Key Points
Severity of ICH is linked to the severity of in utero thrombocytopenia.
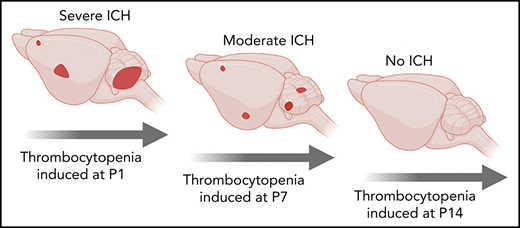
Resilience of the cerebral vasculature to thrombocytopenia occurs within the first 2 weeks of birth.
Abstract
Whether increasing platelet counts in fetal and neonatal alloimmune thrombocytopenia (FNAIT) is effective at preventing intracerebral hemorrhage (ICH) has been a subject of debate. The crux of the matter has been whether thrombocytopenia is the major driver of ICH in diseases such as FNAIT. We recently demonstrated in mice that severe thrombocytopenia was sufficient to drive ICH in utero and in early neonatal life. It remains unclear what degree of thrombocytopenia is required to drive ICH and for how long after birth thrombocytopenia can cause ICH. By inducing a thrombocytopenic range, we demonstrate that there is a large buffer zone of mild thrombocytopenia that does not result in ICH, that ICH becomes probabilistic at 40% of the normal platelet number, and that ICH becomes fully penetrant below 10% of the normal platelet number. We also demonstrate that although the neonatal mouse is susceptible to thrombocytopenia-induced ICH, this sensitivity is rapidly lost between postnatal days 7 and 14. These findings provide important insights into the risk of in utero ICH with varying degrees of thrombocytopenia and into defining the developmental high-risk period for thrombocytopenia-driven ICH in a mouse model of FNAIT.
Introduction
Mouse models have demonstrated that, although platelets are essential for hemostasis, the absence of platelets alone does not cause hemorrhage in the adult.1 By using a model of fetal and neonatal alloimmune thrombocytopenia (FNAIT), which involves injecting an anti-GP1Bα antibody that specifically targets platelets for clearance2 into pregnant or neonatal mice,3 we were able to demonstrate that severe thrombocytopenia in utero or in the neonate is sufficient to cause hemorrhage, including intracerebral hemorrhage (ICH).4
Two important questions in the field of FNAIT are: what level of thrombocytopenia places patients at risk of ICH?5; and, what is the platelet count required to prevent or minimize ICH-associated mortality?5-10 Answers to these questions are critical to understanding what degree of thrombocytopenia should trigger treatment.5,7,11-14 Thus, it is important that we understand what thrombocytopenic threshold results in ICH and the duration of neonatal susceptibility to thrombocytopenia-induced ICH.
By tuning platelet counts in utero, we were able to define 3 levels of thrombocytopenia that have a differential risk of developing into ICH. In addition, we demonstrated that by the second week after birth, the cerebral vasculature has developed resilience to severe thrombocytopenia.
Study design
All mice had a C57BL/6 background. The Walter and Eliza Hall Institute (WEHI) Animal Ethics Committee approved procedures. Theiler’s criteria were used for embryonic staging. Timed matings were set up overnight, and the morning of a positive plug was designated embryonic day 0.5 (E0.5). Immunoglobulin G (IgG [R301]) and anti-GP1Bα (R300) antibodies were purchased from EMFRET Analytics. Antibodies were delivered by intravenous (facial vein) injection at postnatal day 1 (P1) or intraperitoneal injection at E13.5, P7, and P14. Mice were analyzed after 48 hours. Delivery route did not affect experimental outcome (supplemental Figure 1). Flow cytometry was performed as previously described.4 Data were analyzed using FlowJo, and Prism software was used for statistical analysis and graphs.
Results and discussion
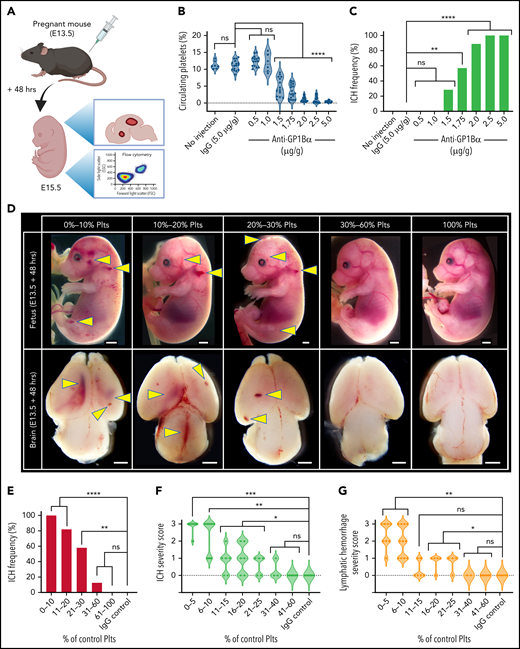
It has previously been shown that platelet counts in adult mice can be altered by varying the concentration of the injected anti-GP1Bα antibody.2,15 Importantly, the platelets that remain after treatment are functionally normal.2,15 To determine how effectively platelet counts could be tuned in utero, 0.5 to 5 μg/g body weight anti-GP1Bα or 5 μg/g IgG control antibody was injected into pregnant mice at E13.5 (a stage of active neurogenesis and structural patterning equivalent to 6 to 20 weeks in human development16). Fetuses were analyzed 48 hours later by measuring platelet frequency by flow cytometry and scoring hemorrhage (Figure 1A). We found that in utero platelet numbers could be reduced in a dose-dependent manner (Figure 1B).
Defining the thrombocytopenic threshold for in utero ICH. (A) Experimental plan. E13.5 pregnant mice were injected with anti-GP1Bα antibody at a series of concentrations and analyzed 48 hours after injection for platelet numbers and hemorrhage phenotype. IgG antibody at 5 µg/g was used as a control. (B) Frequency of circulating platelets in the peripheral blood in E13.5 + 48 hours (E15.5) fetuses. No injection control: n = 7; IgG: 5 µg/g, n = 16; anti-GP1Bα: 0.5 µg/g, n = 15; 1.0 µg/g, n = 6; 1.5 µg/g, n = 14; 1.75 µg/g, n = 14; 2.0 µg/g, n = 18; 2.5 µg/g, n = 15; 5.0 µg/g, n = 15. ****P < .0001. (C) ICH frequency at 48 hours after no injection control: n = 7; IgG: 5 µg/g, n = 16; anti-GP1Bα: 0.5 µg/g, n = 15; 1.0 µg/g, n = 6; 1.5 µg/g, n = 14; 1.75 µg/g, n = 14; 2.0 µg/g, n = 18; 2.5 µg/g, n = 15; 5.0 µg/g, n = 15. **P = .002; ****P = .000007. (D) Representative images of E13.5 + 48 hours (E15.5) fetuses and dissected brains after treatment with IgG control or anti-GP1Bα antibody. Fetuses were classified according to the percentage of normal platelet count. Images shown represent the most frequent severity of hemorrhage observed in each group (scale bars represent 1 mm). (E) Frequency of ICH at platelet counts: 0% to 10%, n = 13; 11% to 20%, n = 17; 21% to 30%, n = 12; 31% to 60%, n = 8; 100%, n = 14; IgG control: n = 14. **P = .004; ****P < .00001. (F) ICH severity scores at platelet counts: 5% to 10%, n = 10; 11% to 15%, n = 6; 16% to 20%, n = 5; 21% to 25%, n = 5; 31% to 40%, n = 3; 41% to 60%, n = 8; 100% IgG: n = 8. Arbitrary values were used to define 3 as severe ICH (intraventricular involvement and/or the presence of large cortical hemorrhage), 2 as moderate ICH (large focal hemorrhage), 1 as mild ICH (small focal hemorrhage), and 0 as no ICH. *P = .05; **P < .001; ***P = .0006. (G) Lymphatic bleed severity scores: 0% to 5%, n = 8; platelet counts: 6% to 10%, n = 11; 11% to 15%, n = 6; 16% to 20%, n = 5; 21% to 25%, n = 4; 31% to 40%, n = 2; 41% to 60%, n = 8; 100% IgG: n = 8. Arbitrary values were used to define 3 as severe hemorrhage (edema, dermal hemorrhage, and extensive blood-filled lymphatics), 2 as moderate hemorrhage (extensive blood-filled lymphatics), 1 as mild hemorrhage (blood-filled lymphatics or focal dermal hemorrhage), and 0 as no hemorrhage. *P < .05; **P = .005. Data were analyzed with one-way analysis of variance (ANOVA) using the Šídák multiple comparisons test (B), or by using contingency table analysis with Fisher’s exact test (C,E-G). P values were adjusted for multiple testing using the Holm-Šídák method (C,E-G). ns, not statistically significant; Plts, platelets.
Defining the thrombocytopenic threshold for in utero ICH. (A) Experimental plan. E13.5 pregnant mice were injected with anti-GP1Bα antibody at a series of concentrations and analyzed 48 hours after injection for platelet numbers and hemorrhage phenotype. IgG antibody at 5 µg/g was used as a control. (B) Frequency of circulating platelets in the peripheral blood in E13.5 + 48 hours (E15.5) fetuses. No injection control: n = 7; IgG: 5 µg/g, n = 16; anti-GP1Bα: 0.5 µg/g, n = 15; 1.0 µg/g, n = 6; 1.5 µg/g, n = 14; 1.75 µg/g, n = 14; 2.0 µg/g, n = 18; 2.5 µg/g, n = 15; 5.0 µg/g, n = 15. ****P < .0001. (C) ICH frequency at 48 hours after no injection control: n = 7; IgG: 5 µg/g, n = 16; anti-GP1Bα: 0.5 µg/g, n = 15; 1.0 µg/g, n = 6; 1.5 µg/g, n = 14; 1.75 µg/g, n = 14; 2.0 µg/g, n = 18; 2.5 µg/g, n = 15; 5.0 µg/g, n = 15. **P = .002; ****P = .000007. (D) Representative images of E13.5 + 48 hours (E15.5) fetuses and dissected brains after treatment with IgG control or anti-GP1Bα antibody. Fetuses were classified according to the percentage of normal platelet count. Images shown represent the most frequent severity of hemorrhage observed in each group (scale bars represent 1 mm). (E) Frequency of ICH at platelet counts: 0% to 10%, n = 13; 11% to 20%, n = 17; 21% to 30%, n = 12; 31% to 60%, n = 8; 100%, n = 14; IgG control: n = 14. **P = .004; ****P < .00001. (F) ICH severity scores at platelet counts: 5% to 10%, n = 10; 11% to 15%, n = 6; 16% to 20%, n = 5; 21% to 25%, n = 5; 31% to 40%, n = 3; 41% to 60%, n = 8; 100% IgG: n = 8. Arbitrary values were used to define 3 as severe ICH (intraventricular involvement and/or the presence of large cortical hemorrhage), 2 as moderate ICH (large focal hemorrhage), 1 as mild ICH (small focal hemorrhage), and 0 as no ICH. *P = .05; **P < .001; ***P = .0006. (G) Lymphatic bleed severity scores: 0% to 5%, n = 8; platelet counts: 6% to 10%, n = 11; 11% to 15%, n = 6; 16% to 20%, n = 5; 21% to 25%, n = 4; 31% to 40%, n = 2; 41% to 60%, n = 8; 100% IgG: n = 8. Arbitrary values were used to define 3 as severe hemorrhage (edema, dermal hemorrhage, and extensive blood-filled lymphatics), 2 as moderate hemorrhage (extensive blood-filled lymphatics), 1 as mild hemorrhage (blood-filled lymphatics or focal dermal hemorrhage), and 0 as no hemorrhage. *P < .05; **P = .005. Data were analyzed with one-way analysis of variance (ANOVA) using the Šídák multiple comparisons test (B), or by using contingency table analysis with Fisher’s exact test (C,E-G). P values were adjusted for multiple testing using the Holm-Šídák method (C,E-G). ns, not statistically significant; Plts, platelets.
To understand the relationship between platelet count and severity of hemorrhage, we determined ICH frequency after injecting various concentrations of anti-GP1Bα, and we found that the incidence of ICH significantly increased with the concentration of anti-GP1Bα (Figure 1C). It became apparent that the important variable was not the dose of antibody injected but the severity of thrombocytopenia induced. We classified embryos according to platelet count and quantified the frequency of hemorrhage in the dermal layer of the skin (which includes lymphatic vessels) and the frequency and severity of ICH (Figure 1D). We found that a platelet count that was 60% of normal was sufficient to fully protect against hemorrhage (Figure 1E-G). At a platelet count that was 30% of normal, ∼60% of mice exhibited ICH. This was not a sex-related difference (supplemental Figure 2). When platelet counts dropped below 10%, all mice had ICH (Figure 1E). Analysis of the severity of hemorrhage (supplemental Figure 3) in mice with platelet counts ranging from 0% to 60% of normal revealed that, although the severity of ICH significantly increased when platelet counts dropped below 40%, the most severe ICH occurred when platelets dropped below 10% (Figure 1F). As we previously noted,4 ICH in the E13.5 + 48 hours (E15.5) fetus occurred within the ventricles and cerebral cortex. Damage to these regions has been associated with significant neurologic damage in children.17-21
Platelets have a well-established developmental role in preserving the separation of the blood and lymphatic circulatory systems.22 As with ICH, the severity of hemorrhage into the lymphatics significantly increased with decreasing platelet counts (Figure 1G).
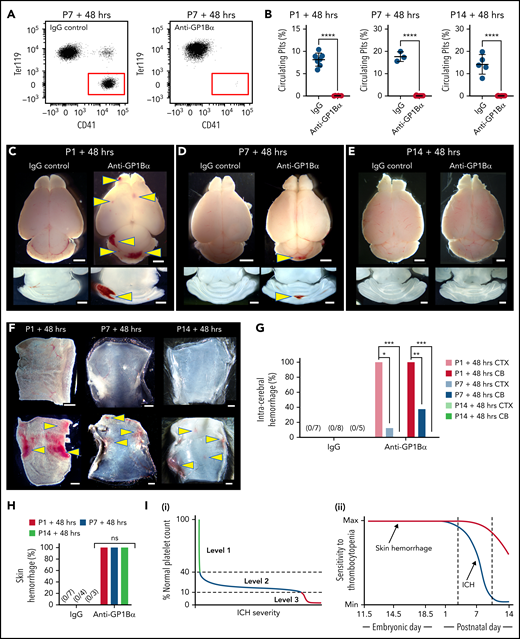
We recently showed that the spatial pattern of ICH is driven by the timing of severe thrombocytopenia; this evolves from the ganglionic eminence in the E12.5 embryo, the cerebral cortex by E16.5, and the cerebellum by P1.4 In the adult, severe thrombocytopenia only drives hemorrhage (including ICH) in an inflammatory context.1 Thus, between birth and adulthood, the cerebral vasculature becomes resilient against thrombocytopenia. To understand when this occurs, severe thrombocytopenia (<5% normal platelets) was induced at P1 (equivalent to 23-32 weeks in human development23), P7, and P14 (equivalent to 36-40 weeks in human development23) (Figure 2A-B), and we scored the frequency of ICH and skin hemorrhage after 48 hours. To ensure that ICH detection had adequate sensitivity, thick sections were cut from dissected brains at all time points. By using this approach, no bleeds were observed in any of the P1 to P14 mice that received IgG control injections. Injection of anti-GP1Bα into P1 mice resulted in fully penetrant ICH involving both the cerebral cortex and cerebellum (Figure 2C,G). When severe thrombocytopenia was induced at P7, cortical hemorrhage was detected in 10% of mice, and cerebellar hemorrhage was detected in 40% (Figure 2D,G). Induction of severe thrombocytopenia at P14 did not result in ICH (Figure 2E,G); thus, the cerebral vasculature had developed an adult-like resilience to thrombocytopenia.
Sensitivity of the cerebral vasculature to induced thrombocytopenia ends within the first weeks after birth. (A) Representative flow cytometry plots of platelets in the peripheral blood of P7 mice 48 hours after treatment with IgG or anti-GP1Bα antibody. (B) Quantification of circulating platelets 48 hours after treatment with IgG or anti-GP1Bα antibody on P1, P7, or P14. P1: IgG control, n = 7; anti-GP1Bα, n = 6. P7: IgG control, n = 3; anti-GP1Bα, n = 11. P14: IgG control, n = 5; anti-GP1Bα, n = 9. Error bars represent mean ± standard deviation. ****P < .0001. (C-E) Representative images of brain and horizontal sections of the cerebellum 48 hours after injection at (C) P1, (D) P7, and (E) P14. P1: IgG control, n = 8; anti-GP1Bα, n = 8. P7: IgG control, n = 6; anti-GP1Bα, n = 14. P14: IgG control, n = 6; anti-GP1Bα, n = 15. Arrows indicate sites of ICH. Scale bars represent 1 mm. (F) Representative images of the dermal skin layer showing hemorrhage (arrows) 48 hours after injection at P1 (IgG, n = 7; anti-GP1Bα, n = 10), P7 (IgG, n = 4; anti-GP1Bα, n = 4), and P14 (IgG, n = 3; anti-GP1Bα, n = 7). (G) Frequency of hemorrhage in the cerebral cortex (CTX) and cerebellum (CB) 48 hours after antibody injection at P1 (IgG, n = 7; anti-GP1Bα, n = 10), P7 (IgG, n = 8; anti-GP1Bα, n = 8), and P14 (IgG, n = 5; anti-GP1Bα, n = 8). *P = .02; **P = .001; ***P = .0001. (H) Frequency of skin hemorrhage at P1, P7, and P14 at 48 hours after injection. (I) Summary schematics illustrating (i) identification of 3 levels of in utero thrombocytopenia that confer increasing risk of ICH and hemorrhage severity; (ii) the development of resilience to thrombocytopenia-induced ICH within the first 2 weeks after birth. Data were analyzed using the unpaired two-tail Student t test (B) or by using contingency table analysis with Fisher’s exact test (G-H). P values were adjusted for multiple testing using the Holm-Šídák method (G-H).
Sensitivity of the cerebral vasculature to induced thrombocytopenia ends within the first weeks after birth. (A) Representative flow cytometry plots of platelets in the peripheral blood of P7 mice 48 hours after treatment with IgG or anti-GP1Bα antibody. (B) Quantification of circulating platelets 48 hours after treatment with IgG or anti-GP1Bα antibody on P1, P7, or P14. P1: IgG control, n = 7; anti-GP1Bα, n = 6. P7: IgG control, n = 3; anti-GP1Bα, n = 11. P14: IgG control, n = 5; anti-GP1Bα, n = 9. Error bars represent mean ± standard deviation. ****P < .0001. (C-E) Representative images of brain and horizontal sections of the cerebellum 48 hours after injection at (C) P1, (D) P7, and (E) P14. P1: IgG control, n = 8; anti-GP1Bα, n = 8. P7: IgG control, n = 6; anti-GP1Bα, n = 14. P14: IgG control, n = 6; anti-GP1Bα, n = 15. Arrows indicate sites of ICH. Scale bars represent 1 mm. (F) Representative images of the dermal skin layer showing hemorrhage (arrows) 48 hours after injection at P1 (IgG, n = 7; anti-GP1Bα, n = 10), P7 (IgG, n = 4; anti-GP1Bα, n = 4), and P14 (IgG, n = 3; anti-GP1Bα, n = 7). (G) Frequency of hemorrhage in the cerebral cortex (CTX) and cerebellum (CB) 48 hours after antibody injection at P1 (IgG, n = 7; anti-GP1Bα, n = 10), P7 (IgG, n = 8; anti-GP1Bα, n = 8), and P14 (IgG, n = 5; anti-GP1Bα, n = 8). *P = .02; **P = .001; ***P = .0001. (H) Frequency of skin hemorrhage at P1, P7, and P14 at 48 hours after injection. (I) Summary schematics illustrating (i) identification of 3 levels of in utero thrombocytopenia that confer increasing risk of ICH and hemorrhage severity; (ii) the development of resilience to thrombocytopenia-induced ICH within the first 2 weeks after birth. Data were analyzed using the unpaired two-tail Student t test (B) or by using contingency table analysis with Fisher’s exact test (G-H). P values were adjusted for multiple testing using the Holm-Šídák method (G-H).
No hemorrhage into the lymphatics was observed when IgG control antibody was injected at P1, P7, or P14, but hemorrhage was detected at all time points after injection of anti-GP1Bα (Figure 2F,H). Of note, hemorrhage seemed more severe at P1.
In summary, we demonstrated that there are at least 3 operational levels of in utero thrombocytopenia: (1) mild thrombocytopenia (41%-60% of normal platelet counts) representing an asymptomatic buffer zone; (2) moderate thrombocytopenia (11%-40% of normal platelet counts) in a group that has a high risk of mild to moderate ICH; and (3) severe thrombocytopenia (<10% of normal platelet counts), which is associated with a high chance of severe ICH (Figure 2Ii). In combination with our previous findings,4 we have also shown that resilience to thrombocytopenia-induced ICH develops within the first 2 weeks after birth (Figure 2Iii). Development of thrombocytopenic resilience coincides with the plateau in growth of brain volume.24 It is possible that platelets prevent bleeding associated with vascular remodeling, similar to that which occurs during mesenteric development.25 Alternatively, platelets might play an as yet unidentified role in cerebral vascular health unrelated to hemostasis.
Although caution should be used when extrapolating specific values from our mouse study to human patients, there are 2 potential clinical implications of our findings. The first is understanding when the human cerebral vasculature develops resilience to thrombocytopenia, which will help avoid the potential negative consequences associated with unnecessary platelet transfusions.5,9 The second is noting that ICH can occur in individuals with even moderate thrombocytopenia. Assuming a normal human neonatal platelet count of 150 × 109/L to 400 × 109/L,8,9,14 a count of <50 × 109/L (which is defined as severe thrombocytopenia) would fit the low end of moderate thrombocytopenia. This would explain why fetuses and neonates with FNAIT have a variable risk of developing ICH. We now need to identify the covariables that drive ICH in this group.
Acknowledgments
The authors thank Samantha Chandler for her consumer advocacy.
This work was supported by National Health and Medical Research Council (NHMRC) project grants (1128993 and 1129012), a Cerebral Palsy Alliance project grant (PG12418), a Speedy Innovation award, a grant from the Independent Research Institutes Infrastructure Support Scheme (361646) from the NHMRC, the Australian Cancer Research Fund, and by Victorian State Government Operational Infrastructure Support. S.T. was supported by a fellowship from the Lorenzo and Pamela Galli Charitable Trust.
Authorship
Contribution: A.M.F. conceived the study, designed and performed experiments, and analyzed the data; M.D. performed experiments; C.B., O.S., and A.T. provided essential input into experimental design and data analysis; S.T. conceived the study, designed experiments, and analyzed the data; and all authors contributed to manuscript preparation.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Samir Taoudi, Walter and Eliza Hall Institute, 1G Royal Parade, Melbourne, VIC 3052, Australia; e-mail: taoudi@wehi.edu.au.
Requests for data sharing may be submitted to Samir Taoudi (taoudi@wehi.edu.au).
The online version of this article contains a data supplement.
There is a Blood Commentary on this article in this issue.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal