TO THE EDITOR:
With enhanced testing availability and evolution of therapeutic strategies, survival of COVID-19–infected patients has improved over time.1-3 Two large series have reported outcomes for patients with chronic lymphocytic leukemia (CLL) infected with COVID-19 from February through May 2020, reporting case fatality rates (CFRs) of 31% to 33%.4,5 Whether patients with CLL have experienced improvement in outcomes over time, as observed in the general population, has remained unknown. To understand change in outcomes over time, we present this follow-up study, which builds upon a previously reported cohort with extended follow up and addition of more recently diagnosed cases.
Emergency use authorization has been granted by the US Food and Drug Administration for several agents for treatment of COVID-19,6-9 and dexamethasone has demonstrated an overall survival (OS) benefit for COVID-19–infected patients requiring oxygen.10,11 These therapeutic studies have included few patients with hematological malignancies, and disease-specific outcomes have not been presented. Given the possible differences in immune response and risk of infection, understanding the benefit of these therapies in a CLL-specific population is crucial.
Early data from a small series suggest that patients with CLL may not consistently generate anti-SARS-CoV-2 antibodies after infection.12 This finding, along with previous reports of inadequate response to vaccines in patients with CLL,13-19 highlight significant questions regarding COVID-19 vaccine efficacy in this population.
In this retrospective study, investigators from 45 centers identified patients with CLL diagnosed with COVID-19 based on polymerase chain reaction detection of SARS-CoV-2 from 17 February 2020 through 1 February 2021. Institutional review board approvals were granted, and the study was conducted in accordance with the Declaration of Helsinki.
A uniform case report form was used to collect baseline demographics, comorbidities, CLL-directed treatment history, date of COVID-19 diagnosis, and the COVID-19 clinical course and management strategy. Information regarding anti-SARS-CoV-2 serology testing during routine care was collected if performed; the specific antibody tested was not mandated or recorded.
Our primary purpose was to report the CFR for a larger group of patients with CLL who were diagnosed with COVID-19 and had a longer follow-up. We further sought to report the CFR stratified by date (the “early cohort,” diagnosed from 17 February through 30 April 2020 and the “later cohort,” diagnosed from 1 May 2020 through 1 February 2021; dates selected to mirror population-based studies1,2), examine outcomes for patients who received specific COVID-19–directed therapies, and describe serology testing results for those tested in routine clinical care.
OS was estimated using the Kaplan-Meier method.20 Univariable Cox regression analyses adjusted for potential confounders were performed (Stata, v 621) to evaluate the relationship between baseline characteristics and COVID-19–directed therapies and OS.
This analysis included 374 patients with CLL who were diagnosed with COVID-19. With median follow-up of 38 days (range, 1-364 days) for the entire group and 63.5 days for survivors (range, 1-364), the CFR was 28%. Hospital admission was required for 75%, and intensive care unit (ICU) admission was required for 27%. Supplemental oxygen was used for 68% of the patients and mechanical ventilation was necessary for 20%. For patients who were admitted to the hospital, the CFR was 36% (99 of 278), whereas it was 4.3% (4 of 92) for those who were not admitted. Age >75 years and cumulative illness rating scale-geriatric (CIRS)22 >6 were independent predictors of poor survival. Sex, hypogammaglobulinemia, and CLL-directed treatment (including a history of any treatment, current treatment, current Bruton tyrosine kinase inhibitor (BTKi) therapy, and prior lines of therapy) were not associated with survival (supplemental Table 1, available on the Blood Web site).
To examine trends over time, we compared updated information for 254 patients diagnosed from 17 February through 30 April 2020 (early cohort) to data for 120 patients more recently diagnosed from 1 May 2020 through 1 February 2021 (later cohort). Comparison of baseline characteristics and markers of COVID-19 severity in these 2 cohorts are presented in Table 1. A larger proportion of patients in the early cohort were admitted (85% vs 55%) and required ICU admission (32% vs 15%). The CFR in the early cohort was 35% vs 11% in the later cohort (P < .001). For patients requiring hospitalization, the CFR was 40% (86 of 213) in the early cohort and 20% (13 of 65) in the later cohort (P = .003). For those who required oxygen, the CFR was 44% vs 25% (P = .015). The proportion of hospitalized patients requiring ICU-level care was lower in the later cohort (37% in early cohort vs 29% in the later cohort), whereas the CFR remained high for the subset of patients who required ICU-level care (52% vs 50%; P = .89). A difference in management of BTKi-treated patients was observed in the early vs the later cohort. In the early cohort, 76% of patients receiving BTKi had their drug therapy suspended or discontinued. In the later cohort, only 20% of BTKi-treated patients had their therapy suspended or discontinued.
