Key Points
Belumosudil, a selective ROCK2 inhibitor, was well tolerated in heavily pretreated subjects, with 44% continuing treatment beyond 1 year.
Belumosudil demonstrated efficacy in patients with SR cGVHD, with responses in all organs and after failure of ibrutinib/ruxolitinib.
Abstract
Belumosudil, an investigational oral selective inhibitor of Rho-associated coiled-coil–containing protein kinase 2 (ROCK2), reduces type 17 and follicular T helper cells via downregulation of STAT3 and enhances regulatory T cells via upregulation of STAT5. Belumosudil may effectively treat patients with chronic graft-versus-host disease (cGVHD), a major cause of morbidity and late nonrelapse mortality after an allogeneic hematopoietic cell transplant. This phase 2 randomized multicenter registration study evaluated belumosudil 200 mg daily (n = 66) and 200 mg twice daily (n = 66) in subjects with cGVHD who had received 2 to 5 prior lines of therapy. The primary end point was best overall response rate (ORR). Duration of response (DOR), changes in Lee Symptom Scale score, failure-free survival, corticosteroid dose reductions, and overall survival were also evaluated. Overall median follow-up was 14 months. The best ORR for belumosudil 200 mg daily and 200 mg twice daily was 74% (95% confidence interval [CI], 62-84) and 77% (95% CI, 65-87), respectively, with high response rates observed in all subgroups. All affected organs demonstrated complete responses. The median DOR was 54 weeks; 44% of subjects have remained on therapy for ≥1 year. Symptom reduction with belumosudil 200 mg daily and 200 mg twice daily was reported in 59% and 62% of subjects, respectively. Adverse events (AEs) were consistent with those expected in patients with cGVHD receiving corticosteroids and other immunosuppressants. Sixteen subjects (12%) discontinued belumosudil because of possible drug-related AEs. Belumosudil, a promising therapy for cGVHD, was well tolerated with clinically meaningful responses. This trial was registered at www.clinicaltrials.gov as #NCT03640481.
Introduction
Chronic graft-versus-host disease (cGVHD) is an immune-mediated inflammatory and fibrotic disorder1 that is characterized by tissue damage2,3 and multisystem organ involvement.2 It is the leading cause of morbidity,4 late nonrelapse mortality (NRM),4,5 and impaired quality of life (QOL)6 after an allogeneic hematopoietic cell transplant (alloHCT).4-6 Chronic GVHD affects up to 70% of all alloHCT recipients,2,4,7-9 and 42% of patients have ≥4 organs involved at the time of diagnosis.10 Patients often progress to more advanced disease, with ∼40% of cGVHD cases classified as severe.11,12
The pleomorphic presentation of cGVHD contributes to disease burden,13 because cGVHD may affect almost every organ.14 Fibrotic manifestations, such as fasciitis, cutaneous sclerosis, and bronchiolitis obliterans syndrome, are particularly difficult to manage and often require prolonged treatment. The clinical manifestations of cGVHD vary in severity and can significantly impact patient QOL after transplant.13 The Lee Symptom Scale (LSS), which measures cGVHD symptoms, has shown that patients, especially those with moderate to severe disease, have substantial symptom burden.15
Despite first-line therapy for National Institutes of Health (NIH)-defined moderate to severe cGVHD with systemic corticosteroids (CSs) alone or in combination with sirolimus or a calcineurin inhibitor (CNI),16,17 ≥70% of patients require subsequent lines of therapy (LOTs) owing to the toxicity and the lack of efficacy associated with current treatments.13,18 Chronic GVHD and current immunosuppressive therapy (IST) are associated with an increased risk of infection, cumulative organ toxicities, subsequent neoplasms and recurrent malignancies.11,19 A meta-analysis of the therapies used in steroid-refractory (SR) cGVHD, including extracorporeal photopheresis (ECP) and IST, demonstrated variable overall response rates (ORRs) that ranged between 30% and 85%.20 More targeted and tolerable approaches are needed that directly address the inflammation and the fibrosis associated with cGVHD without suppressing the immune system.13
Belumosudil is an oral selective Rho-associated coiled-coil–containing protein kinase 2 (ROCK2) inhibitor with 100-fold selectivity for ROCK2 over ROCK1.21 ROCK2 inhibition acts on the dysregulated adaptive immune system and the fibrosis that occurs as a result of aberrant tissue repair.2,21,22 Specifically, ROCK2 inhibition leads to the downregulation of STAT3 phosphorylation and the consequent decreased expression of type 17 T helper (Th17) cell–specific transcription factors.21 Moreover, selective ROCK2 inhibition restores immune homeostasis by shifting the Th17/regulatory T-cell balance via a STAT5-dependent mechanism.21,22 ROCK signaling plays a central role in multiple fibrotic pathways.23 ROCK2 activation by profibrotic mediators, such as lysophosphatidic acid and tumor growth factor-β, results in polymerization of G-actin to F-actin.23,24 This frees myocardin-related transcription factors and leads to the activation of profibrotic gene expression.23 This promotes the differentiation of fibroblasts into myofibroblasts and increases the production of collagen,23 both of which are key features of fibrotic diseases.24
Treatment of cGVHD with belumosudil was evaluated in a phase 2a dose-finding study (KD025-208) that included 3 dose cohorts. In that study, belumosudil demonstrated a pooled ORR of 65%, improvements in QOL (as measured by the LSS) and reductions in CS use in subjects with cGVHD after failure of 1 to 3 prior systemic LOTs.25 Given these positive results, we designed and conducted a pivotal randomized phase 2 study (ROCKstar; KD025-213) to further evaluate the efficacy and safety of belumosudil in subjects with SR cGVHD.
Methods
Subject eligibility
This phase 2 randomized multicenter study enrolled subjects at 28 centers in the United States. Eligible subjects were alloHCT recipients aged ≥12 years with persistent cGVHD manifestations after receiving 2 to 5 prior systemic LOTs. Subjects were required to be receiving stable CS therapy for 2 weeks prior to screening and to have a Karnofsky or Lansky Performance Status Scale score ≥ 60. Certain concurrent immunosuppressive medications were allowed, because drug-drug interactions were not anticipated. Subjects were excluded if they had a relapse of their underlying malignancy, had a forced expiratory volume in 1 second (FEV1) ≤ 39% or an NIH lung symptom score of 3, had developed posttransplant lymphoproliferative disease, had liver transaminases (aspartate aminotransferase [AST] or alanine transaminase [ALT]) >3 times the upper limit of normal, had a total bilirubin > 1.5 times the upper limit of normal for any reason, or were currently receiving ibrutinib.
Study design and oversight
Screening for eligibility was conducted within 14 days of cycle 1 day 1. Treatment consisted of belumosudil 200 mg daily or 200 mg twice daily administered orally in subjects with cGVHD. Randomization was stratified (1:1) by cGVHD severity and prior exposure to ibrutinib. Belumosudil was administered continuously until clinically significant progression of cGVHD or unacceptable toxicity. Progression was defined using an organ-specific cGVHD response assessment, as defined by the 2014 NIH Consensus Development Project on Criteria for Clinical Trials in cGVHD, referred to as the 2014 NIH Consensus Criteria. After ≥2 weeks on belumosudil, CS therapy could be tapered at the discretion of the investigator.
This study was supported by Kadmon Corporation, who provided the study drug, conducted quality assurance, developed the analysis plan, analyzed the results, and funded professional writing assistance. The study protocol (supplemental Appendix; available on the Blood Web site) was approved by the institutional review board/independent ethics committee at each center, and written informed consent was provided by all subjects. The study was conducted in accordance with the principles of the Declaration of Helsinki and Good Clinical Practice guidelines (NCT03640481).
Study end points
The primary end point was best ORR at any time, defined as the proportion of subjects who achieved complete response (CR) or partial response (PR) according to the 2014 NIH Consensus Criteria.26,27 All responses were assessed by the study site investigators.
Secondary end points included duration of response (DOR), time to response, changes in LSS summary score, failure-free survival (FFS), CS dose reductions, and overall survival (OS). DOR was measured from the time of initial PR or CR until documented progression from best response of cGVHD, time from initial response to start of additional systemic cGVHD therapy, or death. The 7-day LSS summary score was calculated based on the developer recommendations and was compared with the score from baseline28; an improvement ≥7 points was considered clinically meaningful.29 FFS was defined as the interval between the start of belumosudil and the addition of a new cGVHD therapy, relapse, or NRM. The safety of belumosudil was evaluated by adverse event (AE) and serious AE (SAE) assessments. Relative dose intensity (RDI) was used as a surrogate measure of drug tolerability and was defined as actual dose intensity/planned dose intensity, where dose intensity was defined as the cumulative dose over the duration of exposure (mg/d). Actual dose intensity captured the sum of actual doses received over the duration of exposure and incorporated dose reductions and/or interruptions.
Statistical analysis
The sample size was based on the primary efficacy end point (best ORR), with 1 planned interim analysis and a target ORR of 55%. With a target sample size of 63 subjects per treatment arm and an estimated 10% dropout rate, each treatment arm was estimated to have ∼90% power to yield a 95% confidence interval (CI) of ORR that excluded 30% as the lower bound. Based on consultation with key opinion leaders, a 30% ORR was considered clinically meaningful in this heavily pretreated population with cGVHD and unmet medical needs. The Hochberg procedure was used for multiplicity adjustment for the primary end point of best ORR. The primary analysis was conducted using the modified intent-to-treat (mITT) population, defined as randomized subjects who received ≥1 dose of belumosudil. Interim, primary, and follow-up analyses were planned at ∼2, 6, and 12 months, respectively, after 126 subjects were enrolled in the mITT population. Here, we report data from the 12-month analysis.
Results
Subject characteristics
A total of 132 subjects was enrolled between October of 2018 and August of 2019; data through 19 August 2020 are reported.
Overall, baseline demographics and clinical characteristics were comparable across treatment arms (Table 1). At enrollment, the median subject age was 56 years (range, 21-77). The median time from cGVHD diagnosis to enrollment was 28 months (range, 2-162). Thirty-one percent of subjects had moderate cGVHD at screening, and 67% had severe cGVHD, based on the 2014 NIH Consensus Criteria; 52% had involvement of ≥4 organs. Thirty-six percent of subjects had lung involvement at baseline, with 38% of these subjects having an NIH lung symptom score of 2. Subjects were previously treated with a median of 3 systemic LOTs. Seventy-two percent of subjects (n = 79) had cGVHD refractory to their last systemic LOT, 34% (n = 45) had previously received ibrutinib, 29% (n = 38) had previously received ruxolitinib, and 72% (n = 95) had received ≥3 prior LOTs. The baseline median CS dose was 0.2 mg/kg per day (range, 0.03-1.07) of prednisone equivalent. The baseline mean CS dose was 0.25 mg/kg per day (range, 0.03-1.07) of prednisone equivalent.
Baseline demographics and clinical characteristics
| Characteristic . | Belumosudil, 200 mg daily (n = 66) . | Belumosudil, 200 mg twice daily (n = 66) . | Total (N = 132) . |
|---|---|---|---|
| Age, median (range), y | 53 (21-77) | 57 (21-77) | 56 (21-77) |
| Males | 42 (64) | 33 (50) | 75 (57) |
| Indication for transplant | |||
| AML | 28 (42) | 25 (38) | 53 (40) |
| ALL | 7 (11) | 12 (18) | 19 (14) |
| MDS | 8 (12) | 5 (8) | 13 (10) |
| CML | 5 (8) | 3 (5) | 8 (6) |
| Myelofibrosis | 3 (5) | 2 (3) | 5 (4) |
| CLL | 2 (3) | 2 (3) | 4 (3) |
| Non-Hodgkin lymphoma and DLBCL | 3 (5) | 4 (7) | 7 (5) |
| Other | 7 (11) | 11 (17) | 18 (14) |
| Conditioning intensity | |||
| Myeloablative | 41 (62) | 42 (64) | 83 (63) |
| Nonmyeloablative | 22 (33) | 22 (33) | 44 (33) |
| Unknown | 3 (5) | 2 (3) | 5 (4) |
| Stem cell source | |||
| Peripheral blood | 57 (86) | 63 (96) | 120 (91) |
| Bone marrow | 6 (9) | 3 (5) | 9 (7) |
| Cord blood | 0 | 0 | 0 |
| Unknown | 3 (5) | 0 | 3 (2) |
| HLA matching of donor/recipient | |||
| Matched | 57 (86) | 62 (94) | 119 (90) |
| Partially matched | 8 (12) | 3 (5) | 11 (8) |
| Unknown | 0 | 1 (2) | 1 (1) |
| Missing | 1 (2) | 0 | 1 (1) |
| CMV-positive serostatus (donor/recipient) | |||
| +/+ | 23 (35) | 16 (24) | 39 (30) |
| i) +/− | 3 (5) | 8 (12) | 11 (8) |
| ii) −/+ | 18 (27) | 17 (26) | 35 (27) |
| iii) −/− | 13 (20) | 16 (24) | 29 (22) |
| 1 unknown | 3 (5) | 3 (5) | 6 (5) |
| Unknown/unknown | 5 (8) | 6 (9) | 11 (8) |
| Missing | 1 (2) | 0 | 1 (1) |
| iv) Time from cGVHD diagnosis to enrollment, median (range), mo | 25 (2-162) | 30 (4-144) | 29 (2-162) |
| NIH cGVHD severity* | |||
| Severe | 46 (70) | 43 (65) | 89 (67) |
| Moderate | 18 (27) | 23 (35) | 41 (31) |
| Mild | 2 (3) | 0 | 2 (2) |
| Organ involvement | |||
| No. of organs involved, median (range) | 4 (0-7) | 4 (2-7) | 4 (0-7) |
| ≥4 organs involved | 33 (50) | 35 (53) | 68 (52) |
| Skin | 55 (83) | 55 (83) | 110 (83) |
| Joints/fascia | 51 (77) | 49 (74) | 100 (76) |
| Eyes | 48 (73) | 49 (74) | 97 (74) |
| Mouth | 30 (46) | 42 (64) | 72 (55) |
| Lungs | 24 (36) | 23 (35) | 47 (36) |
| Esophagus | 19 (29) | 12 (18) | 31 (24) |
| Upper GI | 13 (20) | 10 (15) | 23 (17) |
| Lower GI | 6 (9) | 7 (11) | 13 (10) |
| Liver | 9 (14) | 4 (6) | 13 (10) |
| Baseline global severity rating | |||
| 0 | 1 (2) | 0 | 1 (1) |
| 1 | 0 | 0 | 0 |
| 2 | 2 (3) | 1 (2) | 3 (2) |
| 3 | 3 (5) | 2 (3) | 5 (4) |
| 4 | 8 (12) | 3 (5) | 11 (8) |
| 5 | 6 (9) | 8 (12) | 14 (11) |
| 6 | 12 (18) | 14 (21) | 26 (20) |
| 7 | 11 (17) | 20 (30) | 31 (24) |
| 8 | 19 (29) | 14 (21) | 33 (25) |
| 9 | 4 (6) | 3 (5) | 7 (5) |
| 10 | 0 | 1 (2) | 1 (1) |
| Median Karnofsky Performance Status | |||
| 60-70 | 10 (15) | 19 (29) | 29 (22) |
| 80-90 | 52 (79) | 43 (65) | 95 (72) |
| 100 | 4 (6) | 4 (6) | 8 (6) |
| Prior therapy characteristics | |||
| Median prior LOTs, n | 3 | 4 | 3 |
| 2 prior LOTs | 23 (35) | 14 (21) | 37 (28) |
| 3 prior LOTs | 13 (20) | 17 (26) | 30 (23) |
| 4 prior LOTs | 15 (23) | 14 (21) | 29 (22) |
| 5 prior LOTs | 14 (21) | 19 (29) | 33 (25) |
| ≥6 prior LOTs | 1 (2) | 2 (3) | 3 (2) |
| Refractory to prior LOT | 44 (79) | 35 (65) | 79 (72) |
| Prior systemic cGVHD therapy type | |||
| CS (prednisone) | 65 (99) | 65 (99) | 130 (99) |
| Tacrolimus | 40 (61) | 42 (64) | 82 (62) |
| ECP | 31 (47) | 32 (49) | 63 (48) |
| Sirolimus | 29 (44) | 33 (50) | 62 (47) |
| Ibrutinib | 22 (33) | 23 (35) | 45 (34) |
| Ruxolitinib | 20 (30) | 18 (27) | 38 (29) |
| MMF | 18 (27) | 15 (23) | 33 (25) |
| Rituximab | 15 (23) | 13 (20) | 28 (21) |
| MTX | 3 (5) | 3 (5) | 6 (5) |
| Cyclosporine | 4 (6) | 1 (2) | 5 (4) |
| Imatinib | 3 (5) | 1 (2) | 4 (3) |
| Ixazomib | 0 | 1 (2) | 1 (1) |
| Ofatumumab | 0 | 1 (2) | 1 (1) |
| Concomitant systemic cGVHD therapy type† | |||
| CS | 65 (99) | 66 (100) | 131 (99) |
| CNI | 24 (36) | 25 (38) | 49 (37) |
| ECP | 17 (26) | 22 (33) | 39 (30) |
| Sirolimus | 17 (26) | 18 (27) | 35 (27) |
| MMF | 11 (17) | 2 (3) | 13 (10) |
| Imatinib | 1 (2) | 1 (2) | 2 (2) |
| Rituximab | 1 (2) | 0 | 1 (1) |
| Ruxolitinib | 1 (2) | 0 | 1 (1) |
| Other systemic cGVHD therapies | 9 (14) | 13 (20) | 22 (17) |
| Prednisone-equivalent dose at enrollment, median (range), mg/kg/d | 0.20 (0.03-0.95) | 0.20 (0.03-1.07) | 0.20 (0.03-1.07) |
| Characteristic . | Belumosudil, 200 mg daily (n = 66) . | Belumosudil, 200 mg twice daily (n = 66) . | Total (N = 132) . |
|---|---|---|---|
| Age, median (range), y | 53 (21-77) | 57 (21-77) | 56 (21-77) |
| Males | 42 (64) | 33 (50) | 75 (57) |
| Indication for transplant | |||
| AML | 28 (42) | 25 (38) | 53 (40) |
| ALL | 7 (11) | 12 (18) | 19 (14) |
| MDS | 8 (12) | 5 (8) | 13 (10) |
| CML | 5 (8) | 3 (5) | 8 (6) |
| Myelofibrosis | 3 (5) | 2 (3) | 5 (4) |
| CLL | 2 (3) | 2 (3) | 4 (3) |
| Non-Hodgkin lymphoma and DLBCL | 3 (5) | 4 (7) | 7 (5) |
| Other | 7 (11) | 11 (17) | 18 (14) |
| Conditioning intensity | |||
| Myeloablative | 41 (62) | 42 (64) | 83 (63) |
| Nonmyeloablative | 22 (33) | 22 (33) | 44 (33) |
| Unknown | 3 (5) | 2 (3) | 5 (4) |
| Stem cell source | |||
| Peripheral blood | 57 (86) | 63 (96) | 120 (91) |
| Bone marrow | 6 (9) | 3 (5) | 9 (7) |
| Cord blood | 0 | 0 | 0 |
| Unknown | 3 (5) | 0 | 3 (2) |
| HLA matching of donor/recipient | |||
| Matched | 57 (86) | 62 (94) | 119 (90) |
| Partially matched | 8 (12) | 3 (5) | 11 (8) |
| Unknown | 0 | 1 (2) | 1 (1) |
| Missing | 1 (2) | 0 | 1 (1) |
| CMV-positive serostatus (donor/recipient) | |||
| +/+ | 23 (35) | 16 (24) | 39 (30) |
| i) +/− | 3 (5) | 8 (12) | 11 (8) |
| ii) −/+ | 18 (27) | 17 (26) | 35 (27) |
| iii) −/− | 13 (20) | 16 (24) | 29 (22) |
| 1 unknown | 3 (5) | 3 (5) | 6 (5) |
| Unknown/unknown | 5 (8) | 6 (9) | 11 (8) |
| Missing | 1 (2) | 0 | 1 (1) |
| iv) Time from cGVHD diagnosis to enrollment, median (range), mo | 25 (2-162) | 30 (4-144) | 29 (2-162) |
| NIH cGVHD severity* | |||
| Severe | 46 (70) | 43 (65) | 89 (67) |
| Moderate | 18 (27) | 23 (35) | 41 (31) |
| Mild | 2 (3) | 0 | 2 (2) |
| Organ involvement | |||
| No. of organs involved, median (range) | 4 (0-7) | 4 (2-7) | 4 (0-7) |
| ≥4 organs involved | 33 (50) | 35 (53) | 68 (52) |
| Skin | 55 (83) | 55 (83) | 110 (83) |
| Joints/fascia | 51 (77) | 49 (74) | 100 (76) |
| Eyes | 48 (73) | 49 (74) | 97 (74) |
| Mouth | 30 (46) | 42 (64) | 72 (55) |
| Lungs | 24 (36) | 23 (35) | 47 (36) |
| Esophagus | 19 (29) | 12 (18) | 31 (24) |
| Upper GI | 13 (20) | 10 (15) | 23 (17) |
| Lower GI | 6 (9) | 7 (11) | 13 (10) |
| Liver | 9 (14) | 4 (6) | 13 (10) |
| Baseline global severity rating | |||
| 0 | 1 (2) | 0 | 1 (1) |
| 1 | 0 | 0 | 0 |
| 2 | 2 (3) | 1 (2) | 3 (2) |
| 3 | 3 (5) | 2 (3) | 5 (4) |
| 4 | 8 (12) | 3 (5) | 11 (8) |
| 5 | 6 (9) | 8 (12) | 14 (11) |
| 6 | 12 (18) | 14 (21) | 26 (20) |
| 7 | 11 (17) | 20 (30) | 31 (24) |
| 8 | 19 (29) | 14 (21) | 33 (25) |
| 9 | 4 (6) | 3 (5) | 7 (5) |
| 10 | 0 | 1 (2) | 1 (1) |
| Median Karnofsky Performance Status | |||
| 60-70 | 10 (15) | 19 (29) | 29 (22) |
| 80-90 | 52 (79) | 43 (65) | 95 (72) |
| 100 | 4 (6) | 4 (6) | 8 (6) |
| Prior therapy characteristics | |||
| Median prior LOTs, n | 3 | 4 | 3 |
| 2 prior LOTs | 23 (35) | 14 (21) | 37 (28) |
| 3 prior LOTs | 13 (20) | 17 (26) | 30 (23) |
| 4 prior LOTs | 15 (23) | 14 (21) | 29 (22) |
| 5 prior LOTs | 14 (21) | 19 (29) | 33 (25) |
| ≥6 prior LOTs | 1 (2) | 2 (3) | 3 (2) |
| Refractory to prior LOT | 44 (79) | 35 (65) | 79 (72) |
| Prior systemic cGVHD therapy type | |||
| CS (prednisone) | 65 (99) | 65 (99) | 130 (99) |
| Tacrolimus | 40 (61) | 42 (64) | 82 (62) |
| ECP | 31 (47) | 32 (49) | 63 (48) |
| Sirolimus | 29 (44) | 33 (50) | 62 (47) |
| Ibrutinib | 22 (33) | 23 (35) | 45 (34) |
| Ruxolitinib | 20 (30) | 18 (27) | 38 (29) |
| MMF | 18 (27) | 15 (23) | 33 (25) |
| Rituximab | 15 (23) | 13 (20) | 28 (21) |
| MTX | 3 (5) | 3 (5) | 6 (5) |
| Cyclosporine | 4 (6) | 1 (2) | 5 (4) |
| Imatinib | 3 (5) | 1 (2) | 4 (3) |
| Ixazomib | 0 | 1 (2) | 1 (1) |
| Ofatumumab | 0 | 1 (2) | 1 (1) |
| Concomitant systemic cGVHD therapy type† | |||
| CS | 65 (99) | 66 (100) | 131 (99) |
| CNI | 24 (36) | 25 (38) | 49 (37) |
| ECP | 17 (26) | 22 (33) | 39 (30) |
| Sirolimus | 17 (26) | 18 (27) | 35 (27) |
| MMF | 11 (17) | 2 (3) | 13 (10) |
| Imatinib | 1 (2) | 1 (2) | 2 (2) |
| Rituximab | 1 (2) | 0 | 1 (1) |
| Ruxolitinib | 1 (2) | 0 | 1 (1) |
| Other systemic cGVHD therapies | 9 (14) | 13 (20) | 22 (17) |
| Prednisone-equivalent dose at enrollment, median (range), mg/kg/d | 0.20 (0.03-0.95) | 0.20 (0.03-1.07) | 0.20 (0.03-1.07) |
Unless otherwise noted, data are n (%). Percentages may not add to 100% because of rounding.
ALL, acute lymphocytic leukemia; AML, acute myelogenous leukemia; CLL, chronic lymphocytic leukemia; CML, chronic myelogenous leukemia; CMV, cytomegalovirus; DLBCL, diffuse large B-cell lymphoma; GI, gastrointestinal; MDS, myelodysplastic syndrome; MMF, mycophenolate mofetil; MTX, methotrexate.
Disease severity was determined using NIH Global Severity of cGVHD scoring.
Classified as concomitant systemic cGVHD medications on cycle 1 day 1.
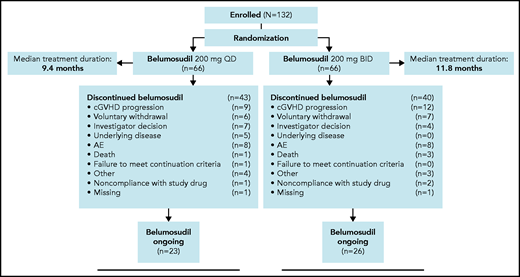
The CONSORT diagram (Figure 1) shows subject disposition. The median duration of treatment was 10 months (range, 0.4-22.0), and the median follow-up was 14 months (range, 1-22). Forty-four percent of subjects had received treatment for ≥12 months. At the time of the data analysis, 37% of subjects continued to receive belumosudil. Reasons for discontinuation included progression of cGVHD (n = 21), voluntary withdrawal (n = 13), AEs (n = 16), physician decision (n = 11), progression of underlying malignancy (n = 5), death due to underlying malignancy or disease progression (n = 4), other (n = 7), and nonadherence to study drug (n = 3).
CONSORT flowchart diagram of enrollment and randomization of subjects with cGVHD. Treatment consisted of oral belumosudil 200 mg daily and 200 mg twice daily in subjects with cGVHD. Randomization was stratified by cGVHD severity and prior exposure to ibrutinib. Reasons for discontinuation are shown in the following figure. Subjects were treated until clinically significant progression of cGVHD or unacceptable toxicity.
CONSORT flowchart diagram of enrollment and randomization of subjects with cGVHD. Treatment consisted of oral belumosudil 200 mg daily and 200 mg twice daily in subjects with cGVHD. Randomization was stratified by cGVHD severity and prior exposure to ibrutinib. Reasons for discontinuation are shown in the following figure. Subjects were treated until clinically significant progression of cGVHD or unacceptable toxicity.
Efficacy
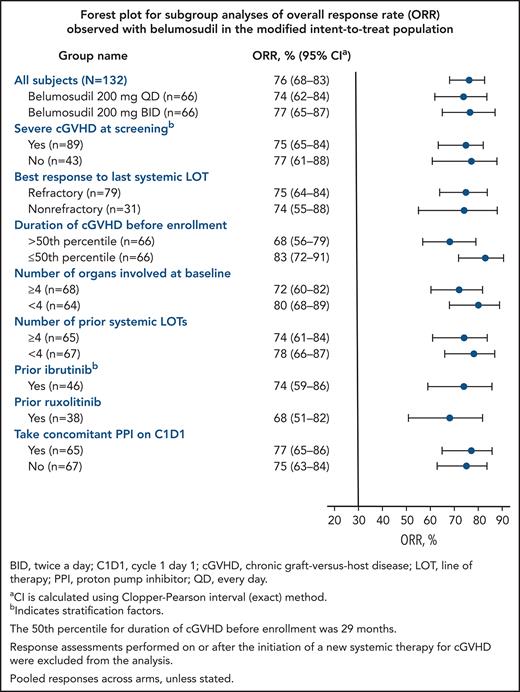
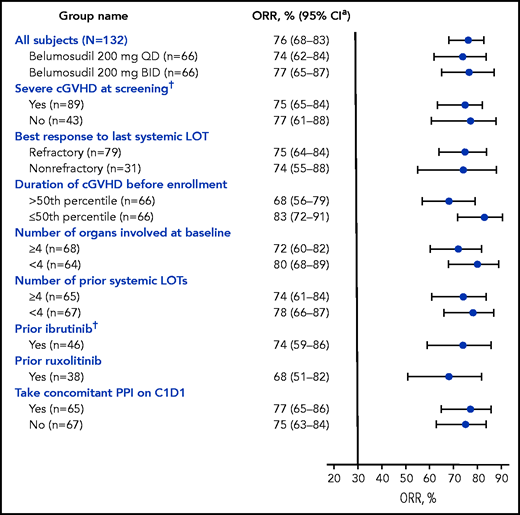
The best ORR for belumosudil 200 mg daily and 200 mg twice daily was 74% (95% CI, 62- 84) and 77% (95% CI, 65-87), respectively (Table 2). High ORRs (61-85%) were observed in all subgroups (Figure 2). Efficacy of belumosudil was maintained, irrespective of prior ibrutinib (n = 46) or ruxolitinib (n = 38) therapy. The ORR for the subgroup with prior ruxolitinib therapy was 68% (95% CI, 51-83). The ORR (95% CI) for the subgroup with prior ibrutinib therapy was 74% (95% CI, 59-86).
Efficacy end points in both arms within mITT population
| Efficacy end point . | Belumosudil, 200 mg daily (n = 66) . | Belumosudil, 200 mg twice daily (n = 66) . | Total (N = 132) . |
|---|---|---|---|
| ORR | 49 (74) | 51 (77) | 100 (76) |
| 95% CI | 62-84 | 65-87 | 68-83 |
| ORR for responses occurring within 6 mo of treatment | 47 (71) | 48 (73) | 95 (72) |
| 95% CI | 59-82 | 60-83 | 64-80 |
| CR | 2 (3) | 1 (2) | 3 (2) |
| PR | 45 (68) | 47 (71) | 92 (70) |
| ORR for responses occurring within 12 mo of treatment | 49 (74) | 50 (76) | 99 (75) |
| 95% CI | 62-84 | 64-86 | 67-82 |
| CR | 4 (6) | 2 (3) | 6 (5) |
| PR | 45 (68) | 48 (73) | 93 (71) |
| Clinically significant improvement from baseline (LSS)* | |||
| Overall | 39 (59) | 41 (62) | 80 (61) |
| Responder, n/N (%) | 34/49 (69) | 36/51 (71) | 70/100 (70) |
| Nonresponder, n/N (%) | 5/17 (29) | 5/15 (33) | 10/32 (31) |
| FFS at 6 mo (95% CI), % | 73 (61-83) | 76 (63-84) | 75 (66-81) |
| FFS at 12 mo (95% CI), % | 57 (44-68) | 56 (43-67) | 56 (47-64) |
| Proportion with CS reduction | 42 (64) | 44 (67) | 86 (65) |
| Median CS reduction from baseline to greatest reduction, % | 38 | 50 | 50 |
| Mean change in CS dose from baseline, % | |||
| Overall | −43 | −48 | −45 |
| Responder | −49 | −58 | −54 |
| Nonresponder | −22 | −10 | −16 |
| CS discontinuation | 13 (20) | 15 (23) | 28 (21) |
| Efficacy end point . | Belumosudil, 200 mg daily (n = 66) . | Belumosudil, 200 mg twice daily (n = 66) . | Total (N = 132) . |
|---|---|---|---|
| ORR | 49 (74) | 51 (77) | 100 (76) |
| 95% CI | 62-84 | 65-87 | 68-83 |
| ORR for responses occurring within 6 mo of treatment | 47 (71) | 48 (73) | 95 (72) |
| 95% CI | 59-82 | 60-83 | 64-80 |
| CR | 2 (3) | 1 (2) | 3 (2) |
| PR | 45 (68) | 47 (71) | 92 (70) |
| ORR for responses occurring within 12 mo of treatment | 49 (74) | 50 (76) | 99 (75) |
| 95% CI | 62-84 | 64-86 | 67-82 |
| CR | 4 (6) | 2 (3) | 6 (5) |
| PR | 45 (68) | 48 (73) | 93 (71) |
| Clinically significant improvement from baseline (LSS)* | |||
| Overall | 39 (59) | 41 (62) | 80 (61) |
| Responder, n/N (%) | 34/49 (69) | 36/51 (71) | 70/100 (70) |
| Nonresponder, n/N (%) | 5/17 (29) | 5/15 (33) | 10/32 (31) |
| FFS at 6 mo (95% CI), % | 73 (61-83) | 76 (63-84) | 75 (66-81) |
| FFS at 12 mo (95% CI), % | 57 (44-68) | 56 (43-67) | 56 (47-64) |
| Proportion with CS reduction | 42 (64) | 44 (67) | 86 (65) |
| Median CS reduction from baseline to greatest reduction, % | 38 | 50 | 50 |
| Mean change in CS dose from baseline, % | |||
| Overall | −43 | −48 | −45 |
| Responder | −49 | −58 | −54 |
| Nonresponder | −22 | −10 | −16 |
| CS discontinuation | 13 (20) | 15 (23) | 28 (21) |
Unless otherwise noted, data are n (%).
Changes in cGVHD symptom burden were measured using LSS. Clinically meaningful improvement in symptom burden was defined as a decrease ≥ 7 points in LSS score.
Forest plot of subgroup analyses of ORR (mITT). High ORRs were observed in all subgroups analyzed in the mITT population, and efficacy was maintained irrespective of prior treatments. The 50th percentile for duration of cGVHD before enrollment was 29 months. Response assessments performed on or after the initiation of a new systemic therapy for cGVHD were excluded from the analysis. Pooled responses across arms, unless stated otherwise. *CI was calculated using the Clopper-Pearson interval (exact) method. †Indicates stratification factors. C1D1, cycle 1 day 1; PPI, proton pump inhibitor.
Forest plot of subgroup analyses of ORR (mITT). High ORRs were observed in all subgroups analyzed in the mITT population, and efficacy was maintained irrespective of prior treatments. The 50th percentile for duration of cGVHD before enrollment was 29 months. Response assessments performed on or after the initiation of a new systemic therapy for cGVHD were excluded from the analysis. Pooled responses across arms, unless stated otherwise. *CI was calculated using the Clopper-Pearson interval (exact) method. †Indicates stratification factors. C1D1, cycle 1 day 1; PPI, proton pump inhibitor.
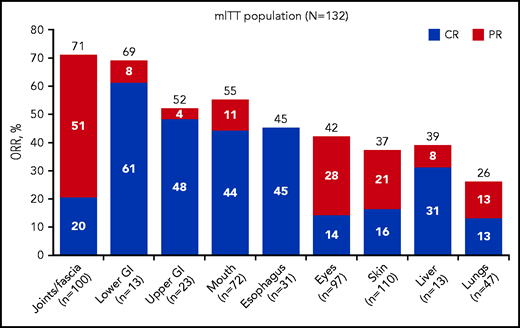
Best ORR, including CR, was evaluated across all affected organs. In the mITT population, organ-specific analyses demonstrated a best ORR of 37% in the skin, 42% in the eyes, 55% in the mouth, 39% in the liver, 26% in the lungs, 71% in the joints/fascia, 52% in the upper gastrointestinal (GI) tract, 69% in the lower GI tract, and 45% in the esophagus (Figure 3; supplemental Table 1). Overall, 7 subjects achieved CR in all affected organs. Of the 12 subjects with lung responses, 3 were scored as CR based on normalization of FEV1 (median increase, 23%; range, 18-25), with an additional 3 CRs based on a reduction in NIH lung symptom score from 1 to 0 in the absence of pulmonary function tests. Six additional subjects had PR, with a >10% increase in FEV1 (median increase for all subjects achieving PR, 10%; range, 0-15) or a reduction in NIH lung symptom score of 1 point when pulmonary function tests were unavailable. Of the 41 subjects with skin responses, 11 had a decrease in sclerotic features, 15 had a decrease in body surface area involvement, and 13 had improvements in body surface area involvement and sclerotic features. Two subjects had skin responses according to the investigator’s clinical assessment, not according to the 2014 NIH Consensus Criteria.
ORR by organ system in the mITT population. Organ-specific analyses in the mITT population demonstrated ORRs in the skin, eyes, mouth, liver, lungs, joints/fascia, upper GI tract, lower GI tract, and esophagus. CR was seen across all affected organs.
ORR by organ system in the mITT population. Organ-specific analyses in the mITT population demonstrated ORRs in the skin, eyes, mouth, liver, lungs, joints/fascia, upper GI tract, lower GI tract, and esophagus. CR was seen across all affected organs.
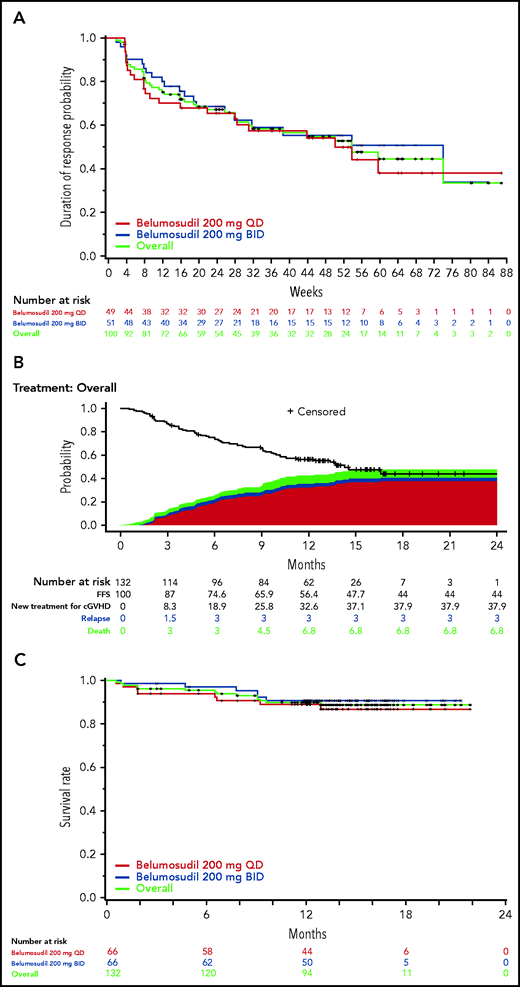
Responses were rapid, with an overall median time to response of 5 weeks (range, 4-66) (Figure 4). Ninety-one percent of responses occurred within 6 months of treatment, with the remaining 9% of responses seen after 6 to 12 months of treatment. Fifty-nine percent of responders maintained responses for ≥20 weeks. The median DOR was 54 weeks in the responder population. The overall FFS rate was 75% (95% CI, 66-81) and 56% (95% CI, 47-64) at 6 and 12 months, respectively (Figure 4). Overall, low rates of NRM (7%) and relapse (3%) were observed. The most common failure event was the initiation of a new systemic cGVHD therapy (38%). The 2-year OS rate was 89% (95% CI, 82-93) (Figure 4).
Durability of response to belumosudil by dose. (A) Kaplan-Meier plot of DOR in the responder population. DOR was defined as the time from response until documented progression or start of another cGVHD systemic treatment; durability data continue to mature. (B) Kaplan-Meier curves of estimated FFS in the mITT population, including reasons for failure. FFS was defined as the absence of cGVHD treatment change, NRM, and recurrent malignancy. (C) Kaplan-Meier curves of estimated OS in the mITT population.
Durability of response to belumosudil by dose. (A) Kaplan-Meier plot of DOR in the responder population. DOR was defined as the time from response until documented progression or start of another cGVHD systemic treatment; durability data continue to mature. (B) Kaplan-Meier curves of estimated FFS in the mITT population, including reasons for failure. FFS was defined as the absence of cGVHD treatment change, NRM, and recurrent malignancy. (C) Kaplan-Meier curves of estimated OS in the mITT population.
During treatment with belumosudil, 65% of subjects reduced their CS dose. The mean CS dose was reduced by 45% in the mITT population, with a mean CS dose reduction of 54% in responders. Twenty-one percent of subjects discontinued CS therapy. In addition, 22% of those subjects successfully discontinued CNI therapy, and 20% and 21% of subjects discontinued sirolimus and mycophenolate mofetil, respectively.
Clinically meaningful improvement (≥7-point reduction) in 7-day LSS summary score from baseline with belumosudil 200 mg daily and 200 mg twice daily was observed in 59% and 62% of the mITT population, respectively. This improvement was observed in 69% and 71% of responders in the belumosudil 200-mg daily and 200-mg twice-daily arms, respectively, as well as in 29% and 33% of nonresponders, respectively.
Safety
Belumosudil was well tolerated, with a median RDI of 99.7%. Eighty-one percent of subjects received an RDI >95%.
AEs were consistent with those expected in patients with cGVHD receiving CS therapy and other ISTs (Table 3). Thirty-eight percent of subjects had ≥1 SAE; the most common was pneumonia (7%). The most common (≥5%) grade 3 or 4 AEs were pneumonia (8%), hypertension (6%), and hyperglycemia (5%). Twenty-four percent of subjects had increased liver function tests (LFTs); at baseline, 5% of subjects had increased γ-glutamyltransferase (GGT), 5% of subjects had increased AST, 3% of subjects had increased ALT, 3% of subjects had increased LFTs, and 1% of subjects had increased bilirubin. The most common liver-related AE was increased GGT (12%). Of the 83 subjects who discontinued treatment, 28 (21%) discontinued because of overall AEs, 16 (12%) discontinued because of possible drug-related AEs, 5 (4%) discontinued because of progression of underlying malignant disease, and 21 (16%) discontinued because of progression of cGVHD. Fourteen subjects died during the study; 2 from multiorgan failure and infection possibly related to belumosudil, 2 from cardiac arrest, 2 from respiratory failure, 1 from hemothorax resulting from lung biopsy, 1 from acute myeloid leukemia recurrence, and 6 during long-term follow-up (>28 days after last dose). Grade ≥3 anemia was reported in 3% of subjects, neutropenia was reported in 2% of subjects, and thrombocytopenia was reported in 2% of subjects. There was 1 case of Epstein-Barr viremia that required treatment and 1 case of cytomegalovirus (CMV) reactivation; both were unrelated to belumosudil treatment.
Safety overview
| AE . | Belumosudil, 200 mg daily (n = 66) . | Belumosudil, 200 mg twice daily (n = 66) . | Total (N = 132) . |
|---|---|---|---|
| Any AE | 65 (99) | 66 (100) | 131 (99) |
| Grade ≥3 AEs | 37 (56) | 34 (52) | 71 (54) |
| Drug-related AEs | 49 (74) | 40 (61) | 89 (67) |
| SAEs | 27 (41) | 23 (35) | 50 (38) |
| Deaths* | 8 (12) | 6 (9) | 14 (11) |
| Drug-related SAEs | 5 (8) | 2 (3) | 7 (5) |
| All grades in ≥20% of subjects (overall) | |||
| Fatigue | 30 (46) | 20 (30) | 50 (38) |
| Diarrhea | 23 (35) | 21 (32) | 44 (33) |
| Nausea | 23 (35) | 18 (27) | 41 (31) |
| Cough | 20 (30) | 17 (26) | 37 (28) |
| Upper respiratory tract infection | 17 (26) | 18 (27) | 35 (27) |
| Dyspnea | 21 (32) | 12 (18) | 33 (25) |
| Headache | 13 (20) | 18 (27) | 31 (24) |
| Peripheral edema | 17 (26) | 13 (20) | 30 (23) |
| Vomiting | 18 (27) | 10 (15) | 28 (21) |
| Muscle spasms | 13 (20) | 13 (20) | 26 (20) |
| Grade ≥3 in ≥5% of subjects in either arm | |||
| Pneumonia | 6 (9) | 4 (6) | 10 (8) |
| Hypertension | 4 (6) | 4 (6) | 8 (6) |
| Hyperglycemia | 3 (5) | 3 (5) | 6 (5) |
| Liver-related AEs | 12 (18) | 19 (29) | 31 (24) |
| GGT increased | 6 (9) | 10 (15) | 16 (12) |
| AST increased | 5 (8) | 8 (12) | 13 (10) |
| ALT increased | 4 (6) | 7 (11) | 11 (8) |
| Blood alkaline phosphatase increased | 4 (6) | 6 (9) | 10 (8) |
| Hypoalbuminemia | 2 (3) | 2 (3) | 4 (3) |
| Transaminases increased | 1 (2) | 1 (2) | 2 (2) |
| Bilirubin conjugated increased | 1 (2) | 0 | 1 (1) |
| LFT increased | 1 (2) | 0 | 1 (1) |
| AE . | Belumosudil, 200 mg daily (n = 66) . | Belumosudil, 200 mg twice daily (n = 66) . | Total (N = 132) . |
|---|---|---|---|
| Any AE | 65 (99) | 66 (100) | 131 (99) |
| Grade ≥3 AEs | 37 (56) | 34 (52) | 71 (54) |
| Drug-related AEs | 49 (74) | 40 (61) | 89 (67) |
| SAEs | 27 (41) | 23 (35) | 50 (38) |
| Deaths* | 8 (12) | 6 (9) | 14 (11) |
| Drug-related SAEs | 5 (8) | 2 (3) | 7 (5) |
| All grades in ≥20% of subjects (overall) | |||
| Fatigue | 30 (46) | 20 (30) | 50 (38) |
| Diarrhea | 23 (35) | 21 (32) | 44 (33) |
| Nausea | 23 (35) | 18 (27) | 41 (31) |
| Cough | 20 (30) | 17 (26) | 37 (28) |
| Upper respiratory tract infection | 17 (26) | 18 (27) | 35 (27) |
| Dyspnea | 21 (32) | 12 (18) | 33 (25) |
| Headache | 13 (20) | 18 (27) | 31 (24) |
| Peripheral edema | 17 (26) | 13 (20) | 30 (23) |
| Vomiting | 18 (27) | 10 (15) | 28 (21) |
| Muscle spasms | 13 (20) | 13 (20) | 26 (20) |
| Grade ≥3 in ≥5% of subjects in either arm | |||
| Pneumonia | 6 (9) | 4 (6) | 10 (8) |
| Hypertension | 4 (6) | 4 (6) | 8 (6) |
| Hyperglycemia | 3 (5) | 3 (5) | 6 (5) |
| Liver-related AEs | 12 (18) | 19 (29) | 31 (24) |
| GGT increased | 6 (9) | 10 (15) | 16 (12) |
| AST increased | 5 (8) | 8 (12) | 13 (10) |
| ALT increased | 4 (6) | 7 (11) | 11 (8) |
| Blood alkaline phosphatase increased | 4 (6) | 6 (9) | 10 (8) |
| Hypoalbuminemia | 2 (3) | 2 (3) | 4 (3) |
| Transaminases increased | 1 (2) | 1 (2) | 2 (2) |
| Bilirubin conjugated increased | 1 (2) | 0 | 1 (1) |
| LFT increased | 1 (2) | 0 | 1 (1) |
All data are n (%).
Six subjects died during long-term follow-up (>28 days after last dose).
Discussion
There remains a clear unmet medical need for targeted and tolerable treatment options in SR cGVHD.11,19 The ROCKstar Study demonstrated promising efficacy and a favorable safety profile for belumosudil therapy in patients with SR cGVHD. The study population, consisting of subjects with severe cGVHD with multiorgan involvement and fibrotic manifestations who were treated after a median of 3 prior systemic LOTs, achieved best ORRs of 74% and 77% in the 200-mg daily and twice-daily treatment arms, respectively.
Responses to belumosudil were sustained and clinically meaningful, regardless of response to prior treatment, severity of cGVHD, and number of organs involved. Responses were observed in all organs, which was clinically significant because CR and PR were achieved in difficult-to-treat organs, such as the lungs and liver, as well as in organs with fibrotic manifestations, such as the skin. cGVHD greatly impairs QOL,6 especially in patients with fibrotic multiorgan involvement, which can be challenging to treat.13,14 The CR and PR observed, along with improvements in LSS, limited interactions, and lack of drug toxicities, are promising results that demonstrate that belumosudil treatment may have the potential to improve overall patient well-being. Seven subjects achieved CR in all affected organs. CR in all affected organs can be difficult to achieve in cGVHD because of the irreversible changes that occur in several organs, most notably the eyes and the lungs.30
The clinical benefit and tolerability of belumosudil therapy demonstrate the potential to halt the expected cycling of therapies for cGVHD seen in clinical practice. Responses were sustained in 59% of responders for ≥20 weeks at the 12-month analysis. The median DOR was 54 weeks in responders at the 12-month analysis.
In a patient population that is vulnerable to AEs and infections from IST,11,19 belumosudil was well tolerated, allowing most subjects to remain on therapy to achieve clinically meaningful results and improvement in QOL, which could be maintained with continued treatment. Only 12% of subjects discontinued belumosudil because of possible drug-related AEs. The median duration of treatment was 10 months (range, 0.4-22.0), and 37% of subjects continued to receive belumosudil after this time point. AEs were manageable, with few grade ≥ 3 SAEs attributable to belumosudil. The SAE rates were comparable between the 2 treatment arms. Many current cGVHD treatment options are immunosuppressive and, consequently, increase the risk of infection13,31 and may cause hematologic toxicities, including leukopenia, anemia, and thrombocytopenia.31-33 Grade ≥ 3 cytopenias were present in <4% of subjects, and there was only 1 report of CMV reactivation that was unrelated to belumosudil treatment. In our clinical practice experience, cytopenias and CMV infection present as serious complications of cGVHD and cGVHD therapeutics; thus, the low rates of grade ≥3 cytopenias and CMV infection rates are promising features of the safety profile of belumosudil.
A limitation of this study was that all subjects received belumosudil. Requiring randomization to best available therapy was not deemed appropriate, because subjects had previously progressed following ≥2 systemic LOTs, where response rates were historically low. Indeed, subjects in this study had already attempted a median of 3 prior lines of best available therapy for cGVHD before enrollment, with the use of ECP (48%), ibrutinib (34%), ruxolitinib (29%), and rituximab (21%), among other agents. The best ORR was 75% in subjects who were refractory to their last LOT.
The search for a more effective and tolerable therapy specifically designed for the treatment of cGVHD has increasingly focused on novel agents that target suspected pathophysiologic pathways.34 Selective ROCK2 inhibition has been shown to impact fibrotic manifestations of cGVHD, which can be difficult to manage.13,22 Belumosudil was first studied in animal models, where it showed efficacy in treating sclerotic skin and bronchiolitis obliterans manifestations, 2 highly morbid phenotypes.22 The safety and efficacy of belumosudil in cGVHD was further established with the KD025-208 dose-finding study.25 Dosing with belumosudil is convenient because of its oral formulation, few drug-drug interactions, and lack of significant AEs, which was confirmed in this study by the high proportion of subjects taking belumosudil for an extended duration. Controlling inflammatory Th17 cells while enhancing regulatory T cells with belumosudil is expected to control cGVHD without significantly increasing immunosuppression, thus sparing responses to infectious pathogens. Based on the similar efficacy and safety observed in this study, 200 mg daily is the preferred dosage for the treatment of SR cGVHD. Although the 200-mg twice-daily dose showed higher responses in certain organs, such as the skin, and slightly fewer AEs, the difference compared with the 200-mg daily dose was not deemed significant. Because of its unique mechanism of action, belumosudil may have broad therapeutic potential beyond cGVHD and is currently being studied in systemic sclerosis, with plans for additional studies in other immune disorders.
Acknowledgments
The authors thank all of the subjects, caregivers, health care professionals, ROCKstar investigators, and employees of Kadmon Corporation who were involved in the ROCKstar study.
This study was supported by Kadmon Corporation, who funded medical writing and editorial assistance, which was provided by Angelli Chua (senior medical writer) and Lindsay Hock (senior medical editor) at RevHealth. Clinical data and statistical support were provided by Zhongming Yang (Director of Biostatistics, Kadmon Corporation).
Authorship
Contribution: C.C., S.J.L., Z.D., M. Jagasia, and S.P. conceived and designed the study, analyzed and interpreted data, and wrote the manuscript and approved its final version; S. Arai collected and assembled data and wrote the manuscript and approved its final version; M.R., B.Z., A.R., A. Salhotra, W.C.-H.. R.M., T.W., M.A., I.P., A. Saad, N.N.S., S. Abhyankar, C.B., J.G., A.I., A. Langston, J.L., M. Juckett, A. Logan, L.S., A.A., D.H. conceived and designed the study, analyzed and interpreted data, and approved the final version of the manuscript; A. Lazaryan, D.E., S.K.A., O.S., L.G., Z.Y. conceived and designed the study, collected, assembled, analyzed, and interpreted data, and wrote and approved the final version of the manuscript; H.W.W. conceived and designed the study, collected and assembled data, and approved the final version of the manuscript; J.R. and H.K. conceived and designed the study, collected and assembled data, and wrote the manuscript and approved its final version; J.I. analyzed and interpreted data and wrote the manuscript and approved its final version; B.R.B. conceived and designed the study and wrote the manuscript and approved its final version; and C.C., S. Arai, Z.D., M. Jagasia, and B.R.B. agreed to be accountable for all aspects of the work, which includes ensuring that questions related to accuracy or integrity of any part of the work are appropriately investigated and resolved.
Conflict-of-interest disclosure: C.C. is a consultant and advisor for Janssen, Mesoblast, Syndax Pharmaceuticals Inc, Omeros, Incyte Corporation, Jazz Pharmaceuticals, Mallinckrodt, CareDx, and Pfizer; has been a pro bono consultant for Kadmon Corporation; and has not received any payment for consulting in the past year. S.J.L. is on a steering committee for Incyte Corporation and has received research funding from Amgen, AstraZeneca, Incyte Corporation, Kadmon Corporation, Novartis Pharmaceuticals Corporation, Pfizer, Syndax Pharmaceuticals Inc., and Takeda. A. Lazaryan is a consultant and advisor for, and has received honoraria from, EUSA Pharma Inc. and has limited equities in Pfizer and Bristol Myers Squibb. A.R. is a consultant and advisor for Amgen and Takeda. Z.D. is a consultant and advisor for Syndax Pharmaceuticals Inc and has received research funding from Incyte Corporation and REGiMMUNE Corporation. A. Salhotra is a consultant and advisor for Syros Pharmaceuticals and Kadmon Corporation and has received research funding from Bristol Myers Squibb. R.M. has received research funding from CSL Behring, Incyte Corporation, and Kadmon Corporation. M.A. is a consultant and advisor for Fate Therapeutics and has received research funding from Pharmacyclics, Kadmon Corporation, and Syndax Pharmaceuticals Inc. I.P. is a consultant and advisor for Syndax Pharmaceuticals Inc, Incyte Corporation, and Kadmon Corporation. A. Saad is a consultant and advisor for Incyte Corporation, CareDx, and Magenta Therapeutics and has received research funding from Amgen, Kadmon Corporation, and Orca Bio. N.N.S. is a consultant and advisor for Eli Lilly, Kite Pharma, Celgene, Legend Biotech, Epizyme, and TG Therapeutics; has received honoraria and/or travel support from Incyte Corporation, Celgene, Eli Lilly, and Miltenyi Biotec; has equity ownership in Exelixis and Geron Corporation; and has received research funding from Eli Lilly and Miltenyi Biotec. S. Abhyankar is a consultant and advisor for Elixell Therapeutics and has received research funding from THERAKOS, Incyte Corporation, and Genentech. C.B. is a consultant and advisor for Novartis Pharmaceuticals Corporation, AlloVir, CRISPR Therapeutics, Bristol Myers Squibb, Juno Therapeutics, and Kadmon Corporation. A.I. is a consultant and advisor for Incyte Corporation. A. Langston has received research funding from Kadmon Corporation, Incyte Corporation, Novartis Pharmaceuticals Corporation, Astellas Pharma, and Bristol Myers Squibb. J.L. has received honoraria from Onconova Therapeutics and Syros Pharmaceuticals. A. Logan is a consultant and advisor for Agios Pharmaceuticals, Amgen, and Pfizer and has received research funding from Autolus Therapeutics, Jazz Pharmaceuticals, Kadmon Corporation, Kite Pharma, and Pharmacyclics. D.H. is a consultant and advisor for Jazz Pharmaceuticals and has received research funding from Radiation Oncology Institute as a public health sciences collaborator for the adolescent and young adult population. H.W.W., J.R., D.E., J.I., O.S., L.G., Z.Y., and H.K. have stock options in and are employees of Kadmon Corporation. S.K.A. has stock options in Kadmon Corporation, was a full-time employee of Kadmon Corporation at the beginning of the study, and now consults for Kadmon Corporation. M. Jagasia is a consultant and advisor for Kadmon Corporation and Incyte Corporation, has received honorarium from Kadmon Corporation, and has stock options in and is an employee of Iovance Biotherapeutics. B.R.B. is a cofounder of Tmunity Therapeutics, is a consultant and advisor for Magenta Therapeutics and Blue Rock Therapeutics and has received research funding from Blue Rock Therapeutics, Children’s Cancer Research Fund, and Kids First Fund. S.P. received research support from the Center for Cancer Research at the National Cancer Institute through the National Institutes of Health Intramural Research Program, which includes Clinical Research Development Agreements with Celgene, Actelion, Eli Lilly, Pharmacyclics and Kadmon Corporation. The remaining authors declare no competing financial interests.
Correspondence: Corey Cutler, Dana-Farber Cancer Institute, 450 Brookline Ave, Boston, MA 02215; e-mail: corey_cutler@dfci.harvard.edu.
Proposals for access to original data should be sent to Corey Cutler (corey_cutler@dfci.harvard.edu). Data on individual participants will not be shared. Deidentified patient-level data are available only with an appropriate request and research statement. The study protocol is included as a data supplement available with the online version of this article.
The online version of this article contains a data supplement.
There is a Blood Commentary on this article in this issue.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
REFERENCES
Author notes
B.R.B. and S.P. contributed equally to this study.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal