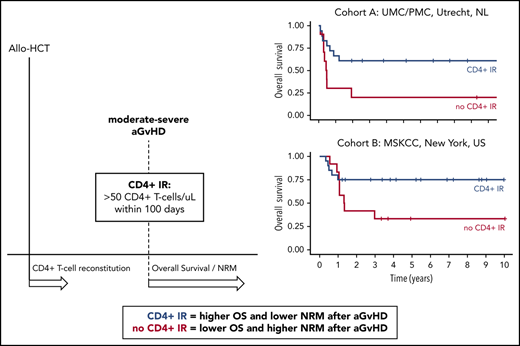
Key Points
Early CD4+ IR predicts OS and NRM after moderate to severe aGVHD.
Approaches to augment early and predictable CD4+ IR could improve survival in patients developing aGVHD.
Abstract
Acute graft-versus-host-Disease (aGVHD) is a major cause of morbidity and mortality after allogeneic hematopoietic cell transplantation (HCT). We previously showed that early CD4+ T-cell immune reconstitution (IR; CD4+ IR) predicts survival after HCT. Here, we studied the relation between CD4+ IR and survival in patients developing aGVHD. Pediatric patients undergoing first allogeneic HCT at University Medical Center Utrecht (UMC)/Princess Máxima Center (PMC) or Memorial Sloan Kettering Cancer Center (MSK) were included. Primary outcomes were nonrelapse mortality (NRM) and overall survival (OS), stratified for aGVHD and CD4+ IR, defined as ≥50 CD4+ T cells per μL within 100 days after HCT or before aGVHD onset. Multivariate and time-to-event Cox proportional hazards models were applied, and 591 patients (UMC/PMC, n = 276; MSK, n = 315) were included. NRM in patients with grade 3 to 4 aGVHD with or without CD4+ IR within 100 days after HCT was 30% vs 80% (P = .02) at UMC/PMC and 5% vs 67% (P = .02) at MSK. This was associated with lower OS without CD4+ IR (UMC/PMC, 61% vs 20%; P = .04; MSK, 75% vs 33%; P = .12). Inadequate CD4+ IR before aGVHD onset was associated with significantly higher NRM (74% vs 12%; P < .001) and inferior OS (24% vs 78%; P < .001). In this retrospective analysis, we demonstrate that early CD4+ IR, a simple and robust marker predictive of outcomes after HCT, is associated with survival after moderate to severe aGVHD. This association must be confirmed prospectively but suggests strategies to improve T-cell recovery after HCT may influence survival in patients developing aGVHD.
Introduction
Acute graft-versus-host-disease (aGVHD) is a life-threatening complication causing high mortality after allogeneic hematopoietic stem cell transplantation (HCT). To limit the risk of aGVHD, all HCT protocols contain some method of prophylaxis for aGVHD, such as cyclosporine A (CsA), mycophenolate mofetil (MMF), prednisolone, or methotrexate after HCT.1 In addition, some protocols include serotherapy for in vivo T-cell depletion (TCD; eg, ATG).1-5 Nevertheless, aGVHD is still common and affects 20% to 60% of patients undergoing allogeneic HCT, depending on particular patient characteristics and treatment protocols.6 Alternative strategies to prevent GVHD in HLA-disparate HCT involve in vivo TCD with posttransplantation cyclophosphamide targeting rapidly activated alloreactive T cells as well as ex vivo TCD techniques, such as CD34+ selection or the more recent α-β T-cell receptor depletion. Recipients of TCD transplants experience relatively low incidence of aGVHD (20% to 30%)7-9 but are prone to high mortality rates when aGVHD treatment strategies fail. A recent outcome review showed that aGVHD still accounts for 10% to 20% of deaths after allogeneic HCT.10
Although knowledge of risk factors for developing GVHD after HCT is currently expanding, there is a need for clinical parameters that can predict the severity of aGVHD and outcomes. Plasma profiles of ST2, REG3α, tumor necrosis factor receptor 1, and interleukin-2 receptor α were recently reported as biomarkers for aGVHD severity and could potentially guide therapy at disease onset.11,12 This is of great interest, because predictors for survival outcomes after aGVHD are scarce. Identifying patients at high risk of severe aGVHD morbidity and mortality may help guide aGVHD treatment and prophylaxis.
Previously, we showed that early CD4+ T-cell immune reconstitution (CD4+ IR) predicts survival after HCT in a variety of transplantation platforms.13-15 CD4+ IR overcomes the aGvHD risk associated with Epstein-Barr virus and human herpesvirus 6 viral reactivation.14 Here, we evaluated the association of CD4+ IR with survival probability after onset of aGVHD in 2 separate centers, the first exclusively performing T-cell replete HCT (bone marrow or cord blood) and the other predominantly ex vivo TCD.
Materials and methods
Patients and blood samples
Pediatric patients undergoing their first allogeneic HCT between 2004 and 2018 within the pediatric transplantation program at the Wilhelmina Children’s Hospital at University Medical Center Utrecht (UMC; 2004-2017) and/or Princess Máxima Center for Pediatric Oncology (PMC; from 2018) in Utrecht, The Netherlands, and between 2008 and 2017 at MSK Kids/Memorial Sloan Kettering Cancer Center (MSK) in New York, New York, who had post-HCT CD4+ T-cell data available were included in this retrospective study. Immune monitoring and clinical outcome data were collected and registered prospectively. The study was approved by local ethical committees and institutional review boards (#05-143 and #11-063k UMC/PMC and #19-379 MSK Kids), with informed consent.
Procedures
Patients were treated in high-efficiency, particle-free, air-filtered, positive-pressure isolation rooms. Conditioning regimens were applied according to center-specific protocols. Patients underwent selective gut decontamination (UMC/PMC) and received infection prophylaxis (UMC/PMC and MSK). At UMC/PMC, GVHD prophylaxis consisted of CsA (targeted at trough levels, 200-250 μg/L for all patients) combined with either 1 mg/kg of prednisolone (cord blood) or 10 mg/m2 of methotrexate (on days +1, +3, and +6; for bone marrow grafts). CsA was continued for at least 3 months after HCT. Prednisolone was tapered after 28 days. At MSK, GVHD prophylaxis consisted of in vitro TCD with CD3 <104/kg (with or without ATG). Thymoglobulin was mostly used as serotherapy in both cohorts. Recipients of conventional transplants received calcineurin inhibitor–based prophylaxis (CsA or tacrolimus) with methotrexate (15 mg/m2 on day +1 and 10 mg/m2 on days +3, +6, and +11) with or without serotherapy. Recipients of cord blood transplants received CsA/MMF prophylaxis. Patients who developed grade 2 to 4 aGVHD were treated with methylprednisolone (2 mg/kg) as first-line therapy. Those who were refractory were treated with best available treatment, mainly mesenchymal stromal cells at both centers but also other therapies (eg, antibodies, adding MMF), depending on availability of mesenchymal stromal cells.
Immune monitoring
At UMC/PMC, absolute numbers of CD4+ T cells (CD3+CD4+CD8−) were measured using TruCOUNT tubes (BD Biosciences, Erembodegem, Belgium)/Sapphire (Abbott) in EDTA-treated whole blood only after leukocyte count reached at least 0.4 × 109 L. Blood CD4+ IR was measured at least every other week up to 12 weeks after HCT and monthly thereafter up to 6 months, followed by every 3 months until 1 year and twice in the second year after HCT. At MSK, absolute numbers of CD4+ T cells (CD3+CD4+CD8−) were measured using a dual-platform method. Flow cytometry was performed on sodium heparin–anticoagulated whole blood with BD FACSCanto flow cytometers using fluorescent antibodies from BD Biosciences, and absolute values were calculated in conjunction with complete blood count results from a Sysmex TX-500 using EDTA-treated whole blood.
Outcomes
Primary outcomes were overall survival (OS) and nonrelapse mortality (NRM), stratified for aGVHD (grade 2-4) or moderate to severe aGVHD (grade 3-4). Presence or absence of adequate CD4+ IR (defined as 2 measurements of CD3+CD4+CD8− ≥50 cells per μL) within the first 100 days after transplantation or before aGVHD onset was investigated as a predictor. NRM was defined as death resulting from a cause other than relapse of malignancy within 5 years of follow-up. OS was defined as time from transplantation to death or last follow-up, with a minimum follow-up of 1 year. GVHD was classified according to the criteria of Glucksberg et al16 and Shulman et al.17
Statistical analysis
Duration of follow-up was defined as the time from HCT to death or last assessment for surviving patients. For the clinical end points OS and NRM, patient (age, sex, indication, cytomegalovirus status recipient) and transplantation characteristics (donor, graft manipulation) as well as CD4+ IR within 100 days after HCT or before aGVHD onset were considered as variables. Definition of CD4+ IR was based on previous studies.13,14,18,19 Probability of OS was calculated using the Kaplan-Meier estimate, and cumulative incidence curves were applied to calculate NRM probability. Relapse was considered a competing risk for NRM. Effect of CD4+ IR within 100 days after HCT on OS and NRM in patients with aGVHD was evaluated with multivariate Cox proportional hazards models and Fine and Gray competing risk models, respectively. Because death and graft failure were competing events for achieving CD4+ IR, we excluded patients without CD4+ IR who died or had a graft failure before median time to CD4+ IR. To evaluate the effect of CD4+ IR before aGVHD onset, we applied a time-to-event Cox proportional hazards model. HCT source (UMC/PMC, cord blood vs bone marrow/peripheral blood stem cells; MSK, TCD or T-cell replete HCT) was considered a covariate for OS and NRM after aGVHD, because this variable is highly likely to affect survival outcomes after aGVHD. Statistical analyses were performed using R software version 3.3 (packages survminer, survival, cmprsk, and flexsurv; R Foundation, Vienna, Austria).
Results
Patients
A total of 591 patients (UMC/PMC, n = 276; MSK, n = 315) were included in this study (Table 1); median age was 7.1 years (range, 0.2-22.7 years) at UMC/PMC and 10.4 years (range, 0.1-35.6 years) at MSK. Incidences of both acute and chronic GVHD were similar at the 2 centers. At UMC/PMC, 73 patients (26.4%) developed grade 2 to 4 aGVHD, and at MSK, 70 patients (22.2%) did so; for stage 3 to 4 aGVHD, cumulative incidences were 29 (10.5%) and 32 patients (10.2%), respectively. In the UMC/PMC cohort, 31 patients (11%) developed chronic GVHD, and in the MSK cohort, 21 patients (7%) did so. Median time to aGVHD was 35 days at UMC/PMC and 55 days at MSK.
Patient characteristics
| . | UMC/PMC (n = 276) . | MSK (n = 315) . |
|---|---|---|
| Median (range) age at transplantation, y | 7.06 (0.16-22.74) | 10.4 (0.1-35.6) |
| Sex, n | ||
| Male | 159 | 188 |
| Female | 117 | 127 |
| HCT source, n | ||
| BM (T-cell replete) | 100 | 61 |
| Unrelated cord blood | 172 | 40 |
| PBSCs | 4 | 6 |
| TCD PBSCs | 0 | 169 |
| TCD BM | 0 | 39 |
| Diagnosis, n | ||
| Malignancy | 139 | 220 |
| Metabolic inborn error | 51 | 1 |
| Hemoglobinopathy | 2 | 11 |
| Primary immune deficiency | 52 | 42 |
| BM failure | 29 | 40 |
| Autoimmune disease | 3 | 1 |
| aGVHD incidence by grade, n | ||
| 2 | 44 | 38 |
| 3 | 19 | 23 |
| 4 | 10 | 9 |
| Chronic (all) GvHD, n | 31 | 18 |
| Extensive | 7 | 11 |
| Conditioning regimen, n | ||
| TBI based | 26 | 112 |
| Chemotherapy based* | 250 | 203 |
| Serotherapy in conditioning, n (%) | 198 (72) | 227 (72) |
| Median (range) time to grade 2-4 aGVHD, d | 34 (6-205) | 55 (14-303) |
| Median (range) follow-up, d | 1293 (14-5017) | 750 (11-3780) |
| . | UMC/PMC (n = 276) . | MSK (n = 315) . |
|---|---|---|
| Median (range) age at transplantation, y | 7.06 (0.16-22.74) | 10.4 (0.1-35.6) |
| Sex, n | ||
| Male | 159 | 188 |
| Female | 117 | 127 |
| HCT source, n | ||
| BM (T-cell replete) | 100 | 61 |
| Unrelated cord blood | 172 | 40 |
| PBSCs | 4 | 6 |
| TCD PBSCs | 0 | 169 |
| TCD BM | 0 | 39 |
| Diagnosis, n | ||
| Malignancy | 139 | 220 |
| Metabolic inborn error | 51 | 1 |
| Hemoglobinopathy | 2 | 11 |
| Primary immune deficiency | 52 | 42 |
| BM failure | 29 | 40 |
| Autoimmune disease | 3 | 1 |
| aGVHD incidence by grade, n | ||
| 2 | 44 | 38 |
| 3 | 19 | 23 |
| 4 | 10 | 9 |
| Chronic (all) GvHD, n | 31 | 18 |
| Extensive | 7 | 11 |
| Conditioning regimen, n | ||
| TBI based | 26 | 112 |
| Chemotherapy based* | 250 | 203 |
| Serotherapy in conditioning, n (%) | 198 (72) | 227 (72) |
| Median (range) time to grade 2-4 aGVHD, d | 34 (6-205) | 55 (14-303) |
| Median (range) follow-up, d | 1293 (14-5017) | 750 (11-3780) |
BM, bone marrow; PBSC, peripheral blood stem cell.
Clofarabine, fludarabine, and busulfan.
In both cohorts, multivariate analysis identified CD4+ IR as a predictor of NRM and OS after moderate to severe aGvHD (Table 2; supplemental Table 1 for univariate analysis, available on the Blood Web site). Achieving CD4+ IR did not decrease the risk of developing grade 2 to 4 aGVHD, which occurred in 26% of patients with vs 27% without CD4+ IR (P = .16) at UMC/PMC and in 21% of those with vs 22% without CD4+ IR (P = .77) at MSK. Nevertheless, CD4+ IR was related to lower grade 3 to 4 aGVHD risk in the UMC/PMC cohort, occurring in 9% of patients with vs 26% without CD4+ IR (P = .005), but not in the MSK cohort, where it occurred in 9.6% of those with vs 12% without CD4+ IR (P = .23). However, outcomes for those who developed aGVHD with vs without CD4+ IR differed. Patients with aGVHD who achieved CD4+ IR within 100 days after HCT had comparable NRM and survival chances to patients without aGVHD (UMC/PMC: NRM, 21% vs 18%; P = .65; OS, 63% vs 76%; P = .19; MSK: NRM, 5% vs 9%; P = .99; OS, 61% vs 76%; P = .43).
OS and NRM after grade 2-4 and 3-4 aGVHD
| aGVHD . | UMC/PMC: CD4+ IR vs no CD4+ IR within first 100 d, % . | P . | MSK: CD4+ IR vs no CD4+ IR within first 100 d, % . | P . | UMC/PMC: CD4+ IR vs no CD4+ IR before aGVHD diagnosis, % . | P . |
|---|---|---|---|---|---|---|
| Grade 2-4 | ||||||
| NRM | 21 vs 43 | .04* | 5 vs 54 | .006** | 18 vs 34 | .006** |
| OS | 63 vs 48 | .13 | 61 vs 36 | .03* | 77 vs 48 | <.001*** |
| Grade 3-4 | ||||||
| NRM | 30 vs 80 | .02* | 5 vs 67 | .02* | 12 vs 74 | <.001*** |
| OS | 61 vs 20 | .04* | 75 vs 33 | .12 | 78 vs 24 | <.001*** |
| aGVHD . | UMC/PMC: CD4+ IR vs no CD4+ IR within first 100 d, % . | P . | MSK: CD4+ IR vs no CD4+ IR within first 100 d, % . | P . | UMC/PMC: CD4+ IR vs no CD4+ IR before aGVHD diagnosis, % . | P . |
|---|---|---|---|---|---|---|
| Grade 2-4 | ||||||
| NRM | 21 vs 43 | .04* | 5 vs 54 | .006** | 18 vs 34 | .006** |
| OS | 63 vs 48 | .13 | 61 vs 36 | .03* | 77 vs 48 | <.001*** |
| Grade 3-4 | ||||||
| NRM | 30 vs 80 | .02* | 5 vs 67 | .02* | 12 vs 74 | <.001*** |
| OS | 61 vs 20 | .04* | 75 vs 33 | .12 | 78 vs 24 | <.001*** |
Stratified by adequate CD4+ IR within first 100 d or before aGVHD diagnosis. Multivariate Cox proportional hazards models were applied.
P < .05, **P < .01, ***P < .001.
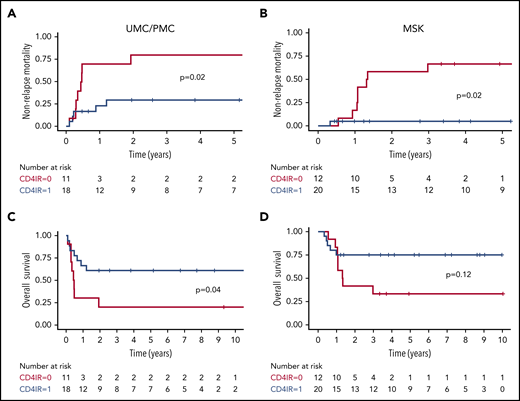
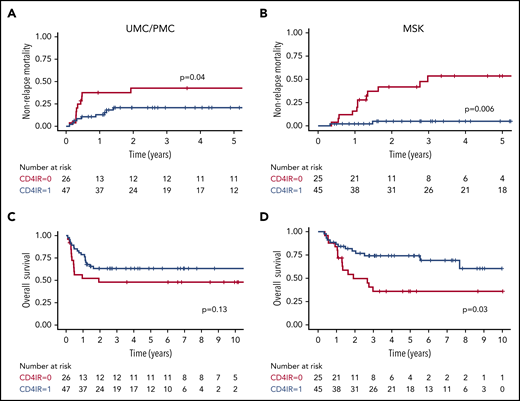
For patients with severe grade 3 to 4 aGVHD, CD4+ IR within the first 100 days after HCT was related to decreased NRM at UMC/PMC (30% vs 80%; P = .02; Figure 1A) and MSK (5% vs 67%; P = .02; Figure 1B). This lower NRM was associated with higher OS of 61% vs 20% (P = .04; Figure 1C) at UMC/PMC and a trend toward higher OS in the MSK cohort (75% vs 33%; P = .12; Figure 1D). For the larger group of patients with grade 2 to 4 aGVHD, these relationships were also significant but less strong in the UMC/PMC cohort, and they were observed in the MSK cohort (UMC/PMC: NRM, 21% vs 43%; P = .04; Figure 2A; OS, 63% vs 48%; P = .13; Figure 2C; MSK: NRM, 5% vs 54%; P = .006; Figure 2B; OS, 61% vs 36%; P = .03; Figure 2D).
NRM and OS after grade 3 to 4 aGVHD, stratified by adequate CD4+IR within the first 100 days after HCT. Cumulative incidence plots showing NRM after grade 3 to 4 aGVHD in the UMC/PMC cohort (A) and the MSK cohort (B). Kaplan-Meier curves showing OS in patients in the UMC/PMC cohort (C) and the MSK cohort (D). CD4+ IR was defined as ≥50 CD4+ T cells per μL in 2 consecutive measurements within the first 100 days after HCT (blue lines) and with no CD4+ IR (red lines).
NRM and OS after grade 3 to 4 aGVHD, stratified by adequate CD4+IR within the first 100 days after HCT. Cumulative incidence plots showing NRM after grade 3 to 4 aGVHD in the UMC/PMC cohort (A) and the MSK cohort (B). Kaplan-Meier curves showing OS in patients in the UMC/PMC cohort (C) and the MSK cohort (D). CD4+ IR was defined as ≥50 CD4+ T cells per μL in 2 consecutive measurements within the first 100 days after HCT (blue lines) and with no CD4+ IR (red lines).
NRM and OS after grade 2 to 4 aGVHD, stratified by adequate CD4+IR within the first 100 days after HCT. Cumulative incidence plots showing NRM after grade 2 to 4 aGVHD in the UMC/PMC cohort (A) and the MSK cohort (B). Kaplan-Meier curves showing OS in patients in the UMC/PMC cohort (C) and the MSK cohort (D). CD4+ IR was defined as ≥50 CD4+ T cells per μL in 2 consecutive measurements within the first 100 days after HCT (blue lines) and with no CD4+ IR (red lines).
NRM and OS after grade 2 to 4 aGVHD, stratified by adequate CD4+IR within the first 100 days after HCT. Cumulative incidence plots showing NRM after grade 2 to 4 aGVHD in the UMC/PMC cohort (A) and the MSK cohort (B). Kaplan-Meier curves showing OS in patients in the UMC/PMC cohort (C) and the MSK cohort (D). CD4+ IR was defined as ≥50 CD4+ T cells per μL in 2 consecutive measurements within the first 100 days after HCT (blue lines) and with no CD4+ IR (red lines).
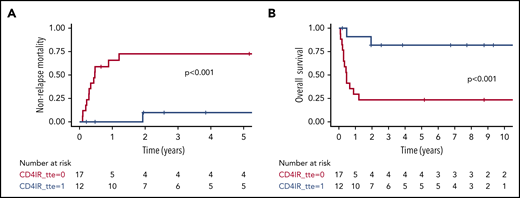
In time-to-event analysis, we evaluated the predictive value of CD4+ IR before aGVHD diagnosis with regard to survival after aGVHD. Because of limited measurements available in the first few weeks after HCT in the MSK cohort, we only included the UMC/PMC cohort in this analysis. CD4+ IR before aGVHD was even more strongly predictive of survival outcomes (grade 3-4 aGVHD: NRM, 12% vs 74%; P < .001; Figure 3A; OS, 78% vs 24%; P < .001; Figure 3B; grade 2-4 aGVHD: NRM, 18% vs 34%; P = .006; Figure 4A; OS, 77% vs 48%; P < .001; Figure 4B). Patients achieving CD4+ IR before aGVHD also had comparable survival to those without aGVHD (NRM, 18% vs 18%; P = .51; OS, 77% vs 76%; P = .56).
NRM and OS after grade 3 to 4 aGVHD, stratified by adequate CD4+IR before aGVHD diagnosis. Cumulative incidence plot showing NRM after grade 3 to 4 aGVHD in the UMC/PMC cohort (A) and Kaplan-Meier curve showing OS (B). CD4+ IR was defined as ≥50 CD4+ T cells per μL in 2 consecutive measurements before aGvHD diagnosis (blue lines) and with no CD4+ IR (red lines).
NRM and OS after grade 3 to 4 aGVHD, stratified by adequate CD4+IR before aGVHD diagnosis. Cumulative incidence plot showing NRM after grade 3 to 4 aGVHD in the UMC/PMC cohort (A) and Kaplan-Meier curve showing OS (B). CD4+ IR was defined as ≥50 CD4+ T cells per μL in 2 consecutive measurements before aGvHD diagnosis (blue lines) and with no CD4+ IR (red lines).
NRM and OS after grade 2 to 4 aGVHD, stratified by adequate CD4+IR before aGVHD diagnosis. Cumulative incidence plot showing NRM after grade 2 to 4 aGVHD in the UMC/PMC cohort (A) and Kaplan-Meier curve showing OS (B). CD4+ IR was defined as ≥50 CD4+ T cells per μL in 2 consecutive measurements before aGvHD diagnosis (blue lines) and with no CD4+ IR (red lines).
NRM and OS after grade 2 to 4 aGVHD, stratified by adequate CD4+IR before aGVHD diagnosis. Cumulative incidence plot showing NRM after grade 2 to 4 aGVHD in the UMC/PMC cohort (A) and Kaplan-Meier curve showing OS (B). CD4+ IR was defined as ≥50 CD4+ T cells per μL in 2 consecutive measurements before aGvHD diagnosis (blue lines) and with no CD4+ IR (red lines).
Discussion
We show that a threshold of CD4+ IR of 50 CD4+ T cells per μL within 100 days after transplantation or before aGVHD onset increases the probability of surviving this severe complication of allogeneic HCT in 2 distinct transplantation strategies (dual-center study). This level of CD4+ IR at time of onset might, therefore, be a useful predictor of mortality in patients with moderate to severe aGVHD. Patients without CD4+ IR who develop aGVHD may need different aGVHD treatment algorithms to improve survival chances. For instance, patients without CD4+ IR might benefit from prolonged aGVHD prophylaxis or more aggressive therapy upon development of aGvHD. It would be of great interest to evaluate CD4+ IR as a predictive marker in a prospective study, especially because this same threshold of adequate CD4+ IR has also been related to lower risks of viral reactivations,14 virus-related morbidity and mortality,14 and relapse.13
The mechanism by which adequate CD4+ IR protects against death after aGVHD may relate to recovery of specific subsets of CD4+ T cells or reflect a more balanced reconstitution in general. It is striking that CD4+ IR shows the same predictive value for surviving aGVHD after conventional HCT as well as after TCD HCT, especially because T-cell reconstitution is highly delayed in the latter, and risk and severity of aGVHD differ.20 With conventional HCT grafts, a higher proportion and early recovery of regulatory T cells (Tregs) and greater T-cell receptor diversity have been related to reduced aGVHD risk and better prognosis.21-25 These associations with aGVHD have not been studied in the setting of TCD or haploidentical HCT, where the mechanisms governing expansion of alloreactive T cells and recovery of Tregs may differ.26 There is a poor understanding of aGVHD development after TCD transplantation,27-30 but expansion of both polyclonal and oligoclonal alloreactive T cells has been demonstrated. Although CD4+ IR did not seem to protect against aGVHD itself in our cohorts, it may translate to lower mortality after severe aGVHD. An explanation might be the induction of Tregs during steroid treatment for GVHD, which requires an adequate amount of CD4+ T cells.31 Possibly, achieving adequate CD4+ IR reflects a balanced T-cell IR with higher Treg counts and broader TCR diversity, resulting in balanced homeostasis and better control of immune dysregulation, thereby decreasing the risk of steroid-refractory aGVHD and subsequent mortality. This would be in line with the observations that CD4+ IR is also associated with better survival after viral reactivation14 and acute myeloid leukemia relapse13 post-HCT (ie, adequate CD4+ IR provides less dysregulation [aGVHD] and at the same time instigates productive immunity). In a subgroup of 15 patients (UMC/PMC cohort), we were able to perform additional Treg analyses. Those with increasing Tregs within 100 days after HCT (n = 11) were survivors, whereas the 4 patients without an increase in Tregs died as a result of a GVHD-related cause (data not shown). This indicates that without CD4+ IR, it is difficult to have an adequate amount of Tregs.
Improving CD4+ IR probability after HCT is an important strategy to enhance HCT success. One of the strategies to improve the chance of achieving adequate CD4+ IR is optimizing and individualizing conditioning regimens to allow for better and more predictable IR. A major factor hampering CD4+ IR is residual ATG exposure (or other serotherapy) after HCT,32,33 which can be limited by targeted dosing.15,34-36 In addition, fludarabine has also recently been shown to affect CD4+ IR probability,37 indicating CD4+ IR might further improve with individualized fludarabine dosing.38 Approaches to enhance expansion of and regulation by CD4+ Tregs include low-dose interleukin-2 therapy39-41 and infusion of graft-derived or third-party Tregs.39-41 Alternatively, sirolimus or other mammalian target of rapamycin inhibitors might be useful, because they can selectively suppress effector memory T cells while expanding Tregs, resulting in better immune regulation.42,43 Effects of such approaches on CD4+ IR and subsequent mortality risk after severe aGvHD in HCT recipients would be of further interest.
In summary, we found that in patients who developed aGVHD, early CD4+ IR (≥50 cells per μL) was associated with reduced GVHD-related mortality. Although this association was found in 2 independent cohorts, the findings are limited by the retrospective nature of this study and the absence of more in-depth characterization of the number, ratio, diversity, and origin of reconstituting T cells. In ongoing and future studies, it will be important to prospectively validate these findings. It could also help us to determine the exact mechanisms by which early adequate CD4+ IR protects against mortality after aGVHD. Unraveling differences in the biology of aGVHD in those with and without CD4+ IR will allow an understanding of how CD4+ IR predicts survival chances after moderate to severe aGVHD and will provide tools to further improve HCT outcomes. In turn, strategies to better predict IR may affect HCT success, including the effect of GVHD-directed treatment.
For original data, please e-mail the corresponding author.
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgment
This work was supported by Children Cancer-free Foundation (KiKa) project #142.
Authorship
Contribution: S.N., J.J.B., J.L., and C.d.K. designed the study; C.d.K. and S.P. wrote the manuscript; C.d.K., J.L., C.S., and I.v.R. analyzed the data; A.H., C.L., J.J.B., and S.N. provided critical comments; and all authors read and approved the final version of the manuscript.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Jaap Jan Boelens, 1275 York Ave (Scholar 417), New York, NY 10065; e-mail: boelensj@mskcc.org.
REFERENCES
Author notes
C.d.K. and S.P. contributed equally to this work as first authors.
S.N. and J.J.B. contributed equally to this work as senior authors.