Key Points
Agkisacucetin β chain contacts GPIbα receptor at the β-switch loop in the LRR domain.
Agkisacucetin α chain contacts GPIbα receptor C-terminal peptide after the LRR domain.
Abstract
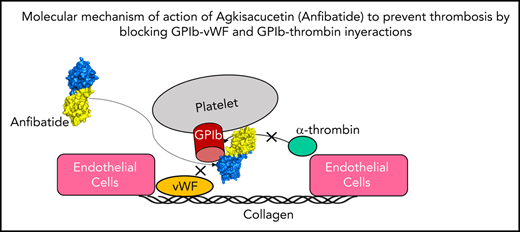
Agkisacucetin, a snake C-type lectin-like protein isolated from the venom of Deinagkistrodon acutus (formerly Agkistrodon acutus), is a novel antithrombotic drug candidate in phase 2 clinical trials. Agkisacucetin specifically recognizes the platelet surface receptor glycoprotein Ib α chain (GPIbα) to block GPIb and von Willebrand factor (VWF). In this study, we solved the crystal structure of the GPIbα N-terminal domain (residues 1-305) in complex with agkisacucetin to understand their molecular recognition mechanism. The crystal structure showed that agkisacucetin primarily contacts GPIbα at the C-terminal part of the conserved leucine-rich repeat (LRR) domain (LRR-6 to LRR-8) and the previously described “β-switch” region through the β chain. In addition, we found that agkisacucetin α chain contacts part of the GPIbα C-terminal peptide after the LRR domain through complementary charge interactions. This C-terminal peptide plays a key role in GPIbα and thrombin recognition. Therefore, our structure revealed that agkisacucetin can sterically block the interaction between the GPIb receptor and VWF and thrombin proteins to inhibit platelet function. Our structural work provides key molecular insights into how an antithrombotic drug candidate recognizes the GPIb receptor to modulate platelet function to inhibit thrombosis.
Introduction
Thrombosis is the most common underlying pathology of the 3 major cardiovascular disorders: ischemic heart disease, stroke, and venous thromboembolism; it is also a frequent complication in patients with COVID-19.1,2 The development of safe and effective antithrombotic drugs is urgently needed. The adhesion of blood platelets to sites of vascular injury is mediated by von Willebrand factor (VWF), a large multimeric plasma glycoprotein that interacts with subendothelial matrix proteins and platelet surface receptor glycoprotein Ib (GPIb) α chain of the GPIb-IX-V complex, particularly at high shear stress.3 Platelets adherent to injured tissues also provide a surface for the assembly of the prothrombinase complex that leads to the generation of α-thrombin. Through its interaction with GPIb on platelets, α-thrombin induces platelet aggregation and activation by signaling through protease-activated receptors. VWF and GPIb are key targets for the development of antithrombotic drugs. Arterial thrombosis still occurs in VWF-knockout mice, but complete vessel occlusion is prevented or postponed.4 In contrast, thrombus formation is completely abolished in GPIb-knockout mice.5
Snake venom C-type lectins (snaclecs) are an important family of proteins that are similar in sequence and structure, but they show diverse effects by binding to different platelet receptors or coagulation factors.6,7 They all share a basic structure of αβ heterodimers linked covalently via a disulfide bond. In contrast to typical C-type lectin proteins, snaclecs do not contain the classic calcium/sugar-binding loop.8 Agkisacucetin, purified from Deinagkistrodon acutus venom, is a simple heterodimeric snaclec that binds to GPIbα and inhibits GPIbα-VWF interaction without causing GPIb clustering and signaling and platelet agglutination.9,10 By targeting the key GPIb receptor, agkisacucetin inhibits ristocetin-induced platelet aggregation and has a beneficial role in ischemic stroke and reperfusion injury without the bleeding side effect. Agkisacucetin is currently in phase 2 clinical trials under the trade name Anfibatide.11 Crystal structures have been reported for agkisacucetin alone, GPIbα alone, and GPIbα-VWF and GPIbα-thrombin complexes.9,12,13 However, it is not clear how precisely agkisacucetin interacts with GPIbα. In this study, we report the first crystal structure of agkisacucetin in complex with GPIbα receptor N-terminal domain to better understand the molecular mechanism of action of agkisacucetin for antithrombotic therapy.
Study design
Binding of agkisacucetin to GPIbα measured by surface plasmon resonance spectroscopy
The binding affinity of agkisacucetin to GPIbα was measured using a Biacore system (Biacore T200; GE Healthcare). The temperature was set at 25°C for all steps. Agkisacucetin and Fc-GPIbα (1-289) wild-type and mutant proteins were dialyzed against 1× phosphate-buffered saline (PBS) and concentrated to 1 mg/mL. According to the Biacore manual, a CM5 chip was immobilized with recombinant agkisacucetin protein at 5 µg/mL in sodium acetate buffer (pH 4.5) using an Amine Coupling Kit (GE Healthcare). The CM5 chip was blocked with 1 M ethanolamine buffer (pH 8.5) to inactivate any remaining succinimide esters. After cross-linking, ∼350 resonance units (RU) of agkisacucetin was coupled to the chip. The recombinant GPIbα-Fc was injected at a flow rate of 50 µL/min for 2 minutes. The CM5 chip was regenerated using 10 mM glycine buffer (pH 2.0). Biacore sensorgrams were analyzed using BIAevaluation software. The binding signals at a sensor surface are expressed in RU. One RU is equivalent to 1 picogram of protein per square millimeter on the sensor surface.
Immunoprecipitation experiment
α-Thrombin (0.15 mg) was dissolved in 1× PBS, treated with PPACK (α-thrombin inhibitor), as described previously,12 in a total volume of 10 mL 1× PBS at room temperature for 1 hour, and concentrated to 500 μL. PPACK-treated α-thrombin was labeled with biotin (1 µL of biotin-7-NHS at 20 mg/mL) in 1 mL of 1× PBS, incubated at room temperature for 2 hours, and concentrated down to 1 mg/mL. Twenty microliters of streptavidin beads was loaded onto a mini-column and washed 3 times with 1× PBS. Next, 5 μL of biotinylated α-thrombin (1 mg/mL) was added to the streptavidin beads followed by 10 μL of 1 mg/mL Fc-GPIb protein. Beads were washed 3 times with 1× PBS, and 1× PBS or excess agkisacucetin protein (15 µg) was added to the streptavidin beads before being washed again 3 times with 1× PBS. Forty microliters of 1× sodium dodecyl sulfate loading buffer was incubated with streptavidin beads for 10 minutes to release the bound proteins. After centrifugation, the supernatant was used for western blot analysis.
Duplicate samples were loaded onto 12% sodium dodecyl sulfate–polyacrylamide gels for electrophoresis. After electrophoresis, proteins were transferred from the gel to a nitrocellulose membrane. The membrane was blocked with 5% nonfat milk in 1× PBS with 0.1% Tween 20 (1× TPBS) for 30 minutes at room temperature and washed 3 times with 1× TPBS. One set of samples was incubated with anti-human Fc–horseradish peroxidase antibody (1:1000 in 1× TPBS) at room temperature for 1 hour to examine the level of Fc-tagged GPIb protein. A duplicate set of samples was probed with the streptavidin horseradish peroxidase antibody (1:1000 in 1× TPBS) at room temperature for 1 hour to examine the biotinylated thrombin protein level. After antibody incubation, the membranes were washed 3 times with 1× TPBS and developed using a SuperSignal West Pico Kit (Thermo Fisher Scientific), according to the manufacturer’s instructions.
For details about protein expression and purification, native polyacrylamide gel electrophoresis, crystallization and data collection, and structure determination and refinement see supplemental Information (available on the Blood Web site).
Results and discussion
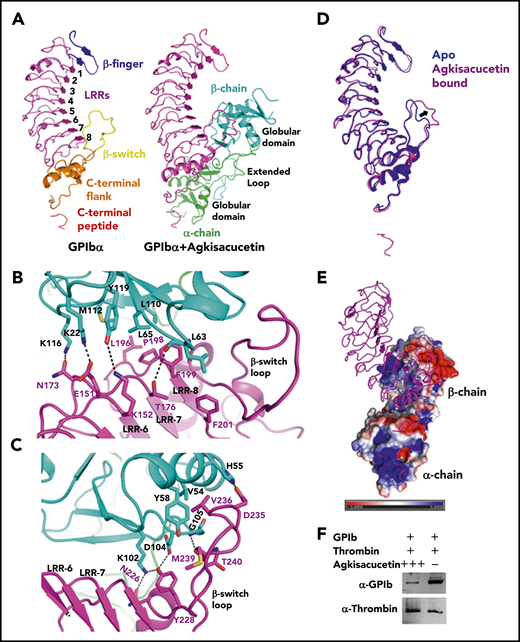
We purified GPIbα and agkisacucetin to homogeneity from CHO mammalian cells and Pichia pastoris. To understand the molecular recognition between agkisacucetin and GPIbα, we crystallized the agkisacucetin-GPIbα complex in P41212 space group with 1 GPIbα and 1 agkisacucetin in the asymmetric unit. The complex structure was refined to a resolution of 3.3 Å with a clear density map (supplemental Figure 1) and refinement statistics (supplemental Table 1). GPIbα and agkisacucetin are bound as a complex with 1:1 stoichiometry (Figure 1A). Typical of leucine-rich repeat (LRR) structures, GPIbα displays an elongated curved shape with 8 central LRRs capped by N-terminal β-finger and C-terminal flank regions at both ends (Figure 1A). It also contains a small visible segment of the C-terminal flexible peptide (residues 267-289) that is separated from the main body of the LRR domain (Figure 1A).13 Agkisacucetin has an overall elongated shape, consisting of 2 symmetrically arranged α and β subunits (Figure 1A). Each subunit has a compact lectin-like globular domain and a long extended loop region; 2 subunits are arranged with 2 globular domains at both ends and the extended loop regions in the middle.9 Agkisacucetin contacts the concave surface of GPIbα toward the C-terminal half of the LRR domain at an ∼50° angle (Figure 1A). The interactions involve 2 sites in GPIbα: the first contact site is near LRRs 6-8 and the previously reported β-switch loop region (residues 227-241) (Figure 1B-C); the second contact site involves residues at the C-terminal flank region and the C-terminal peptide after the C-terminal flank (supplemental Figure 2).13
Crystal structure of the GPIbα and agkisacucetin complex. (A) Overall structure of the GPIbα-agkisacucetin complex is shown as a cartoon representation (right panel). For the complex, GPIbα is purple, and the α and β chains of agkisacucetin are green and cyan, respectively. A separate GPIbα molecule is shown, with the regions colored for clarity (left panel). (B) Detailed contacts between the GPIbα LRR-6, LRR-7, and LRR-8 regions and the agkisacucetin β chain globular domain. Interacting residues are shown as stick models with hydrogen bonds shown as dotted lines. (C) Detailed contacts between the GPIbα β-switch region and the agkisacucetin β chain globular domain. Interacting residues are shown as stick models with hydrogen bonds shown as dotted lines. (D) Superposition of the GPIbα apo structure (Protein Data Bank identification code 1P9A) (blue) and the GPIbα-agkisacucetin complex (purple). The major conformational change in the β-switch region is indicated by an arrow. (E) Overview of the GPIbα-agkisacucetin complex structure, showing the electrostatic potential of agkisacucetin’s surface. GPIbα is shown in cartoon representation. Blue, positive potential; red, negative potential. (F) Agkisacucetin blocks the interaction between GPIb and thrombin. Fc-tagged GPIb, with or without excess agkisacucetin, was incubated with biotinylated thrombin prebound to streptavidin beads. The streptavidin bead–bound protein samples were analyzed by western blot to examine thrombin and GPIb protein levels using specific antibodies. + and – denote presence and absence of a given protein; +++ denotes excess amounts of protein.
Crystal structure of the GPIbα and agkisacucetin complex. (A) Overall structure of the GPIbα-agkisacucetin complex is shown as a cartoon representation (right panel). For the complex, GPIbα is purple, and the α and β chains of agkisacucetin are green and cyan, respectively. A separate GPIbα molecule is shown, with the regions colored for clarity (left panel). (B) Detailed contacts between the GPIbα LRR-6, LRR-7, and LRR-8 regions and the agkisacucetin β chain globular domain. Interacting residues are shown as stick models with hydrogen bonds shown as dotted lines. (C) Detailed contacts between the GPIbα β-switch region and the agkisacucetin β chain globular domain. Interacting residues are shown as stick models with hydrogen bonds shown as dotted lines. (D) Superposition of the GPIbα apo structure (Protein Data Bank identification code 1P9A) (blue) and the GPIbα-agkisacucetin complex (purple). The major conformational change in the β-switch region is indicated by an arrow. (E) Overview of the GPIbα-agkisacucetin complex structure, showing the electrostatic potential of agkisacucetin’s surface. GPIbα is shown in cartoon representation. Blue, positive potential; red, negative potential. (F) Agkisacucetin blocks the interaction between GPIb and thrombin. Fc-tagged GPIb, with or without excess agkisacucetin, was incubated with biotinylated thrombin prebound to streptavidin beads. The streptavidin bead–bound protein samples were analyzed by western blot to examine thrombin and GPIb protein levels using specific antibodies. + and – denote presence and absence of a given protein; +++ denotes excess amounts of protein.
The first site of interaction is the major interaction site, where the globular domain of agkisacucetin β chain contacts GPIbα LRR-6 through LRR-8 (Figure 1B) and the β-switch region (Figure 1C); the interaction buries a total area of 690 Å2. Residues K22, K116, M112, Y119, L63, L65, and L110 from 1 side of the globular domain of agkisacucetin β chain interact with E151 and K152 from LRR-6, N173 and T176 from LRR-7, and L196, P198, F199, and F201 from LRR-8 in GPIbα (Figure 1B). Residues K102, D104, H55, V54, Y58, and G105 from agkisacucetin β chain interact with residues N226, Y228, D235, V236, M239, and T240 from the β-switch loop in GPIbα (Figure 1C). To evaluate the importance of this interface for GPIb and agkisacucetin interaction, we measured the binding affinity of the wild-type, F199A, or Y228A mutant of GPIb protein to agkisacucetin by surface plasmon resonance. GPIb wild-type protein binds to agkisacucetin with a dissociation constant (KD) of 8.7 nM (supplemental Figure 3). GPIb mutants: F199A at LRR-8 and Y228A at the β-switch loop greatly diminished the binding of GPIb to agkisacucetin, supporting the importance of this interface (supplemental Figure 4; supplemental Table 2). The β-switch loop of GPIbα harbors a number of gain-of-function mutations related to platelet-type von Willebrand disease, such as G233V, K237V, and M239V, and is the key interaction site with VWF13 and with OS1, a potent cyclic peptide inhibitor of GPIbα.14 In the GPIbα and VWF complex structure, GPIbα interacts with VWF-D1 at 2 sites. At site 1, LRR-5 through LRR-8 and the C-terminal flank of GPIbα interact with VWF-A1 domain. At site 2, the N-terminal β finger and LRR-1 of GPIbα interact with residues at the bottom face of the A1 domain. The binding interface of agkisacucetin and GPIbα largely overlaps in the region close to the β-switch loop region, the GPIbα site 1 interface with VWF (supplemental Figure 5). The β-switch loop of GPIbα undergoes a dramatic conformational change upon complex formation with VWF-A; it transitions from a loop into 2 antiparallel β strands (supplemental Figure 5A). Interestingly, we also observed a major conformational change in this loop when interacting with agkisacucetin (Figure 1D; supplemental Figure 5B).
The second site of interaction is a minor interaction site, where agkisacucetin uses α chain to contact residues from the C-terminal flank and the C-terminal peptide of GPIbα; the interaction buries a total area of 333 Å2. Residues Y97, G120, and E122 from α chain of agkisacucetin interact with D222 and R218 from the C-terminal flank of GPIbα (supplemental Figure 2). The C-terminal peptide sequence of GPIbα is enriched with negatively charged residues: of residues 272 to 285, which extend out from the main LRR domain and interact with the positively charged surface on the α chain of agkisacucetin, 7 of 14 are acidic residues (Figure 1E). In particular, GPIbα E281, E282, and E285 residues interact with K20 and R35 on the globular domain of agkisacucetin α chain (supplemental Figure 2). Mutations of E281A and E282A of GPIbα had a modest effect on the GPIb-agkisacucetin interaction (supplemental Figure 6; supplemental Table 2), suggesting that these 2 residues are not critical for that interaction. The experimental electron density for the C-terminal anionic peptide was only partial. Future high-resolution structural work is needed to better characterize agkisacucetin’s interaction with the C-terminal peptide of GPIbα. Based on the structure of the GPIbα-thrombin complex, it was proposed that GPIbα first interacts with α-thrombin exosite II, which causes the C-terminal peptide to be dislodged from the main body of GPIbα, allowing it to interact with a second α-thrombin molecule through exposed exosite I, which promotes platelet activation and aggregation.12 The C-terminal peptide of GPIbα is very flexible and adopts different conformations when interacting with thrombin or agkisacucetin (supplemental Figure 7). R218, D222, E281, E282, and E285 are 5 residues of GPIbα that also contact thrombin exosite I. Thus, our complex structure suggests that agkisacucetin’s interaction with GPIbα at the second site could potentially interfere with GPIbα’s interaction with thrombin at exosite I (supplemental Figure 7B-C) and inhibit thrombin-induced platelet aggregation and activation. Using a pull-down assay and a native gel shift assay, we found that agkisacucetin indeed blocks the interaction between GPIbα and thrombin (Figure 1F; supplemental Figures 8 and 10), supporting this hypothesis. On the other hand, thrombin cannot block the interaction between GPIbα and agkisacucetin (supplemental Figure 9), which suggests that agkisacucetin binds more tightly to GPIbα. Presumably, the large surface contact area of agkisacucetin β chain with LRR-6 through LRR-8 and the β-switch region of GPIb is not affected by thrombin (supplemental Figure 7).
In this study, we report the first crystal structure of agkisacucetin in complex with GPIbα N-terminal domain. In addition to confirming that the GPIb-agkisacucetin interaction sterically blocks the GPIbα-VWF interaction, we provide new structural evidence that agkisacucetin could interfere with the GPIbα-thrombin interaction. Our structure provides key molecular insights into the mechanism of action of agkisacucetin: by targeting the essential receptor GPIb, agkisacucetin “kills 2 birds with 1 stone.” VWF and thrombin play important roles in platelet function: the GPIbα-VWF interaction is an early step in platelet adhesion and aggregation, whereas the GPIbα-thrombin interaction is more important for platelet activation and signaling. The relative contributions of these 2 interactions in normal platelet function and thrombosis await further experimentation, which will facilitate the design of better protein-based drugs for antithrombotic therapy.
Coordinates of the agkisacucetin-GPIbα complex have been deposited in the Protein Data Bank under identification code 6XFQ.
Data sharing requests should be sent to Zhongliang Zhu (zlzhu63@ustc.edu.cn).
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
The authors thank the staff at Beamline BL17U1 of the Shanghai Synchrotron Radiation Facility for assistance with data collection.
This work was supported by the Ministry of Science and Technology of China (2017YFA0504903 [L.N.] and 2012CB917200 [Z.Z.]), the National Natural Science Foundation of China (31621002 [L.N.] and 31270770 [Z.Z.]), and, in part, by Hefei National Science Center Pilot Project Funds.
Authorship
Contribution: J.W. and Y. Gao designed the experiments, analyzed the data, and wrote the manuscript; L. Chen, S.H., and L. Cheng performed the experiment of GPIbα and agkisacucetin complex formation; Y. Guo and W.X. performed recombinant expression and purification of agkisacucetin; J.K. revised the manuscript and assisted with the structural analysis; and Z.Z. and L.N. supervised the project.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Jiyuan Ke, H3 Biomedicine Inc, 300 Technology Square, FL5, Cambridge, MA 02139; e-mail: jiyuan_ke@h3biomedicine.com; Zhongliang Zhu, University of Science and Technology of China, School of Life Sciences, 96 Jinzhai Rd, Hefei, Anhui 230026, China; e-mail: zlzhu63@ustc.edu.cn; and Liwen Niu, University of Science and Technology of China, School of Life Sciences, 96 Jinzhai Rd, Hefei, Anhui 230026, China; e-mail: lwniu@ustc.edu.cn.
REFERENCES
Author notes
J.W. and Y. Gao contributed equally to this work.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal