TO THE EDITOR:
Myeloablative conditioning regimens before hematopoietic stem cell transplantation (HSCT) entail a very high risk of infertility.1,2 Prepubertal boys who do not yet produce sperm are offered a testicular biopsy along with cryopreservation of the immature testicular tissue (ITT-CP) to preserve the spermatogonial stem cells that give rise to spermatozoa at puberty.3-6 The use of cryopreserved ITT in nonhuman primates has been shown to lead to full restoration of spermatogenesis and births, thus providing genuine hope for future successes in human fertility.7-9 ITT-CP is an option for prepubertal boys with sickle cell disease (SCD) who are undergoing HSCT to treat severe genotypes (HbSS and HbSβ0 thalassemias). Before HSCT, hydroxyurea (HU) is widely used as a SCD treatment to reduce the frequency of vaso-occlusive and painful crises.10 However, alterations in sperm parameters have been reported in men with SCD,11 and HU is known to worsen this disorder.11,12 There are limited data available regarding the potential effect of SCD and HU on the spermatogonial pool. This is, however, of particular relevance for successful clinical use of ITT in fertility restoration. Several studies to date have reported a reduced spermatogonial pool in immature testicular tissue of prepubertal boys with SCD treated with HU.13-15 To analyze the specific effect of HU on immature testis, we compared the spermatogonial quantity in testicular tissue collected for fertility preservation in prepubertal boys with SCD who had or who had not been exposed to HU. We also compared the results with established reference values in healthy boys.16 This study was approved by the Research Ethics Committee of the Cochin Hospital, Paris, France (AAA-2019-08014) and was conducted in accordance with the Declaration of Helsinki.
Thirty patients with SCD who had undergone ITT retrieval (March 2010 to April 2019) were included. Thirteen patients had not been exposed to HU, and 17 had been administered HU at a median dose of 22.0 mg/kg per day and a median time of exposure of 36.0 months (Table 1; supplemental Table available on the Blood Web site). Six of the exposed patients were on-HU at the time of the ITT-CP, whereas the 11 others were off-HU, with a median washout period of 5.2 months. The surgery was bilateral for 18 patients and unilateral for 12 patients (supplemental Table). MAGE-A4 and GATA-4 markers were used to detect the spermatogonia and Sertoli cells, respectively (supplemental Material and Methods; Figure 1A-C).17,18 The spermatogonial pool was evaluated as the number of spermatogonia (S) per round cross section of seminiferous tubule (T) (S/T ratio), which has been proposed as a standard.13,16 The proportion of seminiferous tubules with Sertoli cells only (SCO), without germ cells, was also quantified.
Histologic analysis of testicular tissue samples retrieved from prepubertal patients with SCD in the context of fertility preservation according to exposure to hydroxyurea
| . | Exposed to HU . | Not exposed to HU . | P . | |||
|---|---|---|---|---|---|---|
| Off-HU . | On-HU . | Total . | P1 . | P2 . | ||
| Patients, n | 11 | 6 | 17 | 13 | ||
| Samples, n | 17 | 8 | 25 | 23 | ||
| Treatment characteristics | ||||||
| Age at HU onset, median (range), y | 5.2 (3.4-10.9) | 4.2 (2.2-9.0) | 5.0 (2.2-10.9) | — | — | .33 |
| HU dosing, median (range), mg/kg/d | 22.2 ± 4.0 (15-28) | 22.5 ± 2.7 (20-25) | 22.0 (15-28) | — | — | .91 |
| HU time of exposure, median (range), mo | 27.6 (8.0-67.0) | 37.5 (9.5-66.0) | 36.0 (8.0-67.0) | — | — | .80 |
| Washout period, median (range), mo | 5.2 (2.2-36.9) | 0.0 | 3.0 (0.0-36.9) | — | — | — |
| Transfusion therapy, median (range), mo | 24.9 (2.4-71.4)$ | 13.2 (3.0-36.0)* | 15.4 (2.4-71.4) | 15.4 (0.9-42.8)† | .70 | .62 |
| ITT histologic analysis | ||||||
| Age at ITT-CP, median (range), y | 10.1 (5.8-15) | 7.9 (4.2-11.3) | 8.8 (4.2-15.0) | 8.0 (4.2-11.8) | .39 | .42 |
| Cross-sections analyzed, mean ± SD (range), n | 77 ± 49 (10-165) | 102 ± 33 (60-143) | 85 ± 45 (10-165) | 87 ± 31 (51-148) | .9 | .26 |
| S/T ratio, mean ± SD (range) | 3.1 ± 4.0 (0.6-14.0) | 1.5 ± 0.7 (0.8-2.4) | 2.5 ± 3.3 (0.6-14.0) | 1.7 ± 0.6 (0.7-2.7) | .61 | .84 |
| SCO tubules, mean ± SD (range), % | 44 ± 23 (0-77) | 40 ± 17 (18-55) | 42 ± 21 (0-77) | 38 ± 16 | .52 | .72 |
| . | Exposed to HU . | Not exposed to HU . | P . | |||
|---|---|---|---|---|---|---|
| Off-HU . | On-HU . | Total . | P1 . | P2 . | ||
| Patients, n | 11 | 6 | 17 | 13 | ||
| Samples, n | 17 | 8 | 25 | 23 | ||
| Treatment characteristics | ||||||
| Age at HU onset, median (range), y | 5.2 (3.4-10.9) | 4.2 (2.2-9.0) | 5.0 (2.2-10.9) | — | — | .33 |
| HU dosing, median (range), mg/kg/d | 22.2 ± 4.0 (15-28) | 22.5 ± 2.7 (20-25) | 22.0 (15-28) | — | — | .91 |
| HU time of exposure, median (range), mo | 27.6 (8.0-67.0) | 37.5 (9.5-66.0) | 36.0 (8.0-67.0) | — | — | .80 |
| Washout period, median (range), mo | 5.2 (2.2-36.9) | 0.0 | 3.0 (0.0-36.9) | — | — | — |
| Transfusion therapy, median (range), mo | 24.9 (2.4-71.4)$ | 13.2 (3.0-36.0)* | 15.4 (2.4-71.4) | 15.4 (0.9-42.8)† | .70 | .62 |
| ITT histologic analysis | ||||||
| Age at ITT-CP, median (range), y | 10.1 (5.8-15) | 7.9 (4.2-11.3) | 8.8 (4.2-15.0) | 8.0 (4.2-11.8) | .39 | .42 |
| Cross-sections analyzed, mean ± SD (range), n | 77 ± 49 (10-165) | 102 ± 33 (60-143) | 85 ± 45 (10-165) | 87 ± 31 (51-148) | .9 | .26 |
| S/T ratio, mean ± SD (range) | 3.1 ± 4.0 (0.6-14.0) | 1.5 ± 0.7 (0.8-2.4) | 2.5 ± 3.3 (0.6-14.0) | 1.7 ± 0.6 (0.7-2.7) | .61 | .84 |
| SCO tubules, mean ± SD (range), % | 44 ± 23 (0-77) | 40 ± 17 (18-55) | 42 ± 21 (0-77) | 38 ± 16 | .52 | .72 |
In the comparison of HU-exposed and nonexposed groups, patients with bilateral surgery account for a single value (see supplemental Materials and Methods). P1, comparison of values between HU-exposed and nonexposed patients, Student t test; P2, comparison of values between off-HU and on-HU patients, Mann-Whitney test.
One missing data point.
Two missing data points.
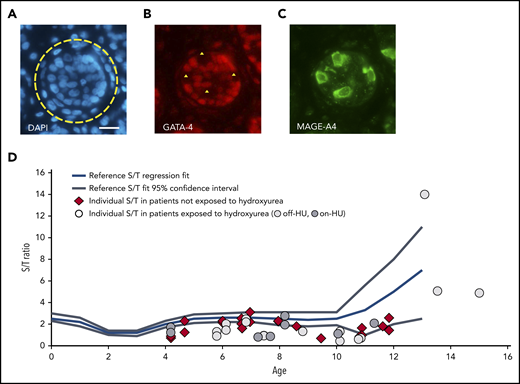
Spermatogonia in the testicular tissue of prepubertal patients with SCD compared with reference values. (A-C) Immunostained section of the testicular tissue of a patient aged 6.1 years who had previously been exposed to hydroxyurea (Off-HU patient 3). (A) Selection of a round tubular cross section (yellow circle). 4′,6-Diamidino-2-phenylindole staining (blue). (B) Expression of GATA-4 nuclear protein in Sertoli cells (red staining). (C) Expression of MAGE-A4 cytoplasmic protein in spermatogonial cells (green staining). Negative images of spermatogonial cells in panel C (yellow triangle). Four spermatogonia could be counted in this selected section. Scale bar = 50 µm. (D) The number of spermatogonia per round tubular cross section (S/T) of patients exposed to hydroxyurea (light gray circle: off-HU; dark gray circle: pon-HU) or not exposed to hydroxyurea (red diamond). Individual S/T values plotted on a meta-regression fit line of S/T reference values during testis development.16 Each mark represents the mean S/T value of a patient. The S/T values of prepubertal patients with SCD were significantly lower than the reference S/T values (Wilcoxon signed-rank test, P<.0001).
Spermatogonia in the testicular tissue of prepubertal patients with SCD compared with reference values. (A-C) Immunostained section of the testicular tissue of a patient aged 6.1 years who had previously been exposed to hydroxyurea (Off-HU patient 3). (A) Selection of a round tubular cross section (yellow circle). 4′,6-Diamidino-2-phenylindole staining (blue). (B) Expression of GATA-4 nuclear protein in Sertoli cells (red staining). (C) Expression of MAGE-A4 cytoplasmic protein in spermatogonial cells (green staining). Negative images of spermatogonial cells in panel C (yellow triangle). Four spermatogonia could be counted in this selected section. Scale bar = 50 µm. (D) The number of spermatogonia per round tubular cross section (S/T) of patients exposed to hydroxyurea (light gray circle: off-HU; dark gray circle: pon-HU) or not exposed to hydroxyurea (red diamond). Individual S/T values plotted on a meta-regression fit line of S/T reference values during testis development.16 Each mark represents the mean S/T value of a patient. The S/T values of prepubertal patients with SCD were significantly lower than the reference S/T values (Wilcoxon signed-rank test, P<.0001).
The HU-exposed and nonexposed groups were comparable in terms of the patient age at ITT-CP and the mean number of tubular cross sections observed (Table 1). Histologic analysis revealed that the spermatogonial pool was not statistically different between the two groups: S/T ratio = 2.5 ± 3.3 vs 1.7 ± 0.6, respectively, (P = .61); SCO = 42 ± 21 vs 38 ± 16%, respectively, (P = .52) (Table). The spermatogonial pool was also not statistically different between the off-HU and the on-HU subgroups (S/T ratio = 3.1 ± 4.0 vs 1.5 ± 0.7, respectively, P = .84) (Table). The latter data warrant additional confirmation with more samples, although it is in accordance with the absence of a correlation between the wash-out delay and the spermatogonial count (r = 0.09, P = .73). The S/T ratio for each of the 48 testicular tissue samples was plotted on the meta-regression fit line of S/T reference values (Figure, panel D).16 Comparison of the S/T ratio of the patients with SCD to the age-related S/T reference values confirmed that the spermatogonial quantity in SCD patients was lower than in healthy boys, with a highly significant P value (Wilcoxon signed-ranks test: P < .0001). Interestingly, the subgroup of the 6 teenagers (≥11 years of age) with a mean S/T value of 5.6 ± 4.6 was not different from the healthy boys for this age group (P = .34). The latter result nevertheless needs to be interpreted with caution because of the small sample size and the large standard deviation. Finally, there was no correlation between the duration of the transfusion therapy and the spermatogonial count (r = −.15, P = .33).
To the best of our knowledge, this study is the largest quantitative study of the spermatogonial pool in boys with SCD. Unlike previous reports in which all the boys with SCD were exposed to HU,13-15 we compared HU-exposed vs nonexposed patients. As a result, the similar S/T ratio and proportion of SCO between the groups indicates that the spermatogonial depletion observed in prepubertal patients with SCD treated with HU was largely related to the disease itself and not to toxicity of the HU. The less frequent division of the spermatogonial stem cells could explain their relative resistance to HU, specifically to the inhibition of DNA synthesis and the induction of cell cycle arrest and apoptosis. An alternative could be that the daily exposure to HU at pharmacologic doses used in SCD boys is too low to induce a lasting detrimental effect on the spermatogonial pool. We emphasize that we could not address the gonadic effect of HU in infancy, because the median age at HU onset in the studied patients was 5 years, with only 1 patient aged 2 years. We therefore cannot exclude that very early HU introduction may have a specific effect on the testis of very young infants. Of note, a research team recently reported a nonaffected spermatogonial pool in 3 SCD patients.19 Other than the very small sample size, this contradictory result could be explained by the fact that the patients were compared with nontreated cancer patients, who may have low spermatogonial counts, unlike healthy boys. SCD is a hemoglobinopathy that affects patients from very early infancy as a result of chronic hemolytic anemia, multiple vaso-occlusive complications, and painful crises.20 The low spermatogonial quantity in patients with SCD may be related to testicular vaso-occlusive subclinical episodes and possible asymptomatic infarction,21,22 as well as testicular perfusion deficit as a result of chronic anemia. The establishment of spermatogonial DNA methylation has been shown to be altered in prepubertal patients with SCD.19 This could be another explanation for the spermatogonial depletion, as correct establishment of the methylation profile is necessary for spermatogonial maintenance. SCD treatments, such as transfusion therapy, did not provide any beneficial effect on the spermatogonial count. This could be because of the complexity of the mechanisms involved in the spermatogonial depletion affecting SCD. We noted, however, that spermatogonial mitotic expansion, physiologically described in peripubertal healthy boys,16 also occurred for the teenagers of our series. Despite the limitation of the small sample size, this suggests that the spermatogonia in young patients with SCD retain a certain capacity to self-renew and to expand at puberty.
In conclusion, we showed that depletion of the spermatogonial pool in prepubertal patients with severe SCD genotypes is related to the disease itself and not to HU toxicity. In the absence of adverse side effects on the spermatogonial quantity, concerns regarding HU gonadotoxicity should hence not affect treatment decisions in young patients with severe SCD genotypes.
Contact the corresponding author for original data.
The online version of this article contains a data supplement.
Acknowledgments
A.-S.G. is supported by a grant from ARC Fundation pour la Recherche contre le Cancer (DOC20180507400). The human Spermatogonial Stem Cell research project (A.-S.G., L.R., J.-P.W., P.F., and V.B.-L.) is funded by grants from the Agence de Biomédecine and Electricité De France. It also benefits from donations of the Laurette Fugain and the Entraide aux Greffés de Moelle Osseuse Associations.
Authorship
Contribution: A.-S.G. and V.B.-L. designed and performed the experiments; A.-S.G., V.B.-L., C. Poirot, and D.V. analyzed and interpreted the data; A.-S.G., V.B.-L., C. Poirot, and P.F. were in charge of the preparation, drafting, and editing of the manuscript; C. Poirot performed fertility preservation counseling for all patients; all other authors contributed to the data collection and to critically reviewing the manuscript; and all authors agreed to the final version of the manuscript.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Virginie Barraud-Lange, Assistance Publique-Hôpitaux de Paris, Hôpitaux Universitaires Paris Centre, CHU Cochin, Laboratoire d’Histologie Embryologie Biologie de la Reproduction CECOS, 123 Boulevard de Port Royal, 75014 Paris, France; e-mail: virginie.barraud-lange@aphp.fr.
REFERENCES
Author notes
C. Poirot and V.B.-L. contributed equally to this study.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal