In this issue of Blood, Goncalves et al1 elegantly demonstrate that patients with interleukin-2 receptor γ (IL2RG)– or JAK3-deficient severe combined immunodeficiency (SCID), despite being successfully treated by allogeneic stem cell transplantation may still have “holes” in their immune response that lead to specific abnormalities.
This study of patients with inborn errors of immunity teaches us about the critical role of specific elements of immunity, as well as the consequences of dysfunction if a particular element is missing or overactive. After the neural complex, the immunological network is the most complicated biological system. It is perhaps not surprising that a tool as blunt as allogeneic hematopoietic stem cell transplantation might not completely reconstitute normal immunity. Few centers worldwide would have the ability to perform a study on this cohort of patients with inborn errors of immunity, especially with the long posttransplant follow-up.
This study emphasizes the critical importance of long-term follow-up of patients transplanted for inborn errors of immunity. Specifically, the investigators found that patients with IL2RG- or JAK3-deficient SCID, who have been treated with allogeneic stem cell infusion without pretransplant cytoreductive chemotherapy, have a specific immune defect in natural killer (NK) cells and type 2 innate lymphoid cells (ILC2s) but not innate lymphoid cell (ILC) precursors. They also found a reduction in circulating NK cells, ILC2s, and T helper 2 (Th2) cells. Previously, the investigators suggested that these findings implied redundancy within the immune system and that innate lymphoid cells (ILCs) may be unnecessary if T lymphocytes are present and B lymphocytes are functional.2 The observations were extended in the study by Goncalves et al. They found a decrease in the type 2 cytokines IL-5 and IL-13 (but not IL-4) in the nasal mucosa, and a strong reduction in total nasopharyngeal mucosal immunoglobulin A (IgA). This correlated with a pattern of nasopharyngeal microbe coating with IgG and IgD alone, in contrast with healthy individuals and patients with other genetic forms of SCID in whom nasopharyngeal microbes were coated with IgA in combination with IgD, and, to a lesser extent, with IgG. Furthermore, in the group with IL2RG-/JAK3-deficient SCID, the total nasopharyngeal bacterial load was increased, but with diminished microbiological diversity and a predominance of pathobionts, suggesting that ILC2s are required for complete immunological surveillance.1
JAK-3 deficiency (and by implication, IL2RG deficiency, because IL2RG is upstream of the JAK3 signaling pathway) blocks ILC differentiation in the bone marrow at the ILC precursor stage, and blocks NK cell development at the pre–NK cell progenitor stage.3 Therefore, it is perhaps not surprising that these particular subsets of cells are missing in nonconditioned patients with IL2RG-/JAK3-deficient SCID. Myeloid engraftment in this group is rare in the absence of pretransplant conditioning, and T lymphocyte reconstitution is dependent on later-stage differentiated donor T-lymphocyte precursors populating the thymus. Thus, the block in ILC2 and NK cell development fails to correct.4
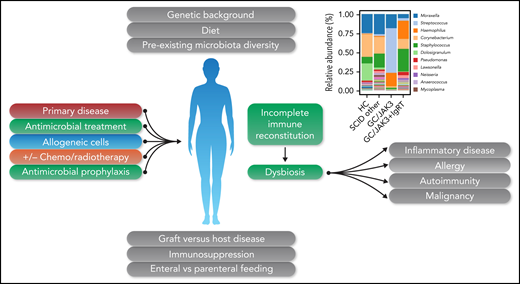
Type 2 cytokines are produced by ILC2s, as well as Th2 cells, and ILC2s influence T lymphocyte responses, including aiding Th2 cell differentiation by cytokine secretion and having important roles in regulatory T lymphocyte homeostasis. ILC2s effect the functions of eosinophils, macrophages, and dendritic cells and other cells of the innate immune system. ILC2s are important for tissue barrier defense but are also a major component of healthy adipose tissue, with an important role in the maintenance of lean body mass.5 Given the importance of ILC2s in mucosal and intestinal health, the findings of Goncalves et al are potentially important. First, they show that, far from being redundant, these cells have important roles in respiratory mucosal homeostasis. The study did not look at the viral microbiome or the intestinal microbiome, but it is likely that abnormalities would be uncovered. Given the important role of ILC2s, it is possible that the long-term absence of these cells could lead to inflammatory or allergic manifestations, autoimmunity, and, even, malignancy, particularly in the context of the major changes in the microbiome during transplantation (see figure). Dysbiosis may be enhanced by pretransplant parameters, including underlying disease, alterations in gut function, and frequent use of treatment and/or prophylactic antimicrobials. The occurrence of graft-versus-host disease, prolonged immunosuppression, and alterations in diet and route of nutrition may also impact microbiotal diversity. The coexistence of “minor” immunological defects, such as those described in the study by Goncalves et al, may magnify the potential pathological consequences. Although the patients were not described as having any specific clinical symptoms, in light of these findings it will be important to take a careful history to determine whether subtle symptoms might have been missed. The critical importance of long-term follow-up of these patients is again emphasized, because some of these findings, such as autoimmunity or malignancy, may take decades to manifest. Finally, with regard to this subset of innate immune cells, patients with rare genetic IL2RG-/JAK3-deficient SCID may not be the only affected population. JAK inhibitors also impair the development of ILC immunity,3 and a significant and growing number of patients are receiving this new class of agents to treat inflammatory or autoinflammatory conditions. Does long-term treatment with these drugs lead to dysbiosis; if so, does it have an adverse clinical consequence? Perhaps we had better find out.
Factors occurring before and during hematopoietic stem cell transplantation may lead to dysbiosis and potentially significant long-term sequelae. Figure 5D, in the article by Goncalves et al that begins on page 2585, showing decreased bacterial diversity, has been incorporated into the figure. GC, gamma chain SCID; HC, healthy control; IgRT, immunoglobulin replacement therapy. Professional illustration by Somersault18:24.
Factors occurring before and during hematopoietic stem cell transplantation may lead to dysbiosis and potentially significant long-term sequelae. Figure 5D, in the article by Goncalves et al that begins on page 2585, showing decreased bacterial diversity, has been incorporated into the figure. GC, gamma chain SCID; HC, healthy control; IgRT, immunoglobulin replacement therapy. Professional illustration by Somersault18:24.
Conflict-of-interest disclosure: The author declares no competing financial interests.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal