeting of inflammasome effector activity in the SCA disease process has been limited to human transgenic SCA models using IL-1β blockade.22
Canakinumab is a fully human monoclonal antibody targeting IL-1β with antiinflammatory effects showing benefits in a number of rheumatic disorders, including cryopyrin-associated periodic syndromes, systemic juvenile idiopathic arthritis, and acute gouty arthritis.23 Most recently, canakinumab was found to decrease rates of cardiovascular events following myocardial infarction, associated with hsCRP reductions, but independently of any lipid-lowering effects.24 Therefore, canakinumab, an established, targeted therapy for chronic, inflammasome-mediated diseases, provides opportunity to explore the role played by IL-1β–mediated inflammation in patients with SCA.
Methods
Study purpose
This study was designed to explore the hypothesis that inflammation associated with SCA contributes to clinical symptoms of pain and other disease manifestations experienced in an ambulatory setting.
Study design
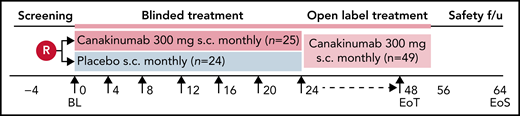
This was an international, multicenter, randomized, placebo-controlled, double-blind, parallel-group phase 2 trial conducted between 5 April 2017 and 27 April 2020 (#NCT02961218). The trial consisted of a 30-day screening phase and a 24-week blinded treatment phase, followed by an optional 24-week open-label treatment phase (Figure 1). End-of-treatment visit occurred approximately 8 weeks after last dose received for all patients, regardless of whether participating in the optional, open-label phase or having stopped treatment early. The study was conducted according to International Conference on Harmonization of Good Clinical Practice guidelines, the principles of the Declaration of Helsinki, and all applicable country-specific regulatory guidelines.
Study design. Patients were blindly randomized on a 1:1 ratio to receive subcutaneous administration every 4 weeks of either canakinumab 300 mg (4 mg/kg for weight ≤40 kg) or placebo. Study visit schedule was as follows: Screening period before randomization for up to 28 days that included recording of daily pain frequency and intensity by eDiary for at least 1 week. On Day 0, patients underwent baseline assessments and dosing. Study visits thereafter occurred every 4 weeks up to the final blinded assessment at Week 24, after which patients had the option to receive open label canakinumab 300 mg subcutaneously every 4 weeks until Week 48, with final study outcome assessments made on Week 52. Patients underwent a safety follow-up visit or telephone call 8 weeks after receiving the last study dose.
Study design. Patients were blindly randomized on a 1:1 ratio to receive subcutaneous administration every 4 weeks of either canakinumab 300 mg (4 mg/kg for weight ≤40 kg) or placebo. Study visit schedule was as follows: Screening period before randomization for up to 28 days that included recording of daily pain frequency and intensity by eDiary for at least 1 week. On Day 0, patients underwent baseline assessments and dosing. Study visits thereafter occurred every 4 weeks up to the final blinded assessment at Week 24, after which patients had the option to receive open label canakinumab 300 mg subcutaneously every 4 weeks until Week 48, with final study outcome assessments made on Week 52. Patients underwent a safety follow-up visit or telephone call 8 weeks after receiving the last study dose.
Study population
A younger patient population was recruited to reduce the presence of irreversible tissue damage (eg, degenerative joint disease, stroke) and thus was more likely to show clinical improvement in response to targeted antiinflammatory treatment. The study population included participants 8 to 20 years of age with homozygous HbSS or HbSβ0-thalassemia who experienced in the year prior screening at least 2 vaso-occlusive pain episodes, defined as pain without non–sickle cell identifiable cause, requiring analgesia, and interfering with participants’ normal daily routine. In addition, study participants were required at screening to have hsCRP >1.0 mg/L and detectable background pain as recorded by electronic patient-reported outcome device (eDiary; www.ert.com), defined as either average daily pain score >1 (see section on Endpoints) without analgesic use over a period of ≥7 days or ≥1 episode of pain requiring analgesia within a 14-day period. Participants receiving HU were eligible if stable dosing ≥60 days prior to screening. Participants receiving regular RBC transfusion therapy or having received a transfusion in the past 3 months prior to screening were excluded. All participants ≥18 years provided written informed consent before study participation, with parent or legal guardian written informed consent required along with child’s assent for participants <18 years. Protocol and consent forms were approved by local ethics committee at each participating trial site.
Study treatment randomization
Participants meeting eligibility criteria were randomly assigned (1:1 ratio) to receive 6 doses during the double-blinded period of either 300 mg (or 4 mg/kg for participants <40 kg) canakinumab (provided as 150 mg/1 mL of liquid in vials) or matching placebo, administered subcutaneously every 28 days. During the open-label period, patients received canakinumab every 28 days while remaining blinded to their prior assigned treatment arm. Patients were stratified by HU use at baseline (yes or no).
Endpoints
Primary endpoint was reduction of daily SCA-related pain averaged over weeks 8 to 12 compared with baseline levels recorded over the screening period. Patients with SCA have been shown to discriminate accurately between pain related to their disease and other types of pain.25 Also, low-to-moderate levels of SCA-related pain are commonly managed at home for many children with the disease and are likely to be underreported in routine outpatient follow-up appointments.26,27 However, daily diaries using shorter recall periods can help reduce memory bias in reporting lower levels of pain managed at home. Therefore, we used eDiaries to record patients’ daily self-assessed SCA-related pain levels throughout the 24 weeks of this study using a numerical visual analog scale (VAS) from 0 to 10 (0 = no pain, 10 = worst pain).28-30 The reported values were rescaled to the equivalent of a 10-cm scale and used to determine average daily pain over 4-week intervals.
Secondary and exploratory endpoints included daily eDiary-documented degree of perceived fatigue using a VAS from 0 to 10 (0 = not at all, 10 = extremely) averaged over 4-week intervals,30 analgesic use, and school or work attendance (supplemental Methods). The incidence and duration of acute vaso-occlusive events were objectively predefined in this study as SCA pain-related hospital admissions documented in the safety database as a serious adverse event (SAE).
Laboratory markers of SCA-associated inflammation were measured monthly and included hsCRP, total leukocyte count, and absolute numbers of circulating neutrophils and monocytes. In addition, downstream markers of inflammasome activation were measured at baseline and weeks 4, 12, and 24 and included serum levels of soluble vascular cell adhesion molecule-1 (VCAM-1; www.proteinsimple.com), CXCL10/interferon protein-10 (IP-10) and IL-18 binding protein (IL-18bp) (www.rndsystems.com), IL-18 (www.mblintl.com), and plasma levels of soluble P-selectin (www.mesoscale.com). Monthly measurement of hemolysis markers included reticulocyte count and levels of hemoglobin, haptoglobin, and lactose dehydrogenase as well as levels of total, direct, and indirect bilirubin.
Safety assessments were performed during the screening and treatment phases and during the follow-up period, including physical examination, vital signs, clinical laboratory tests (chemistry panel, complete blood count and differential, and urinalysis), and 12-lead electrocardiogram. Body height and weight were measured at baseline, and weights were measured thereafter at each visit.
Statistical analysis
Data were analyzed using SAS software, version 9.4 (SAS Institute Inc). Data collected after blood transfusion were excluded from analyses except for safety parameters.
Change from baseline in the average daily pain was analyzed using a Bayesian model for repeated measures including the 4-week intervals average daily pain VAS scores up to week 24. The model included baseline pain scores as a covariate, treatment group, and time as fixed factors as well as interactions of time by treatment group and time by baseline pain score. Noninformative priors were used for the fixed effects and weakly informative prior for the covariance. Due to the high percentage of participants using HU, this stratification factor was not included in the model. Posterior means with 90% credible interval were presented by visit. The following decision criteria were evaluated:
- •
Posterior probability of canakinumab treatment to reduce average pain scores more than placebo treatment is >90%.
- •
The posterior probability of canakinumab treatment to reduce average pain scores by ≥1 cm more than treatment with placebo is >50%.
The same analysis was applied to average daily fatigue VAS scores. The percentage of days participants were absent from school or work in every 4-week interval was summarized by treatment arm on a change from baseline scale. Time to first SCA-related hospitalization was presented as a Kaplan Meier graph. Biomarkers, including hsCRP, absolute counts of leukocytes, neutrophils, and monocytes, weight changes, and the hemolysis parameters of hemoglobin and reticulocyte percentage were analyzed using a Mixed effect Model for Repeated Measures (MMRM; supplemental Methods). A similar MMRM model was used to analyze the change from baseline for post hoc analyses of body weight according to baseline body mass index (BMI) percentile and for supplementary data outcomes of IL-18, IL-18bp, IP-10, sVCAM-1, P-selectin, and oxygen percent saturation (SaO2).
Results
This report describes results from the 24-week blinded treatment study period.
Study patients
Overall, baseline demographics were well matched for age, BMI, gender, and ethnicity across the 2 treatment groups (Table 1). Of 25 patients randomized to canakinumab and 24 to placebo, data for the 24-week blinded period are available for 23 and 19 patients, respectively (supplementary Data). Two patients discontinued in the canakinumab arm due to subject/guardian decision, and 5 discontinued in the placebo arm due to either decision by patient/guardian (n = 1) or physician (n = 2) or were lost to follow-up (n = 2). There were no AE- or drug-related discontinuations. All patients, except for 1 placebo arm patient, were receiving HU background therapy of comparable median daily dose levels between the 2 study arms. Compared with the placebo group at baseline, patients assigned to the canakinumab group trended toward higher disease activity during the screening period, as reflected by trends toward lower hemoglobin levels and higher daily levels of pain and fatigue and opiate/nonopiate analgesic use (Table 1). Correspondingly, median hsCRP baseline values also trended higher in the canakinumab group.
Demographics and baseline disease characteristics of enrolled study patients
| . | . | Canakinumab n = 25 . | Placebo n = 24 . |
|---|---|---|---|
| Age, y | Mean (SD) | 15.8 (2.69) | 15.6 (3.28) |
| 8-11 | 2 | 2 | |
| Age groups, n | 12-17 | 14 | 14 |
| 18-20 | 9 | 8 | |
| Gender, n | Male | 15 | 13 |
| Race, n | Black | 12 | 13 |
| White | 12 | 10 | |
| Other | 1 | 1 | |
| BMI (kg/m2) | Median (range) | 20.50 (15.9-30.4) | 19.25 (15.4-26.8) |
| hsCRP (mg/L) | Median (range) | 4.0 (0.5-67.4) | 3.0 (0.7-19.3) |
| Daily pain (average VAS) | Median (range) | 2.9 (0-7) | 2.0 (0-6) |
| Daily fatigue (average VAS) | Median (range) | 3.8 (0-8) | 2.6 (0-6) |
| Use during screening, patient n (%) | Non-opiate analgesics Opiates | 20 (80.0) 9 (36.0) | 18 (75.0) 5 (20.8) |
| Daily HU dose (mg) | Median (range) | 1000 (500-1600) | 1000 (250-1500) |
| Hemoglobin (g/L) | Median (range) | 92.0 (65-126) | 92.5 (74-129) |
| . | . | Canakinumab n = 25 . | Placebo n = 24 . |
|---|---|---|---|
| Age, y | Mean (SD) | 15.8 (2.69) | 15.6 (3.28) |
| 8-11 | 2 | 2 | |
| Age groups, n | 12-17 | 14 | 14 |
| 18-20 | 9 | 8 | |
| Gender, n | Male | 15 | 13 |
| Race, n | Black | 12 | 13 |
| White | 12 | 10 | |
| Other | 1 | 1 | |
| BMI (kg/m2) | Median (range) | 20.50 (15.9-30.4) | 19.25 (15.4-26.8) |
| hsCRP (mg/L) | Median (range) | 4.0 (0.5-67.4) | 3.0 (0.7-19.3) |
| Daily pain (average VAS) | Median (range) | 2.9 (0-7) | 2.0 (0-6) |
| Daily fatigue (average VAS) | Median (range) | 3.8 (0-8) | 2.6 (0-6) |
| Use during screening, patient n (%) | Non-opiate analgesics Opiates | 20 (80.0) 9 (36.0) | 18 (75.0) 5 (20.8) |
| Daily HU dose (mg) | Median (range) | 1000 (500-1600) | 1000 (250-1500) |
| Hemoglobin (g/L) | Median (range) | 92.0 (65-126) | 92.5 (74-129) |
SD, standard deviation.
Inflammatory markers
hsCRP and absolute counts of leukocytes, neutrophils, and monocytes have been reported predictive of long-term morbidity and mortality in populations with SCD.8,9,12 In this study, progressive reductions in these systemic inflammation markers occurred during the first 3 months of canakinumab treatment compared with patients in the placebo arm (Figure 2A-D). Moreover, marked fluctuations in hsCRP levels experienced by patients receiving placebo throughout the 24-week blinded treatment period were substantially attenuated in patients receiving canakinumab (Figure 2E). However, no effect by canakinumab blockade of IL-1β was observed upon circulating levels of endothelial cell activation marker sVCAM-1 or soluble P-selectin or on IL-18 and its pathway-associated markers IL-18bp and IP-10 (supplemental Data).
Treatment effects on markers of systemic inflammation. Time course over the 24-week, blinded study period is shown as geometric mean ratio to baseline (90% confidence intervals [CI]) obtained from MMRM analyses for serum hsCRP (A) and circulating total leukocytes (B), neutrophils (C), and monocytes (D) in patients receiving canakinumab (red line) or placebo (blue line). Dotted horizontal line references baseline levels. Baseline median (range) values for canakinumab/placebo, respectively, in counts of 109/L are as follows: total leukocytes 10.7 (range, 3.5-20.0)/9.1 (range, 4.55-18.20); neutrophils 5.5 (range, 1.57-10.42)/5.0 (range, 2.18-11.19); monocytes 0.9 (range, 0.26-2.50)/0.8 (range, 0.24-2.04). Baseline values for hsCRP are found in Table 1. Spaghetti plots (E) show percent change from baseline of hsCRP for individual patients in the 2 treatment groups as indicated.
Treatment effects on markers of systemic inflammation. Time course over the 24-week, blinded study period is shown as geometric mean ratio to baseline (90% confidence intervals [CI]) obtained from MMRM analyses for serum hsCRP (A) and circulating total leukocytes (B), neutrophils (C), and monocytes (D) in patients receiving canakinumab (red line) or placebo (blue line). Dotted horizontal line references baseline levels. Baseline median (range) values for canakinumab/placebo, respectively, in counts of 109/L are as follows: total leukocytes 10.7 (range, 3.5-20.0)/9.1 (range, 4.55-18.20); neutrophils 5.5 (range, 1.57-10.42)/5.0 (range, 2.18-11.19); monocytes 0.9 (range, 0.26-2.50)/0.8 (range, 0.24-2.04). Baseline values for hsCRP are found in Table 1. Spaghetti plots (E) show percent change from baseline of hsCRP for individual patients in the 2 treatment groups as indicated.
Patient-reported outcomes: daily pain scores
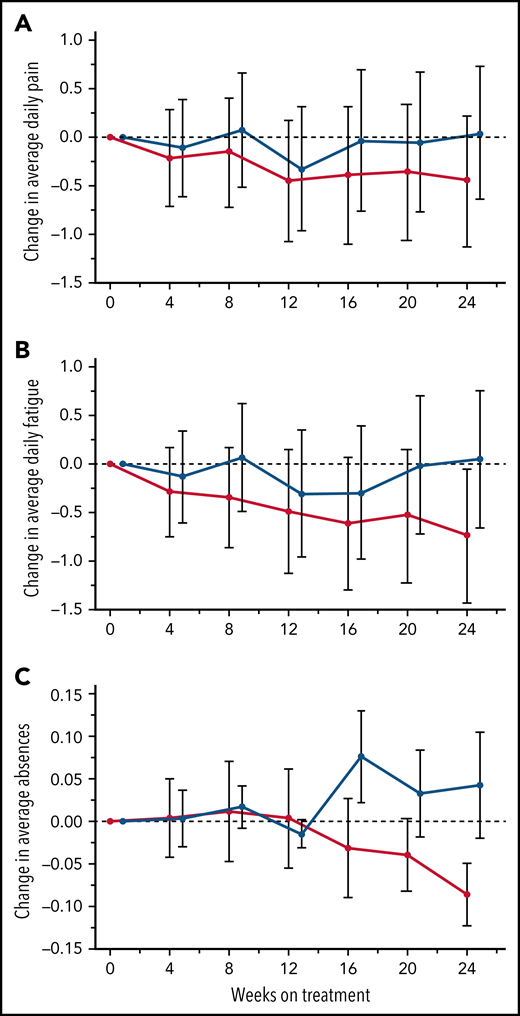
The primary endpoint, daily pain scores, recorded by eDiary and averaged over 4-week periods, decreased with canakinumab over the first 12 weeks and were maintained thereafter to Week 24 (Figure 3A). In contrast, pain levels recorded for placebo-treated patients remained largely unchanged except for a transient reduction at Week 12. However, canakinumab-associated pain reductions did not reach the significance criterion versus placebo and did not achieve a study-predefined, clinically meaningful treatment difference of 1.0 cm on the change from baseline scale.
Treatment effects on patient recorded outcomes. Absolute change from baseline over time with standard deviations are shown for eDiary outcomes during the blinded treatment period for study participants receiving canakinumab (red line) or placebo (blue line) using Bayesian models for average daily pain (A), average daily fatigue (B), and mean change from baseline ± SE for average daily absence from school or work (C). Dotted horizontal line references baseline levels.
Treatment effects on patient recorded outcomes. Absolute change from baseline over time with standard deviations are shown for eDiary outcomes during the blinded treatment period for study participants receiving canakinumab (red line) or placebo (blue line) using Bayesian models for average daily pain (A), average daily fatigue (B), and mean change from baseline ± SE for average daily absence from school or work (C). Dotted horizontal line references baseline levels.
Patient-reported outcomes: analgesic use
Use of analgesics over the 24-week period as recorded by eDiary revealed reduced average opioid use within the canakinumab treatment arm of 26.6 days (range, 0-173) compared with 47.8 days (range, 0-217) in the placebo treatment arm. In contrast, average non-opioid analgesic use was slightly increased in the canakinumab group at 112.2 days (range, 0-210) compared with 106.0 days (range, 0-217) in the placebo arm.
Patient-reported outcomes: fatigue and school/work absences
Average daily fatigue VAS scores fell steadily for patients on canakinumab over the 24-week period (Figure 3B). In contrast, no consistent change occurred for this parameter in patients treated with placebo, resulting in the canakinumab group achieving by Week 20 a clinically meaningful difference vs placebo.31 Canakinumab treatment was also associated with a substantial reduction in rate of school/work absences from baseline compared with placebo, apparent from 16 weeks onwards in the change from baseline scale (Figure 3C). However, the overall mean number of missed days over the 24-week treatment period was numerically higher for the canakinumab group vs placebo (2.2 vs 1.86 days) because patients treated with canakinumab initially had more missed days than patients treated with placebo, possibly related to the observed imbalances in baseline disease severity (Table 1).
Oxygen saturation and hemolysis parameters
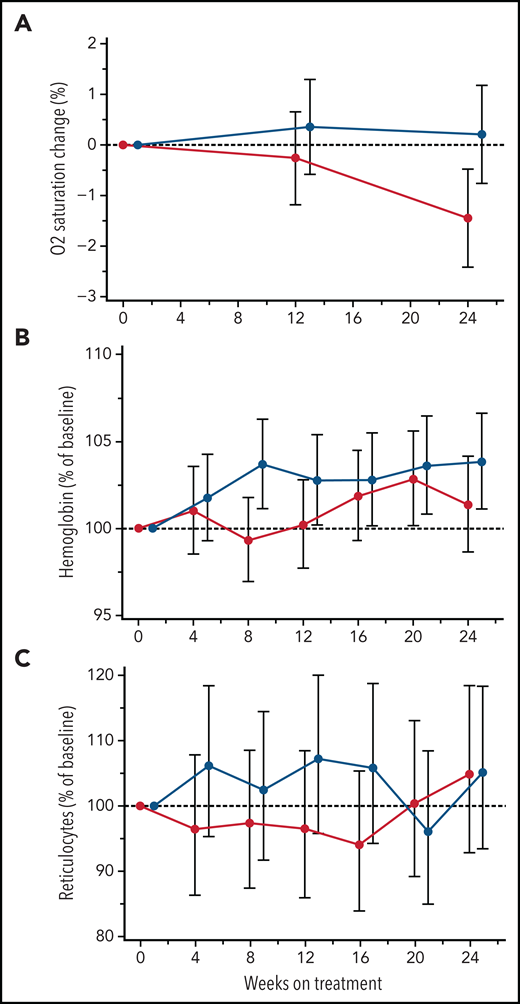
Low SaO2 is associated with higher rates of hemolysis and markers of inflammation32-34 and has been used as a predictor of elevated cerebral flow velocities.35 In this study, canakinumab treatment did not improve mean SaO2 levels (Figure 4A). Similarly, canakinumab treatment was not associated with sustained or significant change compared with placebo in hemoglobin levels (Figure 4B) or reticulocyte percentage (Figure 4C) or other hemolysis parameters (data not shown). During the study, 5 patients in the canakinumab group and 6 patients in the placebo group received ≥1 blood transfusions. Notably, only 2 patients in the canakinumab arm received blood transfusions for acute SCA complications, with the remaining 3 patients having received elective transfusions (eg, prior to a planned procedure). In contrast, 5 patients treated with placebo experienced acute, severe SCA complications requiring blood transfusions.
Treatment effects on blood oxygen percent saturation and hemolysis parameters. Change from baseline in mean absolute values for blood oxygen percent saturation (A) and hemoglobin concentration (B) and reticulocyte percentage (C) obtained from MMRM analysis shown as geometric mean ratio to baseline (90% CI) over the first 24 weeks of treatment in patients on canakinumab (red line) and placebo (blue line). Baseline mean/median (range) values for canakinumab and placebo, respectively, in blood oxygen percent saturation are as follows: 96.8/96 (range, 93-100) and 97.7/98 (range, 92-100). Patient data truncated after having received a blood transfusion.
Treatment effects on blood oxygen percent saturation and hemolysis parameters. Change from baseline in mean absolute values for blood oxygen percent saturation (A) and hemoglobin concentration (B) and reticulocyte percentage (C) obtained from MMRM analysis shown as geometric mean ratio to baseline (90% CI) over the first 24 weeks of treatment in patients on canakinumab (red line) and placebo (blue line). Baseline mean/median (range) values for canakinumab and placebo, respectively, in blood oxygen percent saturation are as follows: 96.8/96 (range, 93-100) and 97.7/98 (range, 92-100). Patient data truncated after having received a blood transfusion.
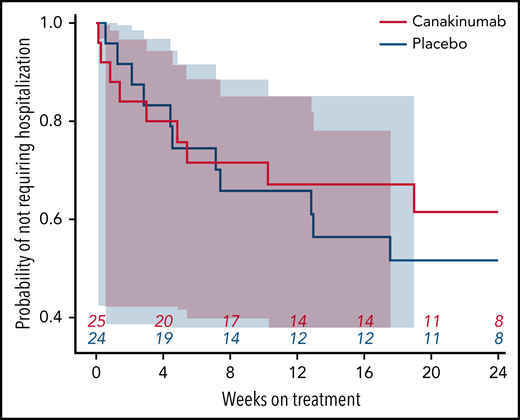
Hospital admissions
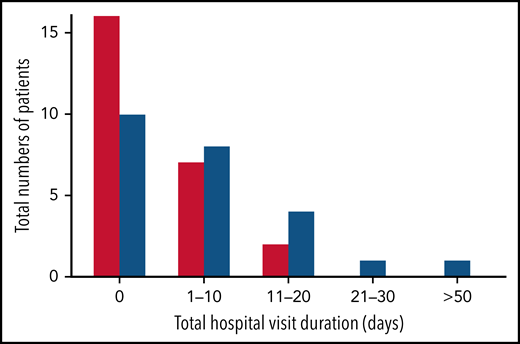
Nearly all study hospitalizations, recorded as SAEs, resulted from SCA-related pain crises or complications thereof. Numbers of overall hospitalizations (SAEs; Table 2) were less in the canakinumab arm compared with placebo arm (Table 2), resulting in a mean SAE rate of 0.9 per patient vs 1.1, respectively. The time to first admission did not show a treatment effect (Figure 5) although uncertainty in this endpoint was high. The overall time spent in hospital was less in patients receiving canakinumab (Figure 6), resulting in an annualized hospitalization duration (mean/median/maximum) of 5.7/0/37.4 days for patients treated with canakinumab and 15.7/7.6/126.8 days for patients in the placebo arm. Furthermore, all hospitalizations in the canakinumab arm were due to acute pain crisis (n = 10), whereas the placebo group also included hospitalization for severe SCA complications, including ACS (n = 1), priapism (n = 1), and osteonecrosis (n = 1), in addition to admissions for acute pain crisis (n = 11).
Number of AEs and SAEs
| . | Canakinumab . | Placebo . |
|---|---|---|
| Number of AEs occurring in the number of patients | 101 in 21 | 150 in 23 |
| AE severity grade | ||
| Mild | 68 in 17 | 86 in 18 |
| Moderate | 26 in 15 | 55 in 19 |
| Severe | 7 in 4 | 9 in 3 |
| Study drug–related AEs | 4 in 3 | 4 in 3 |
| SAEs | 19 in 11 | 42 in 15 |
| . | Canakinumab . | Placebo . |
|---|---|---|
| Number of AEs occurring in the number of patients | 101 in 21 | 150 in 23 |
| AE severity grade | ||
| Mild | 68 in 17 | 86 in 18 |
| Moderate | 26 in 15 | 55 in 19 |
| Severe | 7 in 4 | 9 in 3 |
| Study drug–related AEs | 4 in 3 | 4 in 3 |
| SAEs | 19 in 11 | 42 in 15 |
Time to first SCA-related hospitalization. Probability of not requiring a SCA-related hospitalization during the blinded treatment period is shown as a Kaplan-Meier graph for patients receiving canakinumab (red line) or placebo (blue line), with the number of patients at risk shown at 4-week intervals. Correspondingly shaded areas represent 95% CI.
Time to first SCA-related hospitalization. Probability of not requiring a SCA-related hospitalization during the blinded treatment period is shown as a Kaplan-Meier graph for patients receiving canakinumab (red line) or placebo (blue line), with the number of patients at risk shown at 4-week intervals. Correspondingly shaded areas represent 95% CI.
Total hospitalization duration. Numbers of study participants requiring hospitalization within a treatment arm are shown for canakinumab (red bar) or placebo (blue bar) according to overall duration of time spent in hospital. All hospital visit durations are summed for each patient. Time ranges include zero (0) (ie, no time spent in hospital) and time periods ranging from 1 to 10 days, 11 to 20 days, or >50 days. There was no study participant in either treatment group whose total time in hospital was between 30 and 50 days.
Total hospitalization duration. Numbers of study participants requiring hospitalization within a treatment arm are shown for canakinumab (red bar) or placebo (blue bar) according to overall duration of time spent in hospital. All hospital visit durations are summed for each patient. Time ranges include zero (0) (ie, no time spent in hospital) and time periods ranging from 1 to 10 days, 11 to 20 days, or >50 days. There was no study participant in either treatment group whose total time in hospital was between 30 and 50 days.
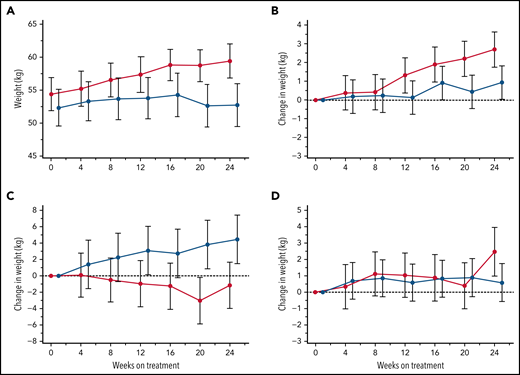
Body weight change
Patient body weights, monitored at each visit for potential dose adjustments in this largely pediatric study population, revealed an incidental finding of consistent weight gains within the canakinumab group over the 24-week treatment period not observed in patients receiving placebo (Figure 7A). Additional post hoc subset analyses of patients aged <18 years revealed that BMI <20th percentile was predictive of substantial weight gain in patients treated with canakinumab compared with patients in the placebo arm (Figure 7B), while the converse was present in patients with BMI >80th percentile (Figure 7C). Mean weight gains of patients having intermediate BMI (ie, 20th-80th percentile range) did not differentiate by treatment arm (Figure 7D). Results were consistent across both genders but not for patients >18 years (data not shown).
Treatment effects on body weight. Average body weights (kg) are shown at 4-week intervals over the 24-week, blinded study period for all patients who received either canakinumab (red line) or placebo (blue line) (A). Post hoc MMRM analysis was applied to study participants aged <18 years and segregated by baseline BMI percentile of <20th percentile (B), 20th to 80th percentile (C), and >80th percentile (D). Standard error of the means (A) and standard deviations (B-D) are shown, and a dotted horizontal line references normalized baseline levels.
Treatment effects on body weight. Average body weights (kg) are shown at 4-week intervals over the 24-week, blinded study period for all patients who received either canakinumab (red line) or placebo (blue line) (A). Post hoc MMRM analysis was applied to study participants aged <18 years and segregated by baseline BMI percentile of <20th percentile (B), 20th to 80th percentile (C), and >80th percentile (D). Standard error of the means (A) and standard deviations (B-D) are shown, and a dotted horizontal line references normalized baseline levels.
Safety
Occurrence of AEs was high (251 events) in this adolescent and young adult study population, with the most commonly reported AEs involving pain. Similar to the above-noted group differences in SAE occurrences, patients treated with canakinumab also reported AEs less frequently (Table 2). None of the AEs in either group led to discontinuation of study treatment. Patients in the placebo treated arm more commonly experienced AEs due to complications of SCA, including ACS (n = 3), priapism (n = 3), and bone infarction (n = 1), compared with only 1 patient in the canakinumab arm experiencing an AE for a sickle cell complication (ACS). Six patients were reported to have drug-related AEs, including 4 AEs occurring in 3 patients treated with canakinumab and 4 AEs in 3 patients in the placebo arm. Importantly, there was no evidence for increased neutropenia associated with canakinumab use concurrent with HU treatment. Furthermore, despite the potential for immune suppression by canakinumab, treated patients had an overall lower rate of infections (n = 8 vs 12) compared with patients receiving placebo. Of particular note, upper respiratory tract infections overwhelmingly occurred in the placebo group (n = 6) compared with the canakinumab group (n = 1).
Discussion
This proof-of-concept study explored the hypothesis that inflammation is a major contributor to the clinical signs, symptoms, and sequelae of SCA, a SCD subset characterized by high rates of hemolysis, elevated hsCRP levels, and a more severe disease course. Canakinumab blockade of IL-1β in these study participants resulted in significant reduction of systemic inflammation markers, including hsCRP and absolute numbers of circulating leukocytes, neutrophils, and monocytes, findings also observed in a range of inflammatory conditions responding to IL-1β blockade by canakinumab.24,36,37 In our study, these reductions in inflammatory markers occurred primarily over the first 12 weeks and were maintained thereafter. Although the trial did not achieve its prespecified primary endpoint for eDiary-reported daily pain score, trends were observed in patients in the canakinumab vs placebo arm of reduced scores for pain as well as for fatigue, together with greater improvements in school/work attendance compared with patients in the placebo arm. Other potential consequences of reduced systemic inflammation by canakinumab included reductions in number of SCA-related AEs and SAEs and number and duration of hospital admissions.
Importantly, canakinumab-induced reduction of inflammation was well tolerated in this population, with no increased risk of infection. No patient developed significant neutropenia or leukopenia despite concomitant treatment with HU; the observed reductions in these cell types with canakinumab treatment were considered not due to a direct myelosuppressive effect but rather indicative of generalized reductions in inflammation, as seen in canakinumab clinical trials for other conditions.24,38,39 Increases in hsCRP and leukocyte counts have been associated with adverse outcomes in SCD, and reduction of these levels may have long-term benefits per se.13,14 High baseline hsCRP levels have been associated with increased frequency of acute pain in children with SCD.9 Similarly, high leukocyte numbers are associated with severe disease complications, including increased frequency of pain,40 increased risk of hemorrhagic stroke,41 earlier death,13 and higher frequency of pain on HU.42 High leukocyte counts were also an important part of a composite score predicting outcomes in children with SCD,43 with relative risk of adverse outcome increased by 2.17 for each 10 × 109/L increase in total leukocyte numbers.
Recent research has shown SCA pathophysiology to have a strong inflammasome-driven component via high rates of constitutive intravascular hemolysis that overwhelm scavenger capacity of the body for heme and free iron, resulting in chronic activation of endothelial cells and vascular inflammation.14,20,21 In mouse models, free heme induces IL-1β production and neutrophil migration dependent on caspase-1 cleavage, thus demonstrating inflammasome activation.20 In the same report, hemolysis-induced lethality in an animal model was greatly reduced in inflammasome-deficient mice (ie, lacking nlrp3, asc, or caspase-1). In our study, canakinumab had little or no effect on hemolysis rates, suggesting that its potential therapeutic benefits in treated patients may occur by blocking inflammation generated by hemolysis and other disease-related sources. In experiments using human transgenic SCA mouse models, anti–IL-1β antibody treatment markedly ameliorated intravascular flow impedance induced by hypoxia.22 However, canakinumab treatment in our clinical study did not completely shut down inflammasome activity, as evidenced by lack of effect on serum levels of IL-18 or IL-18bp in these patients. Moreover, the endothelial cell activation markers sVCAM-1 and P-selectin also remain unaffected by canakinumab treatment, consistent with IL-18-associated effects on these endothelial cell activation markers.36,44 Thus, additional reductions of inflammation in this patient population may potentially be achieved by more complete blockade of inflammasome output.
Canakinumab treatment showed evidence for clinical benefits in this study. Perhaps most impressively, there was a reduction in daily fatigue scores. Previous studies have shown that fatigue is a significant problem in teenagers and young adults with SCD.45 A study of 60 adolescents and young adults with SCD found that 69% reported feeling unusually tired or fatigued in the preceding week and that increased fatigue was associated with reduced quality of life.46 Increased fatigue in patients with SCD has also been associated with increased numbers of painful episodes47 but has not been clearly linked to inflammation, although such links have been shown in other inflammatory conditions such as rheumatoid arthritis48 and cancer.49 Moreover, the IL-1 family of cytokines has been linked to perception of fatigue, and therapeutic IL-1 blockade has been associated with reduction of severe fatigue in a range of inflammatory diseases.50
The effect by canakinumab on pain is more difficult to interpret, having failed to achieve the statistical significance criterion over placebo arm or meet a prespecified, minimally clinically relevant difference. However, reported average analgesic use in the canakinumab arm is reduced for opioids while increased for non-opioid analgesics compared with use in the placebo arm, suggesting reduced pain management requirements in patients receiving canakinumab. It is possible that eDiary entries by patients are reduced during their more severe pain episodes at home or in hospital,26 although no difference was observed between the 2 treatment arms in time-to-first hospitalization for pain crisis. However, patients treated with canakinumab had fewer hospitalizations and hospitalizations of overall shorter duration, and they experienced fewer serious SCA-related complications, suggesting less severe disease activity compared with patients receiving placebo who required hospitalization during the study.
The observed canakinumab-treatment effects upon serially obtained body weights, overall and according to baseline BMI percentile, was not anticipated; thus, serial collection of patients’ heights was not specified in the protocol. Metabolic studies in SCD have shown energy expenditures to be increased at rest and decreased with activity51 and to affect not only height and weight but also patient distribution of body mass between fat and muscle compartments.52 Poor weight gain in combination with delayed sexual and skeletal maturation are clinical features of pediatric SCD, more commonly seen in patients with SCA (homozygous HbSS or HbSβ0-thalassemia) and linked to indicators of disease activity such as anemia, presence of SCA-related complications, and health care use rates.53,54 Improved gains in height and weight have been observed in pediatric patients with SCD responding to effective treatment with HU or transfusion therapy.55,56 Weight gains associated with IL-1 pathway blockade have also been observed in patients treated with anakinra with the chronic inflammatory disease cryopyrin-associated periodic syndrome.57 Thus, canakinumab-related effects on body weight identified by post hoc analysis in our study patients warrant further, more dedicated investigation of the potential impact of IL-1β blockade upon SCA disease metabolism.
This is the first clinical SCA trial to study a drug selectively targeting the proinflammatory effects of IL-1β. As such, our results give novel insights into the role that inflammation plays in the symptomatology of the disease. Inflammation appears to contribute to fatigue and other daily manifestations of SCA, and the antiinflammatory effects of selective IL-1β blockade by canakinumab may reduce the severity of these acute and chronic disease manifestations. In the longer term, based on association studies of inflammatory markers, one might predict that canakinumab treatment could reduce organ damage and improve survival, although clearly this requires formal investigation. These patients were also taking HU, suggesting that canakinumab offers additional therapeutic benefit over this established standard-of-care treatment. It is important to note that canakinumab produced significant laboratory changes and potentially associated clinical benefits in SCA by targeting inflammation alone, with no evidence of improvements in other established therapeutic targets, including hemolysis, anemia, blood oxygenation, or levels of soluble adhesion molecules VCAM-1 and P-selectin. This novel action suggests that IL-1β blockade in SCA might work synergistically in combination with other emerging agents, such as crizanlizumab6 and voxelotor,7 or agents enhancing HbF levels.
Few significant AEs were associated with canakinumab in this study, suggesting it is safe and well tolerated in children and young adults with SCA. IL-1β is one of the main proinflammatory cytokines, not only implicated in the pathophysiology of inflammatory and autoimmune diseases but also playing an important part in host defense against infection.58 SCD is associated with a significantly increased risk of infections, with hyposplenism causing a particular problem with invasive bacterial infection from encapsulated organisms.59 Over the 6 months of this study, there was no suggestion of increased infections in the canakinumab arm, which is in keeping with safety data from other clinical trials of canakinumab.24,60 It is also important to note that 48 of 49 patients in this study were taking HU, and there was no evidence that adding canakinumab caused additional immunosuppression or other side effects such as neutropenia.
In conclusion, these study results suggest that selective blockade of IL-1β–mediated inflammation by canakinumab in adolescent and young adults with SCA could lead to therapeutic benefits without major safety issues. The antiinflammatory effects of canakinumab appeared in this study to be most impactful clinically upon the important symptom of fatigue. Larger randomized controlled trials are needed to establish these effects, and suitable clinical endpoints could include fatigue, school/work attendance, daily pain score, and, in the case of pediatric and young adult patients, growth and development.
Acknowledgments
This study was funded by Novartis Pharma AG, and the sponsor was responsible for the overall conduct of the study as well as the collection, analyses, and interpretation of the data.
Authorship
Contribution: S.J.O. and D.C.R. developed the protocol and, with statisticians K.M. and D.H., analyzed results, generated a clinical study report, and prepared the manuscript; S.J.O. provided medical oversight of the trial; E.M. managed conduct of the trial and contributed to data analysis; D.C.R., Y.K., G.L., Selma Unal, C.D., B.S.P., B.K., S.T., I.O., J.M., Sule Unal, J.B., R.G., B.R.F., B.P.D.I., A.K., and C.L. participated in protocol review, patient recruitment, patient management, and collection of clinical outcomes; and all authors reviewed the manuscript with opportunity to provide input.
Conflict-of-interest disclosure: D.C.R. is an investigator and steering committee member of the Novartis Solace Kids study for crizanlizumab. J.M. has received research grants from Novo Nordisk and Roche. B.P.D.I. has received educational grants from Novartis, AstraZeneca, Global Therapeutics, and Pfizer and honoraria from Cyclerion, AstraZeneca, and Novartis. A.K. acts as a medical advisor and lecturer for Novartis, Apopharma, and Bristol-Myers-Squibb and has received Novartis financial compensation for clinical studies. E.M., K.M., D.H., and S.J.O. are full-time employees of Novartis Pharma AG. S.J.O. has equity ownership in Novartis Pharma AG. The remaining authors declare no competing financial interests.
Correspondence: Stephen J. Oliver, Novartis Pharma AG, Novartis Campus, WSJ386.11, 4002 Basel, Switzerland; e-mail: stephen.oliver@novartis.com.
Presented in abstract form at the 61st annual meeting of the American Society of Hematology, Orlando, FL, 9 December 2019.
Novartis is committed to sharing with qualified external researchers, access to patient-level data and supporting clinical documents from eligible studies. These requests are reviewed and approved by an independent review panel on basis of scientific merit. All data provided are anonymized to respect privacy of study patients in line with applicable laws and regulations. This trial data availability is according to the criteria and process described on www.clinicalstudydatarequest.com.
The online version of this article contains a data supplement.
There is a Blood Commentary on this article in this issue.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.

![Treatment effects on markers of systemic inflammation. Time course over the 24-week, blinded study period is shown as geometric mean ratio to baseline (90% confidence intervals [CI]) obtained from MMRM analyses for serum hsCRP (A) and circulating total leukocytes (B), neutrophils (C), and monocytes (D) in patients receiving canakinumab (red line) or placebo (blue line). Dotted horizontal line references baseline levels. Baseline median (range) values for canakinumab/placebo, respectively, in counts of 109/L are as follows: total leukocytes 10.7 (range, 3.5-20.0)/9.1 (range, 4.55-18.20); neutrophils 5.5 (range, 1.57-10.42)/5.0 (range, 2.18-11.19); monocytes 0.9 (range, 0.26-2.50)/0.8 (range, 0.24-2.04). Baseline values for hsCRP are found in Table 1. Spaghetti plots (E) show percent change from baseline of hsCRP for individual patients in the 2 treatment groups as indicated.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/139/17/10.1182_blood.2021013674/4/m_bloodbld2021013674f2.png?Expires=1769094864&Signature=lC2OXlTeJniYOFX~7KNNFXo7v3Cet4erqjuuYwtdFB0BgymYZ1xqWZa2YmwsOMdreM4-rOfzSBwg6QUoASFZjOEI2KxuKdl2JNX5szZXJml0RW7g3BHwSXCKG1x-QgGjjg~ZFxTH0W9Y3RhI58yy1~Daem1A64NLLF89tIrYNgR8XqHgNcH35e2~bmqdkSiRIBzg7owaUAL1Pr-Q4HPoVk7ypYP4ECtAZBPUE1uY8nt4NeY3J~qEXFpE1BAZ2qmwrAKpWVhPibvdlgQTKI0mI8nJuLHrfjoofWEkDGIhWudYdQnRbdA6IDdHiVBTUjocFtnO8ILv3sEVvaUVUAhBFg__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)
This feature is available to Subscribers Only
Sign In or Create an Account Close Modal