Baseline characteristics and COVID-19 management
| . | Early cohort (n = 254) . | Later cohort (n = 120) . | Entire cohort (N = 374) . | |||
|---|---|---|---|---|---|---|
| . | Proportion, unless otherwise specified, % . | Patients with available data, n . | Proportion, unless otherwise specified, % . | Patients with available data, n . | Proportion, unless otherwise specified, % . | Patients with available data, n . |
| Baseline characteristics | ||||||
| Age at CLL diagnosis, median in years (range) | 62.5 (31 - 92) | 248 | 59 (29-86) | 119 | 61 (29-92) | 367 |
| Age at COVID-19 diagnosis, median in years (range) | 70 (36 - 98) | 254 | 65.5 (29-93) | 120 | 68 (29-98) | 374 |
| Male | 64 | 254 | 65 | 120 | 64 | 374 |
| White | 86 | 249 | 85 | 116 | 85 | 365 |
| CIRS,22 median (range) | 8 (4-32) | 229 | 8 (4-21) | 117 | 8 (4-32) | 346 |
| CLL treatment history | 253 | 119 | 372 | |||
| Never treated | 44 | — | 47 | — | 45 | — |
| Prior therapy | 56 | — | 53 | — | 55 | — |
| Lines of therapy for previously treated patients, median (range) | 1.5 (1-8) | 136 | 1 (1-7) | 61 | 1 (1-8) | 197 |
| Receiving therapy at time of COVID-19 diagnosis | 39 | 254 | 34 | 119 | 38 | 373 |
| Receiving BTK inhibitor at time of COVID-19 diagnosis | 29 | 253 | 21 | 119 | 26 | 372 |
| Receiving venetoclax at time of COVID-19 diagnosis | 8 | 253 | 9 | 119 | 8 | 372 |
| COVID-19 management | ||||||
| Admitted | 85 | 252 | 55 | 119 | 75 | 371 |
| ICU admission | 32 | 250 | 15 | 107 | 27 | 357 |
| Imaging performed | 89 | 245 | 60 | 108 | 80 | 353 |
| Pneumonia on imaging | 88 | 224 | 62 | 82 | 81 | 306 |
| Supplemental oxygen | 78 | 250 | 45 | 112 | 68 | 362 |
| Mechanical ventilation | 25 | 246 | 9 | 111 | 20 | 357 |
| Steroids administered | 42 | 244 | 39 | 112 | 41 | 356 |
| . | Early cohort (n = 254) . | Later cohort (n = 120) . | Entire cohort (N = 374) . | |||
|---|---|---|---|---|---|---|
| . | Proportion, unless otherwise specified, % . | Patients with available data, n . | Proportion, unless otherwise specified, % . | Patients with available data, n . | Proportion, unless otherwise specified, % . | Patients with available data, n . |
| Baseline characteristics | ||||||
| Age at CLL diagnosis, median in years (range) | 62.5 (31 - 92) | 248 | 59 (29-86) | 119 | 61 (29-92) | 367 |
| Age at COVID-19 diagnosis, median in years (range) | 70 (36 - 98) | 254 | 65.5 (29-93) | 120 | 68 (29-98) | 374 |
| Male | 64 | 254 | 65 | 120 | 64 | 374 |
| White | 86 | 249 | 85 | 116 | 85 | 365 |
| CIRS,22 median (range) | 8 (4-32) | 229 | 8 (4-21) | 117 | 8 (4-32) | 346 |
| CLL treatment history | 253 | 119 | 372 | |||
| Never treated | 44 | — | 47 | — | 45 | — |
| Prior therapy | 56 | — | 53 | — | 55 | — |
| Lines of therapy for previously treated patients, median (range) | 1.5 (1-8) | 136 | 1 (1-7) | 61 | 1 (1-8) | 197 |
| Receiving therapy at time of COVID-19 diagnosis | 39 | 254 | 34 | 119 | 38 | 373 |
| Receiving BTK inhibitor at time of COVID-19 diagnosis | 29 | 253 | 21 | 119 | 26 | 372 |
| Receiving venetoclax at time of COVID-19 diagnosis | 8 | 253 | 9 | 119 | 8 | 372 |
| COVID-19 management | ||||||
| Admitted | 85 | 252 | 55 | 119 | 75 | 371 |
| ICU admission | 32 | 250 | 15 | 107 | 27 | 357 |
| Imaging performed | 89 | 245 | 60 | 108 | 80 | 353 |
| Pneumonia on imaging | 88 | 224 | 62 | 82 | 81 | 306 |
| Supplemental oxygen | 78 | 250 | 45 | 112 | 68 | 362 |
| Mechanical ventilation | 25 | 246 | 9 | 111 | 20 | 357 |
| Steroids administered | 42 | 244 | 39 | 112 | 41 | 356 |
CIRS, cumulative illness rating scale.
Univariable analyses examined associations between administration of specific COVID-19 therapies and OS in all admitted patients and also the subset of admitted patients who required supplemental oxygen (supplemental Tables 2 and 3). Remdesivir (hazards ratio [HR], 0.48; P = .03) and convalescent plasma (HR, 0.50; P = .04) administration were associated with improved OS, whereas admitted patients who received corticosteroids (HR, 1.73, P = .01) and hydroxychloroquine (HR, 1.53; P = .04) had an increased risk of death.
Supplemental Table 4 describes baseline characteristics and the COVID-19 course for those who did vs did not receive corticosteroids. Corticosteroids were associated with increased risk of death when the data were adjusted for admission status (HR, 1.8; 95% confidence interval [CI], 1.2-2.7; P = .007) and the need for mechanical ventilation (HR, 2.0; 95% CI, 1.3-3.1; P = .002), although they were not significantly associated with survival when the data were adjusted for use of supplemental oxygen (HR, 1.4; 95% CI, 0.93-2.2; P = .11). Furthermore, admitted patients treated with corticosteroids in the later cohort did not experience an OS benefit (HR, 2.6; 95% CI ,0.6-11.9; P = .22). Secondary infections were observed in 26% (18 of 69) vs 8% (12 of 154) of those who did vs did not receive corticosteroids and in 26% (26 of 99) vs 8% (6 of 75) of admitted patients who required oxygen and did vs did not receive corticosteroids.
After acute infection, COVID-19 serology was checked in 25% of patients (93 of 374). Of the patients tested, the serology result was positive in 60%, negative in 39%, and equivocal in 1%. The proportions of untreated patients and those treated with BTKi, venetoclax, or hypogammaglobulinemia who developed anti-SARS-CoV-2 antibodies were 74% (29 of 39), 48% (12 of 25), 30% (3 of 10), and 60% (14 of 28), respectively.
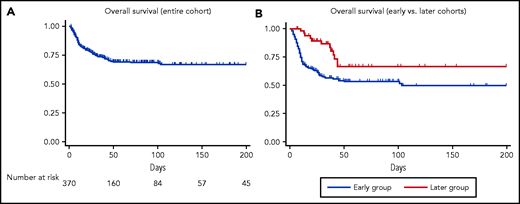
Because the CFR of patients with CLL who were diagnosed with COVID-19 in the spring of 2020 was high (31% to 33%),4,5 we wanted to examine the CFR in a larger cohort with additional follow-up and in a subset of patients diagnosed later in the course of the pandemic. Our findings mirrored population-based studies1-3 with decreasing CFR (35% in those diagnosed before 1 May 2020 vs 11% in those diagnosed after that date). Improvement in OS was also observed in hospitalized patients and in those who required supplemental oxygen, and the proportion of hospitalized patients who needed ICU-level care declined. These trends suggest that patients in the later cohort experienced a less severe clinical course and that the observed difference in CFR over time may not just be due to more frequent testing and identification of less symptomatic patients. (Figure 1).
Overall survival from the time of COVID-19 diagnosis. The entire cohort (A) and stratified by timing of diagnosis (B) for patients who required oxygen.
Overall survival from the time of COVID-19 diagnosis. The entire cohort (A) and stratified by timing of diagnosis (B) for patients who required oxygen.
Although our data corroborate prior studies that demonstrated the benefit of remdesivir6 and the lack of benefit of hydroxychloroquine,23 we interestingly found an OS benefit associated with convalescent plasma24 and a lack of benefit (significantly inferior OS in admitted patients) with corticosteroids.10 Regarding convalescent plasma, patients with CLL have known humoral immunodeficiency, and antibody-based therapies may uniquely benefit this population. The RECOVERY trial demonstrated an OS benefit of dexamethasone in COVID-19 patients requiring oxygen (HR, 0.82; 95% CI, 0.72-0.94) and mechanical ventilation (HR, 0.64; 95% CI, 0.51-0.81).10 In contrast, corticosteroid use was associated with a trend toward inferior OS in patients requiring oxygen and a significant risk of death for intubated patients with CLL. Use of corticosteroids in the earlier cohort may have been reserved for patients with more severe disease, as data regarding use of corticosteroids in COVID-19 were not yet available. Thus, inferior outcomes for steroid-treated patients in this cohort may be an artifact of their use in patients with more severe disease. As RECOVERY trial data were published in July 2020, we hypothesized that patients in the later cohort were more likely to receive corticosteroids in a data-driven, optimal clinical setting. However, corticosteroid use was not associated with improved OS in the later cohort. Although the use of corticosteroids was nonrandomized and is potentially biased by clinical context, the data are hypothesis generating and suggest that COVID-19 directed interventions, particularly immunomodulatory agents, require prospective study, specifically in immunocompromised populations. Although these data are not sufficient to change recommendations for the use of corticosteroids given the demonstrated benefit in a prospective clinical trial, they raise a question about the benefit of immunomodulatory or immunosuppressive therapy in a population at increased risk of infection, as demonstrated in CLL-directed therapeutic trials.
Finally, our multicenter series was consistent with a prior single-center study,12 and 60% of patients with CLL developed positive anti-SARS-CoV-2 serology results after polymerase chain reaction diagnosis of COVID-19. That study is the largest reported series of serologic testing for patients with CLL and adds further evidence that antibody production after COVID-19 is not uniform in patients with CLL. Coupled with prior reports of decreased responses to other vaccines,13-19 further study is ongoing to gain understanding of the immune response to SARS-CoV-2 vaccination in patients with CLL.
Reassuringly, the overall trend in the CFR for patients with CLL mirrors improved OS for patients with COVID-19 in the general population, but the data highlight opportunities for further investigation into optimal management of COVID-19, immune response after infection, and effective vaccination strategy for patients with CLL.
Acknowledgments
This research was supported, in part, by National Institutes of Health, National Cancer Institute Cancer Center support grant P30 CA008748. L.E.R. was supported by an American Society of Hematology Research Training Award for Fellows outside of the submitted work. I.E.A. receives research support from the Intramural Program of the National Heart, Lung, and Blood, Institute and an American Society of Hematology Scholar Award. T.A.E. was supported by the Oxford National Institute for Health Research (NIHR) Biomedical Research Centre.
Authorship
Contribution: L.E.R. was a joint senior principal investigator, responsible for the study design, and data collection, coordination, analysis, and interpretation and writing the manuscript; T.A.E. assisted with the study design, site coordination, data collection and interpretation, and writing and editing the manuscript; M.C.T., N.L., A.R.C., M.S.D., P.O.B., L.L., K.A.R., J.N.A., R.C., A.L.-G., D.A., J.M.P., N.M.-C., J.A.G-.M., J.-A.H.-R., F.M., C.C.C., A.Ö., L.H., A.N.S., J.L.J., M.R.W., D.E.-S., D.W., S.M., T.M., S.V., E.S., P.M.B., J.P., P.E.M.P., G.F.P., S.F.H., H.P., S. Sundaram, A.S., M.K., R.J., H.W., R.W., A.B., S.L., K.M.I., C.A.P., I.E.A., C.S.U., M.S., S. Skånland, and E.A.C. were involved in data collection and interpretation and edited the manuscript; A.R.M. was a joint senior principal investigator and was responsible for study design and data collection, coordination, analysis, and interpretation and writing the manuscript.
Conflict-of-interest disclosure: L.E.R. has served as a consultant for AbbVie, AstraZeneca, Janssen, LOXO Oncology, Pharmacyclics, Pfizer, TG Therapeutics, Vaniam group, and Verastem; holds a minority ownership in Abbott Laboratories; and has received research funding from Aptose Biosciences and Pfizer outside of the submitted work. T.A.E. has received honoraria and advisory board honoraria from Roche, Gilead, Kite, Janssen, AbbVie, AstraZeneca, LOXO Oncology, BeiGene, and Incyte; receives research support from Gilead and AstraZeneca; has received travel support from Gilead, Takeda, and AbbVie; and has served on a trial steering committee for LOXO Oncology. M.C.T. has received honoraria from MJH Life Sciences, VJ Heme Onc, and Curio Science. N.L. reports grants from Loxo Oncology; grants and personal fees from and consultancy and board of directors or advisory board membership for AbbVie, AstraZeneca, BeiGene, and Genentech; personal fees from and consultancy and board of directors or advisory committee membership for Celgene, Gilead, Janssen, and Pharmacyclics; and grants from Juno, Octernal, Verastem, TG Therapeutics, MingSight, and Octapharma, outside the submitted work. M.S.D. reports grants and personal fees from AbbVie, Ascentage Pharma, BMS, Genentech, MEI Pharma, Novartis, Pharmacyclics, Takeda, TG Therapeutics, Verastem, and AstraZeneca; personal fees from Adaptive Biotechnologies, BeiGene, Celgene, Eli Lilly, Gilead Sciences, Janssen, Merck, Research to Practice, and Syros Pharmaceuticals; personal fees from Zentalis; and grants from Surface Oncology, outside the submitted work. L.L. has served on speakers’ bureaus for Seattle Genetics, Celgene/BMS, KitePharma, BeiGene, Pharmacyclics/Janssen, and AstraZeneca and has participated on advisory boards for Bayer, Seattle Genetics, ADC Therapeutics, AbbVie, Janssen, Pharmacyclics, Kite, and AstraZeneca. K.A.R. receives research funding from Genentech, AbbVie, Janssen, and Novartis; has consulted for Acerta Pharma, Genentech, AbbVie, Pharmacyclics, Innate Pharma, and AstraZeneca; and has received travel funding from AstraZeneca. J.N.A. has received research funding from Genentech, Janssen, and Celgene and has served as a consultant to Pharmacyclics, AbbVie, Genentech, AstraZeneca, Sunesis, and Janssen. R.C. serves as a speaker for Roche, Janssen, BMS, AbbVie, and Takeda; serves on advisory boards for Janssen, Celgene, AbbVie, Servier, Kyowa Kirin, and Takeda; and has received travel funding from Roche, Pfizer, Janssen, Celgene, AbbVie, Servier, and Takeda. J.M.P. reports consultancy with LOXO Oncology, AstraZeneca, Gilead, and BeiGene. J.-A.G.-M. has held a consulting or advisory role for AbbVie, AstraZeneca, Janssen, and Roche; has received research funding from AbbVie and Janssen; and has received speakers’ fees from AbbVie, Astra-Zeneca, and Janssen. J.-A.H.-R. serves as a consultant for Janssen, AbbVie, Roche, Gilead, Celgene, Amgen, and Takeda; is on the speakers’ bureau for Janssen, AbbVie, Roche, Gilead, Celgene, AstraZeneca, Amgen, Takeda, and BeiGene; and receives grants and research support from Celgene. C.C.C. reports personal fees from Loxo Oncology, Novartis, AbbVie, Genentech, MEI Pharma, and Octapharma; and payment to her institution for clinical trials from Loxo Oncology, Gilead, H3 Biomedicine, and Incyte. A.Ö. receives grants for academic research from BeiGene, Kancera; holds stock ownership in Kancera; and has served as a consultant for Sanofi. D.E.-S. has received honoraria from AbbVie, AstraZeneca, Janssen, Roche, and Takeda; has received conference/travel support from AbbVie and Novartis; and has served on advisory boards for AbbVie, ASTEX, AstraZeneca, BeiGene, Janssen, and Kyowa Kirin. L.H. has received research funding from Janssen-Cilag and Gilead Sciences. S.M. served as a consultant or advisor for AstraZeneca, BeiGene, Genentech, Pharmacyclics, and Verastem; has served on the speakers’ bureau for AstraZeneca, BeiGene, Janssen, and Pharmacyclics; and has received research funding from AbbVie, AstraZeneca, BeiGene, Juno, LOXO Oncology, Novartis, Pharmacyclics, and TG Therapeutics. P.M.B. has consulted for PCYC/AbbVie, Genentech, Gilead, Merck, Seattle Genetics, Verastem, AstraZeneca, Celgene, and Morphosys. P.E.M.P. serves as a consultant for AbbVie, AstraZeneca, Atura, Gilead, Janssen, Novartis, Roche, and Tolero Pharmaceuticals; has been a remunerated speaker for AbbVie, AstraZeneca, Gilead, Janssen, Novartis, and Roche; has received travel support from AbbVie, Gilead, Janssen, Novartis, and Roche; and has received research funding from Gilead and Roche. S.F.H. has served as a consultant to Celgene, Bayer, Genentech, Pharmacyclics, Novartis, and AbbVie and has received research funding from DTRM Biopharm, Celgene, and TG Therapeutics. S. Sundaram has served as a consultant for Janssen and received research support from Lymphoma Research Foundation. A.S. has served as a consultant for TG Therapeutics, Alexion, Jazz Pharmaceuticals, Pharmacyclics, AstraZeneca, Janssen, Celgene, AbbVie, Genentech, Kite Pharma, and Novartis and is on the speakers’ bureau for TG Therapeutics, Alexion, Jazz Pharmaceuticals, Pharmacyclics, AstraZeneca, Janssen, Celgene, AbbVie, Genentech, Kite Pharma, BeiGene, Verastem, and Seattle Genetics. M.K. has served as a consultant for AZD, Celgene and Pharmacyclics and is on the speaker’s bureau for Seattle Genetics. R.W. reports receiving travel support and serving on speakers’ bureaus for AbbVie, Janssen, and Gilead. A.B. reports receiving travel support and serving on a speaker’s bureau for Gilead. C.S.U. has consulted for Pharmacyclics, AbbVie, and AstraZeneca. M.S. has received research funding from Mustang Bio, Celgene, Pharmacyclics, Gilead, Genentech, AbbVie, TG Therapeutics, BeiGene, Acerta Pharma, and Merck and has served on advisory boards or as a consultant for AbbVie, Genentech, AstraZeneca, Sound Biologics, Verastem, ADC Therapeutics, BMS, and Atara Biotherapeutics. E.A.C. serves on advisory boards for Novartis, Tessa, BMS, and Kite Pharma, and receives research funding from the Lymphoma Research Foundation. A.R.M. has served as a consultant for Celgene, Acerta, and Janssen; has served as a consultant for and received research funding from AbbVie, Loxo, Genentech, Pharmacyclics, AstraZeneca, Sunesis, and Johnson & Johnson; has received research funding from DTRM Biopharma and Gilead; and has served as a consultant for, received research funding from, and is a Data Safety and Monitoring Board member for TG Therapeutics. The remaining authors report no competing financial interests.
Correspondence: Anthony R. Mato, Memorial Sloan Kettering Cancer Center, 1275 York Ave, New York, NY 10065; e-mail: matoa@msckcc.org.
Original data are available by e-mail request to the corresponding author.
The online version of this article contains a data supplement.
There is a Blood Commentary on this article in this issue.
REFERENCES
Author notes
L.E.R. and T.A.E. contributed equally to this study.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal