In this issue of Blood, Gille et al1 provide intriguing observations regarding spermatogonial progenitor cells in prepubescent males with sickle cell anemia (SCA) and hydroxyurea exposure.
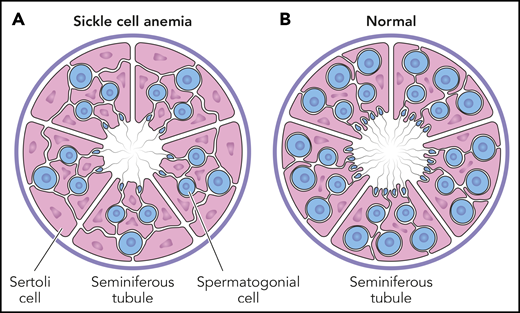
Testicular histology in prepubescent males. The cross-section of a seminiferous tubule contains both spermatogonial progenitor cells (blue) and supportive Sertoli cells (pink). The average number of spermatogonia per tubule (S/T ratio) is significantly less in boys with SCA (A), compared with a normal reference control (B). For boys with SCA, the S/T ratio is not different according to hydroxyurea exposure, indicating that spermatogonial depletion is related to the underlying disease itself, rather than hydroxyurea toxicity. Professional illustration by Patrick Lane, ScEYEnce Studios.
Testicular histology in prepubescent males. The cross-section of a seminiferous tubule contains both spermatogonial progenitor cells (blue) and supportive Sertoli cells (pink). The average number of spermatogonia per tubule (S/T ratio) is significantly less in boys with SCA (A), compared with a normal reference control (B). For boys with SCA, the S/T ratio is not different according to hydroxyurea exposure, indicating that spermatogonial depletion is related to the underlying disease itself, rather than hydroxyurea toxicity. Professional illustration by Patrick Lane, ScEYEnce Studios.
Hematopoietic stem cell transplantation (HSCT) is well recognized as curative therapy for many life-threatening blood disorders, including SCA, but also has known deleterious effects on gonadal function and fertility. Myeloablative conditioning regimens almost inevitably lead to sterility in patients with SCA undergoing transplantation.2 To help preserve fertility, pre-HSCT sperm or egg cryopreservation is routinely offered to adolescents and young adult males and females, respectively. Unfortunately, these are not viable options for prepubertal patients undergoing HSCT. In this setting, more creative experimental solutions involving cryopreservation of immature gonadal tissue itself (testicular biopsies for boys and ovarian fragments after oophorectomy for girls) are needed to maintain the possibility of future gonadal autograft and restored fertility.
Ideally such gonadal preservation would occur before chemotherapy administration, because most myeloablative conditioning regimens include genotoxic agents, meaning they cause damage to DNA. Not all genotoxicity is the same, however, as some treatments are predominately aneugenic (generating abnormal numbers of chromosomes), whereas others are clastogenic (causing structural damage to chromosomes) and others are mutagenic (leading to permanent and transmissible nucleotide changes).3 The latter category of genotoxicity is particularly dangerous: in the case of somatic cells, mutagenesis is a key driver of carcinogenesis; in the case of germ cells, mutation can result in numerous inherited Mendelian diseases.
Where does hydroxyurea fit into this discussion? Hydroxyurea induces fetal hemoglobin and has become standard of care for young patients with SCA. Treatment of affected children as young as 9 months of age is now recommended by the National Institutes of Health (NIH), the American Society of Hematology, and the British Society of Haematology. Hydroxyurea is a potent ribonucleotide reductase inhibitor that causes imbalanced intracellular deoxynucleotide pools; this effect stalls DNA replication forks, which over time can collapse. The exact steps by which double-strand DNA breaks can occur following replication fork collapse are not fully elucidated, but likely involve normal DNA repair mechanisms.4 Because many prepubescent children with SCA now receive hydroxyurea, the issue of drug-related genotoxicity on immature spermatogonia (S) is an important question, especially for boys who may eventually receive HSCT.
In the current study, a network of French investigators describes the effects of hydroxyurea on spermatogonial progenitor cells in prepubescent males with SCA, before undergoing HSCT with a myeloablative conditioning regimen. Over a 10-year period, 30 boys with SCA received immature testicular tissue cryopreservation, including 13 with no previous hydroxyurea treatment and 17 with a median of 36 months of hydroxyurea exposure. To quantify the stem cell pool, immunohistological staining identified both S and seminiferous tubules (T), and a standardized S/T ratio was calculated.
Importantly, the S/T ratio in young patients with SCA was not reduced by hydroxyurea exposure (see figure). Furthermore, within the cohort of boys with hydroxyurea exposure, there was no statistical difference between those currently receiving hydroxyurea and those who stopped treatment an average of 5 months before testicular biopsy. These observations provide direct evidence for the lack of in vivo hydroxyurea-related decreases to the spermatogonial pool in young males with SCA. The authors offer potential explanations for the lack of observed toxicity, including relative quiescence of spermatogonial stem cells and the relatively low pharmacological doses of hydroxyurea.
Of equal importance was the comparison of S/T ratios among children with SCA to age-matched healthy male controls. The spermatogonial stem cell pool was significantly decreased for the entire cohort of children with SCA, regardless of hydroxyurea exposure, indicating that spermatogonial depletion was related to the underlying disease itself, rather than hydroxyurea toxicity. Perhaps vaso-occlusion and infarction occur within testicular tissue of young males more frequently than realized clinically, which damages spermatogonial stem cells and ultimately affects future spermatozoa. This hypothesis helps explain the recent report of substantial quantitative and qualitative semen abnormalities in 15 young men with SCA, with no differences according to prepubertal hydroxyurea exposure.5
Future work in this area will benefit from additional considerations related to hydroxyurea’s genotoxic mode of action. As the authors note, a quiescent cellular state is inherently protective. This is especially true for hydroxyurea, which mainly affects the S-phase of dividing cells. Although hydroxyurea causes measurable genotoxicity in certain specific tissue compartments, its effects are overwhelmingly clastogenic and not mutagenic.6 Because clastogenicity is a highly toxic lesion, affected cells are unlikely to survive and pass on their altered genome, unlike cells exposed to mutagenic agents.
Numerical observations like those reported by Gille and colleagues represent welcome data that inform the ongoing and seemingly endless debate about the long-term safety of hydroxyurea therapy for children with SCA. Despite almost 4 decades of clinical experience and overwhelming cumulative evidence for its efficacy to reduce both morbidity and mortality, the theoretical risks of hydroxyurea treatment still dominate the conversation, even when compared with the known life-threatening risks of nontreatment.
Ultimately, in vitro and preclinical studies can only document drug-related toxicity, whereas safety requires careful and long-term human observations. The NIH-funded Multicenter Study of Hydroxyurea reported that adult patients with SCA and hydroxyurea exposure had >100 offspring with no danger signals related to fertility or teratogenicity.7 Unfortunately, the subsequent NIH-funded pediatric SCA cohorts (HUG-KIDS and BABY HUG, #NCT00006400) were not followed long enough to determine any potential effects on fertility. Currently, the European Sickle Cell Disease Cohort study (ESCORT-HU, #NCT02516579) is a prospective multicenter observational study of children and adults with sickle cell disease receiving hydroxyurea therapy, with >1900 patients (almost half pediatric) enrolled from 63 centers in France, Germany, Greece, and Italy. Initial reports from ESCORT-HU document a lack of hydroxyurea toxicity on pediatric growth and development,8 as well as anecdotal safety to both mother and fetus despite continuous exposure during pregnancy.9 In addition, ongoing hydroxyurea research trials in low-resource settings are collecting vital longitudinal data on growth and development, with >1000 children with SCA currently treated in sub-Saharan Africa and the Caribbean.10 Eventually, these cohorts should provide sufficient numbers for all patients and health care providers to feel safe using hydroxyurea as life-saving therapy for children with SCA.
Conflict-of-interest disclosure: The authors declare no competing financial interests.