TO THE EDITOR:
High cure rates for pediatric acute lymphoblastic leukemia (ALL) have resulted in an additional focus on the mitigation of treatment-related toxicities.1 These toxicities adversely affect the quality of life of patients and survivors.2,3 Osteonecrosis, localized bone death seen most frequently in the hips, knees, and shoulders, can result in significant pain and impaired mobility that frequently requires major joint surgery and may limit physical function throughout survivorship.2,4 Osteonecrosis results from alterations in blood flow to susceptible joints and can lead to collapse of the bone at the joint surfaces; it is associated with intensive glucocorticoid and asparaginase therapy and is most frequently seen in adolescents and young adults.4,5
Genome-wide association studies (GWAS)5-7 and candidate gene analyses8-10 over the last several years have elucidated genetic variation associated with the development of osteonecrosis in children with ALL. Multiple studies have identified associations between glutamate receptor variations and the development of osteonecrosis5,6 ; however, these genetic variations seem to be influenced by variations in therapy. Thus, identification of genetic factors that play a role in large, uncontrolled populations not receiving intensive chemotherapy may offer novel insights into risk factors for this toxicity.
To address this question, we performed GWAS in 2 Children’s Oncology Group trials for children and young adults with newly diagnosed ALL, AALL0434 (#NCT00408005),11,12 which studied T-cell ALL, and AALL0232 (#NCT00075725),5,13 which studied high-risk B-cell precursor ALL. Both trials included nearly identical chemotherapy backbones and randomizations between 2 methods of methotrexate intensification. In addition, the AALL0434 cohort included a randomization for the addition of the novel nucleoside analog nelarabine, which was given without other chemotherapy during 3 phases of therapy. Because of this interruption of the osteonecrosis-causing drugs (dexamethasone and asparaginase),4-6 and to align the chemotherapy regimens between the 2 ALL cohorts, patients receiving nelarabine were excluded.
For the AALL0434 cohort, remission peripheral blood samples from 709 patients with available data and appropriate consent (supplemental Figure 1, available on the Blood Web site) were genotyped by using the Illumina Infinium Omni2.5Exome array as previously described.5 Additional genetic variations were imputed by using the Michigan Imputation server 1000Genome phase3 panel.14 Genetic ancestry was determined by using the computer software package STRUCTURE.15
For analysis, patients who developed Common Terminology Criteria for Adverse Events version 4 avascular necrosis grade 2 or higher were considered cases, with time at risk determined from the on-therapy date to the date of the event. Controls were censored at withdrawal from therapy, death, second malignancy, relapse, or last follow-up, whichever came first. All studies were approved by relevant institutional review boards, and all participants or their parents/legal guardians provided written informed consent.
Before GWAS, multivariable Cox proportional hazards regression was used to identify relevant covariates for inclusion in the association analysis (supplemental Table 1). A total of 9.6 million single nucleotide polymorphism variants (SNPs) with a minor allele frequency of at least 1% were assessed for their association with osteonecrosis in AALL0434 after adjusting for age and ancestry (supplemental Figure 2). SNPs associated with osteonecrosis at P < .01 were selected for further analysis. To prioritize these, we then queried the association of these SNPs from publicly available GWAS result databases based on the UK BioBank database (UKBB).16 SNPs associated within the UKBB with “aseptic necrosis of the bone” (International Classification of Diseases, Tenth Revision, code 733.4) at P < .01 were further evaluated in the AALL0232 cohort (supplemental Table 2).5,13 Association testing used Bonferroni correction for each independent locus to determine the significance P value. SNPs were also assessed in a meta-analysis of the ALL cohorts using Stouffer’s method.
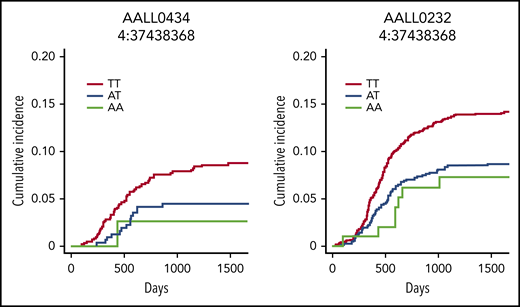
No SNPs reached the genome-wide significance threshold of <5 × 10−8 in the discovery AALL0434 cohort (supplemental Figure 3). Among 24 503 SNPs with values of P < .01, a total of 173 SNPs within 48 independent loci were prioritized for validation in the UKBB cohort (P < .01) (supplemental Figure 2; supplemental Table 2). Of these 173, fourteen SNPs within one locus on chromosome 4 were significantly associated with osteonecrosis in the validation AALL0232 cohort (P < .001). Two SNPs, both significant in the analysis using all 3 cohorts, also met the meta-analysis genome-wide significance threshold using the 2 ALL cohorts (n = 2,994 patients). The lead SNP within this region was rs2973215, a variant within intron 5 of NACHT and WD repeat domain containing 2 (NWD2) (supplemental Figure 4). The T allele (allelic frequency 79.1%) was associated with a hazard ratio of 4.1 (95% confidence interval [CI], 2.12-7.92) in the AALL0434 cohort, a hazard ratio of 1.37 (95% CI, 1.18-1.60) in the UKBB cohort, and a hazard ratio of 1.57 (95% CI, 1.24-1.99) in the AALL0232 cohort (Table 1). An allelic dose effect was observed: the 3-year incidence of osteonecrosis in patients on the AALL0434 trial with a TT diplotype was 7.7%, 4.8% with an AT diplotype, and 2.5% with an AA diplotype (Figure 1). A similar pattern was observed in the ALLL0232 cohort (13.4% vs 8.5% vs 7.5%). NWD2 is a paralog of Angio Associated Migratory Cell Protein (AAMP). AAMP contains a binding domain for heparin and plays a role in both angiogenesis and cellular migration, particularly of endothelial cells; endothelial cell damage is a critical component of the vascular pathology leading to osteonecrosis.17-19 This SNP lies within a weak DNase hypersensitivity site in fetal bone, osteoblasts, and multiple other tissues,20,21 suggesting a potential biological link between the identified variants and the development of osteonecrosis. However, additional research is needed to confirm this theory.
Top SNPs associated with osteonecrosis across the 3 cohorts
| . | . | . | . | AALL0434 (55 cases, 654 controls) . | UK BioBank (542 cases, 391 041 controls) . | AALL0232 (250 cases, 2035 controls) . | . | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| dbSNP . | Chr: pos . | Risk allele . | Alternate allele . | RAF . | HR . | P . | RAF . | HR . | P . | RAF . | HR . | P . | ALL meta P . |
| rs2973215 | 4:37438368 | T | A | 0.799 | 4.1 | 3.29 × 10−5 | 0.812 | 1.37 | 5.68 × 10−5 | 0.791 | 1.57 | .000233 | 3.05 × 10−8 |
| rs1479756 | 4:37437061 | A | G | 0.774 | 4.06 | 1.67 × 10−5 | 0.784 | 1.32 | .000175 | 0.764 | 1.48 | .000583 | 4.35 × 10−8 |
| . | . | . | . | AALL0434 (55 cases, 654 controls) . | UK BioBank (542 cases, 391 041 controls) . | AALL0232 (250 cases, 2035 controls) . | . | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| dbSNP . | Chr: pos . | Risk allele . | Alternate allele . | RAF . | HR . | P . | RAF . | HR . | P . | RAF . | HR . | P . | ALL meta P . |
| rs2973215 | 4:37438368 | T | A | 0.799 | 4.1 | 3.29 × 10−5 | 0.812 | 1.37 | 5.68 × 10−5 | 0.791 | 1.57 | .000233 | 3.05 × 10−8 |
| rs1479756 | 4:37437061 | A | G | 0.774 | 4.06 | 1.67 × 10−5 | 0.784 | 1.32 | .000175 | 0.764 | 1.48 | .000583 | 4.35 × 10−8 |
Chr, chromosome; dbSNP, Single Nucleotide Polymorphism database; HR, hazard ratio; pos, Genome Reference Consortium Human Build 37 position; RAF, risk allele frequency.
Cumulative incidence of osteonecrosis in patients with ALL according to NWD2 genotype. Cumulative incidence of symptomatic osteonecrosis (grade 2 or higher per the Common Terminology Criteria for Adverse Events, version 4) in children treated on AALL0434 (left) and AALL0232 (right). The T (major) allele at rs2973215 in NWD2 was associated with an increased risk of symptomatic osteonecrosis in both cohorts (hazard ratios of 4.1 and 1.57, respectively); patients with 2 copies of the minor A allele had the lowest risk of this toxicity.
Cumulative incidence of osteonecrosis in patients with ALL according to NWD2 genotype. Cumulative incidence of symptomatic osteonecrosis (grade 2 or higher per the Common Terminology Criteria for Adverse Events, version 4) in children treated on AALL0434 (left) and AALL0232 (right). The T (major) allele at rs2973215 in NWD2 was associated with an increased risk of symptomatic osteonecrosis in both cohorts (hazard ratios of 4.1 and 1.57, respectively); patients with 2 copies of the minor A allele had the lowest risk of this toxicity.
This study shows the utility of combining carefully controlled cohorts treated for rare conditions such as childhood leukemia, with uncontrolled datasets representing large populations to identify novel genetic risk variants for disease. Although pediatric ALL is the most common malignancy in childhood, the annual incidence is only 3.5 per 100 000 person-years for children aged <20 years.22 Despite this rarity, large clinical trials with carefully curated data have allowed cure rates to increase to >90% with current therapy.1 One major advantage of studies in ALL is the receipt of protocol-directed therapy, which standardizes exposures across the population. This controlled exposure, combined with the patients’ younger age and thus fewer preexisting conditions, allows association between therapy exposures and subsequent toxicity. These association analyses are furthered by prospective data capture of toxicities using standardized definitions (ie, Common Terminology Criteria for Adverse Events version 4). The association of the NWD2 variant despite the lack of such controls in the UKBB bolsters the robustness of the association. The association between the NWD2 variant and osteonecrosis in the current study, which includes 2 large pediatric ALL cohorts and a large adult population cohort, suggests that the variant influences the risk of osteonecrosis in multiple contexts. Demonstrating the role that treatment plays, the rs2973215 variant was not associated with osteonecrosis in patients treated with nelarabine (P = .15), although this analysis is limited by the rarity of osteonecrosis in this cohort, while the rs10989692 variant near GRIN3A previously associated with osteonecrosis in the AALL0232 cohort5 had a nonstatistically significant trend toward increased risk in the AALL0434 population (hazard ratio, 1.48; 95% CI, 0.85-2.57; P = .16). A clinical analysis of osteonecrosis in AALL0434 will be presented in a forthcoming article.
In summary, our study leverages the strengths of large pediatric clinical trial cohorts along with the power of a large adult cohort to identify a novel association with osteonecrosis. Although treatment still strongly influences the impact of these variants, our finding supports the use of this approach in genomic discovery research.
Requests for deidentified data may be submitted to the corresponding author (Seth E. Karol; e-mail: seth.karol@stjude.org).
The online version of this article contains a data supplement.
Acknowledgments
This research was supported by the American Lebanese Syrian Associated Charities and the National Institutes of Health, National Cancer Institute (K08CA250418, CA142665, CA21765, U10CA180886, and U10CA180899), and the National Institute of General Medical Sciences (GM115279).
The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
Authorship
Contribution: W.Y., M.V.R., and S.E.K. designed the study; W.Y., M.D., Y.L., C.S., Y.D., and L.A.M. analyzed data and performed data analyses; M.D., N.W., S.P.H., M.L.L., E.A.R., E.C.L., W.L.C., S.S.W., K.P.D., and L.A.M. designed the clinical trials; and S.E.K. wrote and revised the manuscript with contributions from all authors.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Seth E. Karol, 262 Danny Thomas Pl, MS 260, Memphis, TN 38105; e-mail: seth.karol@stjude.org.
REFERENCES
Author notes
M.V.R. and S.E.K. contributed equally to this study.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal