In this issue of Blood, Rees et al1 report the results of a randomized, double-blind, placebo-controlled, multicenter phase 2a study that tested the safety and efficacy of the interleukin 1-β (IL-1β) neutralizing antibody canakinumab in children and young adults with sickle cell anemia (SCA), chronic pain, and inflammation. The study’s primary objective was not met because participants receiving the study drug did not experience a significant reduction in average daily pain, but there was a promising reduction of multiple biomarkers of inflammation, number and duration of hospitalizations, and other patient-reported outcomes of pain and fatigue.
The past 5 years have witnessed a proliferation of new treatment approaches for SCA. Seminal studies in humanized transgenic sickle cell mice have dissected a plethora of pathways that link the polymerization of mutated sickle hemoglobin (HbS), the condicio sine qua non for the emergence of the SCA phenotype, to a wide spectrum of vasculotoxic effects and end organ damage. Each of the pathogenic mechanisms of SCA, including endothelial dysfunction, cellular hyperadhesion, ischemia-reperfusion injury, oxidative stress, and sterile inflammation, are now becoming prime targets for new classes of biological agents. Two recently approved drugs, L-glutamine and the anti-P-selectin monoclonal antibody crizanlizumab, target oxidative stress and hyperadhesion, respectively, and lead to a reduction in the rate of vaso-occlusive pain episodes (VOEs).2,3 Yet neither they nor the other currently available disease-modifying drugs approved by the US Food and Drug Administration afford more than 50% reduction in the rate of VOEs, thus leaving patients vulnerable to the devastating effects of the disease; there are still no multipronged therapeutic approaches that target all major disease pathways.
With canakinumab, Rees et al have now opened a new front in the fight against SCA by targeting sterile inflammation. Sterile inflammation is indeed rising to prominence as a druggable pathogenic mechanism in multiple fields, and in particular, in cardiovascular diseases, in which the deleterious effects of a proinflammatory milieu compound those of hemostatic activation and hyperlipidemia. Anti-inflammatory biologicals, whose therapeutic indications were initially confined to rare inflammatory conditions, are being piloted as agents to combat disparate conditions ranging from diabetes to coronary artery disease.4
In addition to the novelty of testing an anti-inflammatory drug in SCA, the study by Rees et al also stands out for the authors’ choice of a somewhat questionable yet intriguing outcome measure. Assessing the impact of new drugs on the incidence of VOEs—typically defined as acute SCA-related pain that leads to a health care encounter—has become a standard approach in clinical research over the past decades, but the incorporation of patient-reported outcomes into the design of clinical trials has lagged.5 Yet pain is a quintessentially subjective outcome measure, and its inherent complexity bears a nuanced investigative approach, particularly in children, whose report of daily pain may never reach the ears of their health care providers.6 Thus, although it was unusual, the choice of average daily pain measured by an electronic patient-reported outcome device (eDiary) as the primary outcome measure was wise.
Arguably, it is not surprising that with a relatively small sample size and a brief intervention, there was no significant reduction in daily pain in the canakinumab group, particularly in light of similarly disappointing results from other studies powered to detect differences in pain.7 Yet the promising findings of a reduction in incidence of SCA-related adverse events and the number and duration of hospitalizations, as well as trends for improvement in pain intensity, fatigue, and absences from school or work, alongside a lack of significant toxicity, augurs well for future studies on the effects of anti-inflammatory drugs in SCA. Indeed, the study may spur research on the nebulous link between inflammation and pain in SCA.
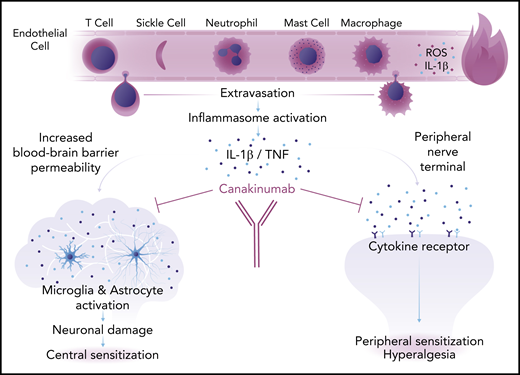
Chronic pain, in particular, is a recalcitrant symptom and a frustrating complication for individuals who have SCA to live with and for health care providers to treat, particularly because its underlying pathology is often elusive. This knowledge gap is not merely an academic concern, and it explains why we have no effective drugs to control chronic pain in SCA, and indeed in most other diseases. The results of this study, even though they are preliminary, highlight the possible role of sterile inflammation in chronic pain, and offer new potential areas of investigation. With sickling of red blood cells being an incessant process in the microcirculation, we can posit that background ischemia-reperfusion events and their downstream inflammatory cascades may incite a low level of daily pain. Alternatively, we may invoke neuroinflammation as a primary effector of pain in SCA, in which overproduction of inflammatory cytokines such as IL-1β, tumor necrosis factor, and interferon-γ lead to recruitment of immune cells, tissue damage, and both central and peripheral sensitization, with the attendant activation of nociceptive receptors and hyperalgesia (see figure).8 This latter hypothesis opens many research avenues with the prospect of modulating both central and peripheral neuroinflammation to alleviate pain.
Proposed mechanism of neuroinflammation in SCA. Sickle cells and inflammatory cells in the circulation release reactive oxygen species (ROS) and proinflammatory cytokines, including IL-1β, that lead to an inflamed, porous endothelium. Inflammatory cells extravasate into the extravascular space where they trigger an inflammatory response. Inflammation causes increased blood-brain barrier permeability and chronic deleterious activation of the microglia and astrocytes that leads to neuronal damage and central sensitization (left). Inflammatory cytokines also activate receptors in peripheral nerve terminals causing peripheral sensitization and hyperalgesia (right). Both central and peripheral sensitization are responsible for chronic pain in SCA. Professional illustration by Somersault18:24.
Proposed mechanism of neuroinflammation in SCA. Sickle cells and inflammatory cells in the circulation release reactive oxygen species (ROS) and proinflammatory cytokines, including IL-1β, that lead to an inflamed, porous endothelium. Inflammatory cells extravasate into the extravascular space where they trigger an inflammatory response. Inflammation causes increased blood-brain barrier permeability and chronic deleterious activation of the microglia and astrocytes that leads to neuronal damage and central sensitization (left). Inflammatory cytokines also activate receptors in peripheral nerve terminals causing peripheral sensitization and hyperalgesia (right). Both central and peripheral sensitization are responsible for chronic pain in SCA. Professional illustration by Somersault18:24.
If IL-1β blockade and other anti-inflammatory treatments with broader impact on the inflammasome do demonstrate value in additional, larger studies, the question remains of how to incorporate them into existing therapeutic strategies. We opine that hydroxyurea, due to its track record of safety and efficacy, low cost, and pleiotropic effects, will likely remain the backbone of therapy. However, multidrug cocktails will become increasingly appealing strategies for such a complex disease, akin to similar approaches for diseases such as rheumatoid arthritis and type II diabetes mellitus. In this vein, hydroxyurea may come to represent the “metformin” of SCA, upon which other treatments may be stacked to achieve better disease control, as was the case for most of the participants in this study who were also receiving hydroxyurea. There is already sound, emerging research evidence that such multidrug strategies may work. A recent study in transgenic humanized SCD mice has shown that IL-1β signaling cooperates with P-selectin to promote the development of toxic neutrophil-platelet aggregates that lead to acute chest syndrome.9 This is an example of how the functional cooperation between 2 proteins, P-selectin and IL-1β, may be harnessed to attempt to surpass that 50% VOE reduction bar that has so far plagued current treatment strategies.
Conflict-of-interest disclosure: E.M.N. served as a consultant for Novartis Pharmaceuticals.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal